Figure 10.1 The matrix of drivers for special formulations.
10.2 Paediatric medication
Children are far from being a homogeneous group. They range from:
- preterm newborn infants (premature: average weight <3.4 kg)
- full-term infants (neonates: 0–27 days)
- infants and toddlers (28 days to 23 months: 3.4–12.4 kg)
- children from 2 to 11 years (12.4–39 kg)
- adolescents from 12 to 16/18 years (on average 39–72 kg for males and 60 kg for females).
Formulations are not designed or selected in isolation from the biological realities. There are age-related developmental changes in the pharmacokinetics1,2 and pharmacodynamics of drugs. Changes occur in gastric acidity, drug clearance and receptor expression. Many of these changes are relevant to drug absorption.
Neonatal stomachs are achlorhydric soon after birth, so the absorption of acid-labile drugs may be increased. The rate of gastric emptying falls as infants get older, being faster in the neonate than in adults, but slower in infants and children than in adults. This has consequences with some formulations. Because the liver takes up a relatively large percentage of body volume in infants, clearance rates often exceed those in adults. It is clear then that extrapolation of results from adult data sets in terms of absorption or the behaviour of both drugs and dose forms is hazardous. Many of these issues are discussed in more detail in a review on the topic of developing paediatric medicines.3
A study over 60 years ago illustrated differences in the absorption of penicillin in neonates and children (Fig. 10.2). One of the key causes is the achlorhydria in neonates so that the acid-labile penicillin survives the passage through the stomach and can explain the results of elevated levels in neonates compared to infants and children up to 13 years old.
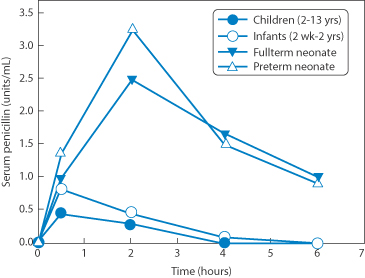
Figure 10.2 Evidence of the different extents of absorption of a weight-related dose of penicillin (a single 22 000 U kg−1) administered to preterm or full term neonates infants and children, the result of the achlorhydria in the neonates.
Reproduced from Huang NN, High RH. Comparison of serum levels following the administration of oral and parenteral preparations of penicillin to infants and children of various age groups. J Pediatr 1953;42:657–669. Copyright Elsevier 1953.
Figure 10.3 illustrates the variety of approaches to paediatric dosing using solutions, powder, granules (accumulation) or dividing solid-dose forms (‘partition’). The scheme makes clear the options available to achieve precise incremental dosing, for example, by using mini-tablets whose quantity for delivery in a capsule can be adjusted appropriately.
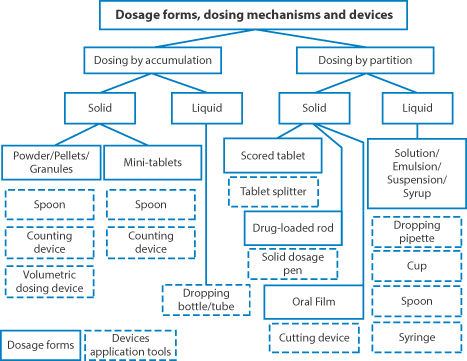
Figure 10.3 A diagram of the approaches to dosing for individual patients using an ‘accumulation’ or ‘partition’ approach.
Reproduced from Wening K, Breitkreuz J. Oral drug delivery in personalized medicine: unmet needs and novel approaches. Int J Pharm 2011;404:1–9. Copyright Elsevier 2011.
10.2.1 Acceptability of paediatric medication
Acceptability is driven by the characteristics of the patient (age, ability, disease type and state) and by the characteristics of a medicinal product, such as:
- palatability
- ease of swallowing (size and shape, integrity of dosage form, e.g. film coating)
- appearance (e.g. colour, shape, embossing)
- complexity of modification prior to administration (if required)
- required dose (e.g. the dosing volume, number of tablets, break marks)
- required dosing frequency and duration of treatment
- selected administration device (if any)
- primary and secondary container closure system
- actual mode of administration.
This list would apply to medicines designed for the elderly also.
10.2.2 Extemporaneous formulations
Extemporaneous formulations are frequently prepared from tablets, capsules and injections to produce medicines suitable for an individual child or for groups of children. In spite of pharmacists’ expertise in formulation and production, preparations made extemporaneously (except in emergencies) without the fullest quality control should be considered unacceptable in the 21st century. Cutting tablets for any age too is not always the optimal way forward. Nonetheless, it is the pharmacist’s role to provide appropriate medicine for individuals and the possibilities to prepare novel dose forms in hospitals and other environments is increasing. Three-dimensional printing of films and tablets will increasingly be a powerful tool.
Figure 10.4 illustrates some of the basic approaches used when converting existing formulations of tablets, capsules and injections into alternative forms. It may be that the contents of an injection can be incorporated directly into a suitable vehicle. The same may be true of soft gelatin capsules, whose contents may be amenable to emulsification depending on their nature. In a survey of extemporaneously prepared dosage forms it was found that 66% were aqueous suspensions, 22% solutions, 4% powders, 1.2% oils and 0.2% capsules,4 most for paediatric patients. These included:
- midazolam
- vancomycin
- clonidine hydrochloride
- diazoxide
- clobazam
- warfarin.
Many had neither chemical nor microbiological supporting data.
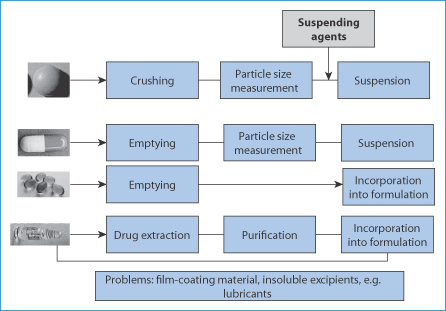
Figure 10.4 Stratagems applied when there is a need to convert existing dosage forms into medicines for children or the elderly: crushing of tablets, emptying hard and soft gelatin capsules or in a few cases extracting the active substance from products when the active is unavailable in pure chemical form. In all cases key parameters should be determined: stability, particle size distribution of granules and powders, purity of drug substance.
Developing paediatric medicines: liquid or solid oral forms?
Liquid formulations are often favoured because of the ease of administration and acceptability to the child. Taste masking is more of an issue with liquid formulations, but solid-dose forms can be difficult to swallow and may lodge in the young oesophagus. Liquid formulations suffer somewhat from variability of measurement of dose, although oral injectors can reduce this frequent domestic error. The teaspoon is far from a standard measure, delivering in use between 2.5 and 9 mL. If tablets are crushed to provide drug for extemporaneous formulations, the process must be standardised as far as possible and the particle size distribution must be evaluated as minimal quality procedures. Often the issue is to reduce doses, but also to provide a dosage form that aids swallowing in both the young and the elderly.
The importance of solvent choice
Figure 10.5 shows clearly the possible effect of solvents in extemporaneous preparations, in this case of oxandrolone (I) which degrades hydrolytically. Earlier chapters discuss the question of stability – the chemical stability of drug substances and excipients and the physical stability of formulations. The example here is simply to illustrate the marked effect in practice of the choice of suboptimal solvents, even the simple solvents discussed in this case.
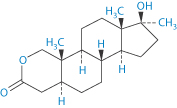
Structure I Oxandrolone
Clearly, given the complexity of some of the vehicles used, this is not a textbook example which explains the data, but in practical terms it highlights the importance of appropriate assays and stability assessments in extemporaneous preparations.
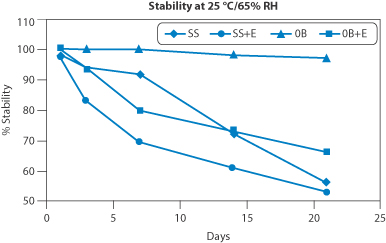
Figure 10.5 The chemical stability of oxandrolone (structure I) prepared with four different solvents: SS, Simple Syrup; SS + E, Simple Syrup with 8% ethanol; OB, Orablend (Paddock Laboratories, USA); OB + E, Orablend with 8% ethanol. Orablend itself comprises purified water, sucrose, glycerin, sorbitol, flavouring, microcrystalline cellulose, carboxymethylcellulose sodium, xanthan gum, carrageenan, calcium sulfate, trisodium phosphate, citric acid and sodium phosphate as buffers, dimethicone antifoam emulsion. Preserved with methylparaben and potassium sorbate.
Reproduced from Garg A et al. Development of an extemporaneous oral liquid formulation of oxandrolone and its stability evaluation. Burns 2012;37:1150–1153. Copyright Elsevier 2012.
Oral liquid captopril formulations for paediatric patients used in UK hospitals have been found to have variable stability, partly as a result of variability of the purity of the captopril used.5 Even with a single source of drug, stability after 1 month at room temperature ranged from 71.5% to 100%, although with storage at 5 ± 3°C the range was narrowed (82–99.7%). At the latter storage temperatures, the formulation is stable for 2 years. Captopril oxidation is catalysed by metal ions, hence the value of ethylenediaminetetraacetic acid and avoidance of contact with metal ions. Apparently, a variety of unlicensed captopril products have been used in 13 tertiary paediatric cardiac centres in the UK and 13 large hospitals referring patients to these centres: four hospitals dispensed captopril tablets by crushing and dissolving in water before administration, and the other 22 used nine different liquid formulations. As the authors conclude,6 this degree of inconsistency raises ‘issues about optimal captopril dosing and potential toxicity, such that its use may influence paediatric cardiac surgical and interventional outcomes’. |
Excipients
There are some restrictions in the range of excipients that can be used in paediatric formulations.7 As neonates do not metabolise alcohol efficiently, ethanol should be used with caution. There have been reports of injury to the gastrointestinal tract of neonates; thus, hypertonic solutions should not be used.
The American Academy of Pediatrics Committee on Drugs recommendation8 is that the quantity of ethanol contained in a single dose of a drug preparation should not produce a blood concentration greater than 25 mg 100 mL–1. A suitable alternative is a less toxic solvent such as glycerin. Preservatives are not contraindicated in formulations for children generally, but should be used only when necessary. However the product may require refrigeration and proper storage conditions must be followed and the hazards associated with administering a preparation that has passed its beyond-use date known. |
Dose in paediatric medication
Low doses can cause many difficulties, not least in the reliable measurement of small volumes per dose. In the case of insulin, the lowest dose that could be reproducibly delivered has been studied.9,10 The smaller the dose, the less accurate it is; it has been suggested that, for the lowest dose, insulin should be given diluted for greater accuracy of dose measurement. Insulin pens accurately delivery 5 U doses but showed no advantage in terms of accuracy over syringes. For other proteins there are questions of solution viscosity that might dictate choice of dosing method (see Chapter 13).
The abstract of a paper by Notterman et al. reads (verbatim): In an 8-month-old infant with tuberculosis meningitis treatment with isoniazid was unsuccessful and was associated with lower than expected plasma concentrations of isoniazid (measured concentration 0.1 microgram/mL). The infant had received isoniazid as a crushed tablet admixed with apple sauce. Oral administration of the parenteral solution of isoniazid (Nydrazid, Squibb) mixed in apple juice produced a higher isoniazid concentration (2.9 micrograms/mL) and the child improved clinically. Pharmacokinetic studies in two subjects were performed after oral administration of three formulations: (1) an isoniazid tablet crushed and mixed with apple sauce, (2) parenteral isoniazid solution mixed with apple juice, and (3) a commercially available syrup containing isoniazid and pyridoxine (P-I-N Forte, Lannett). Of the three oral preparations, the syrup produced the highest peak concentrations (8.3 and 6.9 micrograms/mL). The crushed tablet in apple sauce produced the lowest peak concentrations (1.4 and 2.4 micrograms/mL). Administration of crushed isoniazid tablets with food may be associated with impaired gastrointestinal absorption, lower than expected isoniazid concentrations, and treatment failure. There are issues of the particle size or granule size in the case of the crushed tablets and of the effect of the apple sauce, as suggested, on gastrointestinal (GI) absorption. One of the key issues when using crushed tablets or the contents of capsules is the determination of particle size distributions so that different batches at least consist of the same size as far as possible. Source: Notterman DA et al. Effect of dose formulation on isoniazid absorption in two young children. Pediatrics 1986;77:850–852. |
This case involved not an infant but a 38-year-old female, but it illustrates dramatically the potential dangers in both children and adults of misusing special formulations. Case summary A 38-year-old woman with multiple medical problems presented to the hospital in acute respiratory distress and was diagnosed with acute pulmonary edema and pneumonia. After initial stabilization, her medications were changed to oral hydralazine, labetalol, and nifedipine XL. These medications were crushed and administered through a nasogastric tube. The patient developed worsening bradycardia with hypotension and experienced asystolic cardiac arrest. She was resuscitated; however, the following morning, another dose of labetalol and nifedipine XL was crushed and administered through the nasogastric tube. She again developed worsening bradycardia with hypotension and ultimately died. Source: Schier J. Ann Pharmacother 2002;37:1420–1423. As the author concludes, there may be synergistic effects following simultaneous administration of a beta-blocker and a calcium-channel blocker. The release characteristics of the nifedipine controlled-release product were destroyed on crushing, resulting in the rapid release of the total drug amount. |
Tablets designed for division
The use of mini-tablets, 3 mm in diameter, to aid swallowing has been investigated in children aged 2–6 years.11 Such products also avoid, as we have discussed, the need for subdivision of adult dose forms. Recent developments have also led to new tablets designed for division, as shown in Fig. 10.6.
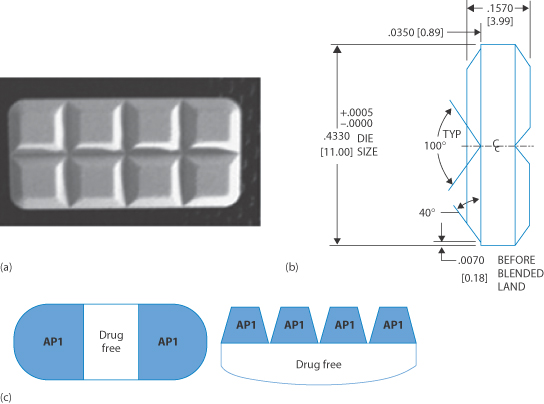
Figure 10.6 Design of rectangular divisible tablets: (a) side view along shortest axis; (b) technical drawing providing the design to make splitting accurate and another prototype system; and (c) systems which provide drug-free regions to aid the accurate breaking of the tablet. API = active pharmaceutical ingredient.
a) and b) from Kayitake E et al Int J Pharm 2009;370:41-47 with permission from Elsevier; c: modified from Solomon L and Kaplan AS, US Patent 2010; 0713547B2
10.2.3 Other routes of administration
Oral mucosal delivery
Delivery to the oral mucosa has been discussed in Chapter 8. Its use in infants has some advantages. Oral transmucosal fentanyl citrate is used as an anaesthetic premedication in children undergoing surgery and painful diagnostic or therapeutic procedures within a monitored care setting. Rapid drug absorption is achieved through the oral mucosa (Fig. 10.7).
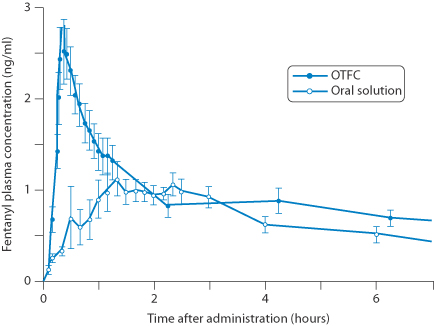
Figure 10.7 An oral transmucosal fentanyl citrate lozenge (OTFC) administered and compared with an oral solution.
Reproduced with permission from: Streisand JB et al. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology 1991; 75:223–229.
Sublingual
The advantages of sublingual and buccal administration have also been discussed in Chapter 8. There can be occasions when this route is appropriate, such as for comatose babies who cannot swallow.
Sublingual sugar has been found to be a useful alternative to intravenous dextrose and superior to orally administered sugar to correct hypoglycaemia in children in the tropics. Bioavailabilities were 84% for sublingual compared to 38% from oral sugar12; one explanation is that the sublingual route is rich in transport systems for sugar. These transporters are saturable and prevent overdose. A 40% dextrose gel has more recently been trialled in hypoglycaemic neonates,13 administered by rubbing the gel on to the buccal surface. It is suggested that dextrose (the D-isomer of glucose) might be more rapidly absorbed sublingually than the disaccharide sucrose (structures below).
|
Subcutaneous injection
The skin of children is thinner and their subcutaneous tissue thickness is lower compared to adults, hence shorter syringe needles (e.g. 4 mm) are employed to avoid unwanted results of injection into intramuscular tissue. Insulin injections should be given into the subcutaneous tissue and optimally avoid deposition into other sites (see Chapter 9). Measurements that have been made of combined skin and subcutaneous tissue show large variations with respect to body mass index at all sites (arm, thigh, abdomen and buttock),14 hence making personalised medicine more difficult. We have discussed above the use of insulin pens and the advantages that these may bring to young diabetic patients.
Intramuscular injection
There is little information on the difference between intramuscular administration of many drugs in different age groups. However, the time to reach peak drug levels after intramuscular injection of ampicillin, cefalexin, cefaloridine and benzylpenicllin is equivalent in neonates, children and adults. It is known that neonates have less muscular mass and subcutaneous fat and higher water content, which should affect solubility and diffusion of drugs deposited in depots.
Transdermal delivery
Patches
Transdermal administration can provide a non-invasive method for paediatric drug delivery. Delgado-Charroe and Guy15 state that ‘the competent skin barrier function in term infants and older children limits both water loss and the percutaneous entry of chemicals including drugs; but the smaller doses required by children eases the attainment of therapeutic concentrations’. Fentanyl, buprenorphine, clonidine, scopolamine, methylphenidate, oestrogens, nicotine and tulobuterol have been formulated as patches for paediatric use. The ‘immature and rapidly evolving skin barrier function in premature neonates represents a significant formulation challenge’ and this group suffers from a lack of approved transdermal formulations.
Microneedles
Microneedles, which have been discussed in Chapter 9, have several advantages over conventional systems. Microneedles fabricated by one of a variety of techniques are now being used in practice to provide a means of administering drugs or vaccines which may be poorly absorbed across the stratum corneum. For the young or old with needle phobia the small size of the microneedle patch and their painless application demonstrate advantages over conventional systems.
Intranasal delivery
Intranasal fentanyl has been used for pain relief in the treatment of burns and procedures during treatment, such as the removal of dressings in paediatric patients as an alternative to oral morphine. The ideal paediatric analgesic should be potent and have a quick onset, a short duration of action and minimal side-effects.
Ophthalmic route
The eye of the newborn is about two-thirds of its adult size; it reaches adult size at ages 3–4 years. There is a ready risk of systemic side-effects with ocular delivery of drops, as drugs after absorption enter a smaller circulating blood volume; it has been found with timolol that in young children blood levels range from 3.5 to 34 ng mL–1, much higher than average levels of 2.45 ng mL–1 in adults. It is thus important that concentrations of active in eye drops are appropriate for the age of the child and that drop size variations (often dictated by the surface tension and viscosity of the drops as well as the nature of the dropper) are minimised.16
Inhalation
Drugs can be delivered via the respiratory tract by the methods discussed in Chapter 9. However, the disparity in the sizes of children and their tolerance to aerosols formed by nebulisers or pressurised devices varies. Recent consensus about the applicability of the various approaches in the range from preterm neonates to adolescent children is summarised in Fig. 10.8.
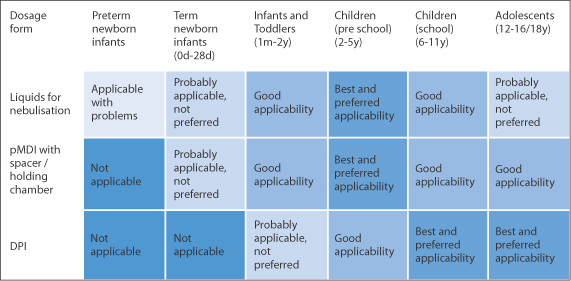
Figure 10.8 The appropriateness of forms of inhalation for various age groups, a figure modified from the EMA Committee for Medicinal Products for Human Use 2006. pMDI, pressurised metered-dose inhaler; DPI, dry powder inhaler.
Reproduced from Leiner S. A respiratory product for children – development and submission experiences. Int J Pharm 2014;469:263–264. Copyright Elsevier 2014.
Spacers (or holding chambers), as shown in Fig. 10.9, are used in inhalation therapy for both adults and children. These chambers reduce the velocity of the aerosol particles and also allow more complete evaporation of solvent so that the particle size is smaller than when used without such as device. Most chambers have one-way valves that open during inspiration, closing during expiration. Spacers are easier to use by children than pressurised metered-dose inhalers.*
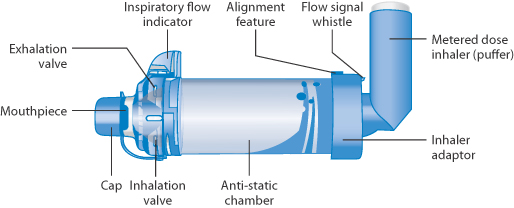
Figure 10.9 Diagram of a holding chamber or spacer for use with a pressurised metered-dose inhaler, showing the inhalation valve mentioned in the text and the adapters for the inhaler.
The reference in the figure legend to the antistatic chamber reveals the issue with some chambers, in which electrostatic charges attract aerosol particles to the surface of the chambers – often plastic – a problem that can be obviated by treating the interior surface of the chamber with detergent or other agents. Figure 10.10 demonstrates the influence of spacers on the lung deposition of the aerosol. The advantages in this case are clear, but with over 100 inhaler/drug combinations available for inhalation therapy it is not easy to provide hard and fast, quantitative rules! However, the facts in some studies are quite stark with lung deposition of salbutamol17: even with chambers reaching maximally around 10%, there is significant variability, in older children reaching around 15% but fluctuating from child to child. The inhaler matters, as does the spacer, as shown in Figs 10.11 and 10.12.
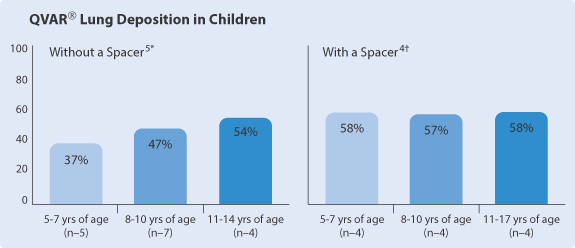
Figure 10.10 The influence of spacers on lung deposition from an aerosol of beclometasone dipropionate.
Reproduced from www.qvar.com, using data from 5*Devadason SG et al. Eur Respir J 2003;21:1007–1011 and 4†Roller CM et al. Eur Respir J 2007;29:299–306.
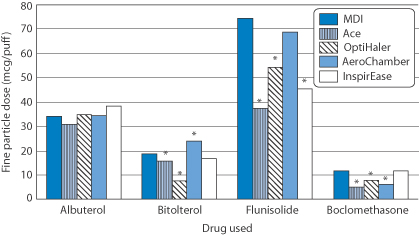
Figure 10.11 The dose of fine particles in a study of four drugs delivered via a range of spacers compared with a metered-dose inhaler (MDI).
Reproduced from Ahrens R et al. Choosing the metered-dose inhaler spacer or holding chamber that matches the patient’s need: evidence that the specific drug being delivered is an important consideration. J Allergy Clin Immunol 1995;96:288–294. Copyright Elsevier 1995.
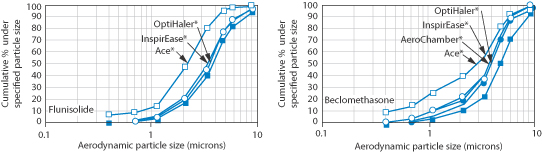
Figure 10.12 The marked effect of some spacers on aerodynamic particle size distribution.
Reproduced from Ahrens R et al. Choosing the metered-dose inhaler spacer or holding chamber that matches the patient’s need: evidence that the specific drug being delivered is an important consideration. J Allergy Clin Immunol 1995;96:288–294. Copyright Elsevier 1995.
10.3 The elderly and their medication
The term ‘elderly’ covers an even longer span of life than does the category ‘paediatric’, and it has been suggested by Wooten18 that there are two subdivisions, the young elderly (from 70 to 85 years) and the old elderly (from 85 years onward).
10.3.1 Changes with age that affect medication
There are many reasons why specialised or tailored medicines are required for the elderly patient. There are physiological and other changes which occur with ageing that often complicate pharmaceutical care, including the following which impact on absorption of drugs, as can be deduced from earlier chapters:
- changes to the oral cavity (e.g. xerostomia: reduction in saliva)
- changes to the oesophagus (e.g. delayed passage of dosage forms)
- decrease in gastric acid production (hence increase in gastric pH)
- reduced gastric emptying time
- intestinal changes (e.g. reduced surface area).
Figure 10.13 lists these and other changes on ageing which impinge on medication. These effects are in addition to the problems of comorbidity and consequent polypharmacy19 (hence drug–drug interactions and compliance) and alterations in physical and biological factors which also affect absorption, distribution, excretion and metabolism and thus pharmacokinetics.
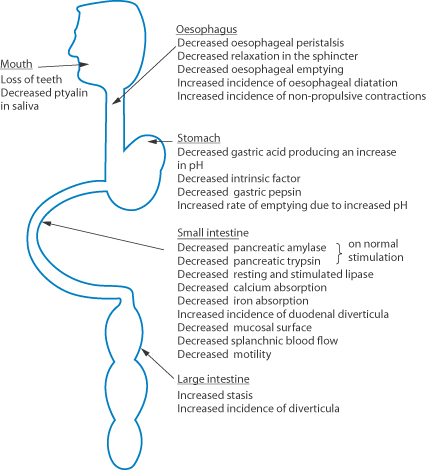
Figure 10.13 The principal changes in gastrointestinal function with age.
Reproduced with permission from Macdonald ET, Macdonald JB. Drug Treatment in the Elderly. Chichester: John Wiley; 1982.
Complicating factors
Xerostomia
Xerostomia and delayed oesophageal emptying may have relevance for the use of certain oral medications, such as fast-dissolving dosage forms or buccal and sublingual forms. Here paucity of fluid intake may slow the release of drug in the oral cavity.
Oesophageal transit
The elderly are more susceptible to the lodging of tablets and capsules in the oesophagus than are younger patients. This is not necessarily the fault of the dose form, but can be the result of taking the tablet with no or too little water. The variability of water intake in 108 female subjects in a trial of a film-coated placebo tablets when they were free to use whatever volume they wished is shown in Fig. 10.14.20 However, as discussed in Chapter 12, some dosage forms can themselves have adhesive properties and adverse effects may be exacerbated in the elderly patient.
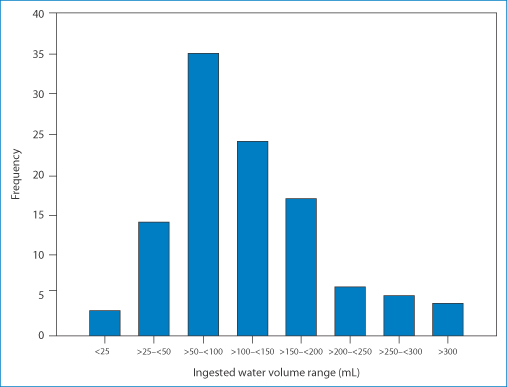
Figure 10.14 Distribution of volume of water ingested by participants swallowing a film-coated placebo tablet in a study of oesophageal transit.
Reproduced from Perkins AC et al. The use of scintigraphy to demonstrate the rapid esophageal transit of the oval film-coated placebo risedronate tablet compared to a round uncoated placebo tablet when administered with minimal volumes of water. Int J Pharm 2001;222:295–239 with permission. Copyright Elsevier 2001.
Difficulties in swallowing are a problem not only in the young but also in the elderly21 with dysphagia** and those in particular with dementia. Patients may simply refuse to take their oral solid medications. Fast-dissolving formulations administered sublingually can be one solution to the problem (Fig. 10.15); such formulations are frequently bioequivalent to the conventional oral forms. The problems of xerostomia may, however, compromise outcomes. There remains the need for a range of different drug formulations, for initial titration and subsequent treatment.22
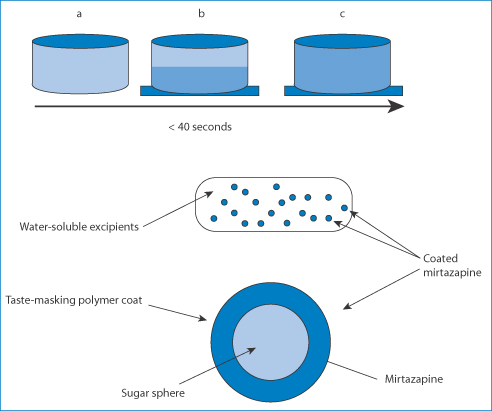
Figure 10.15 Top: the process of dissolution of mirtazapine SolTab in the saliva of the oral cavity: the penetration of water (stages a, b c) takes less than 40 seconds. Bottom: the structure of these fast-dissolving systems.
Reproduced from Frijlink HW. Benefits of different drug formulations in psychopharmacology. Eur Neuropsychopharmacol 2003;13:S77–S84 with permission. Copyright Elsevier 2003.
Alternative routes of administration may be employed. The transdermal route has been promoted for use in the older population. There are apparently few differences in the permeability of the skin: the need to adapt doses for use in the elderly relates more to changes in cardiovascular, renal and hepatic change.23
Achlorhydria
The incidence of achlorhydria is found in up to 20% of elderly patients and hypochlorhydria in 20% of patients over 70 years of age. Around 11% of the elderly have a median fasting gastric pH of above 5.24 Intestinal transit may be slowed in the elderly. The effects of these many changes may be self-cancelling or may be significant. Much will depend on the drug. It is difficult to predict effects, largely because individual patient data are not available, hence the ‘myth of the average’.
10.3.2 Enteral feeding
While the main use of enteral feeding is to enhance nutrition in patients unable to take food normally, enteral feeding tubes also allow the administration of drugs to such patients.25 This poses many pharmaceutical problems, however. Different feeding tubes have different destinations (Fig. 10.16) and hence it is important to choose the correct site for the administration of the drug, depending on the characteristics of the medication. Even though drugs may be absorbed maximally in the intestine, bypassing the stomach may result in poor absorption as the drug will not have the opportunity to dissolve in the acidic environment of the stomach.
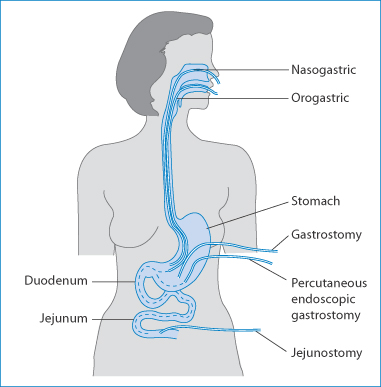
Figure 10.16 Different types of feeding tubes leading to the stomach. Nasoduodenal, nasojejunal and percutaneous jejunostomy tubes extend to the small intestine.
Liquid formulations and solid-dosage forms can be administered this way, and with caution several medications can be given at the same time. Nevertheless, drug–nutrient interactions can take place, there can be problems with the osmolality of the liquids administered, and there can be blockage of the tubes (Table 10.1).
Table 10.1 Some liquid medications that are physically incompatible with most enteral fluids
|
Osmolality of enteral feeding formulae
The osmolality of enteral feeding formulae is important because of its influence on the gastrointestinal tract. Osmolality is expressed in mOsm kg–1 (see Chapter 2, section 2.4.2) and is affected by the concentration of amino acids, carbohydrates and electrolytes. If quantities of a liquid with a higher osmolality than the gut contents are administered, water is drawn into the intestine; such a process leads to diarrhoea, nausea and distension. The osmolality of normal body fluids is 300 mOsm kg–1 and isotonic formulations have values close to this. Table 10.2 lists liquid medications that have osmolalities greater than 300 mOsm kg–1. The exact osmolalites will vary with the exact formulations used and will often differ between various brands of a formulation.
Table 10.2 Some liquid medications with osmolalities greater than 300 mOsm kg–1
|
Reproduced with permission from Dickerson RN, Melnik G. Osmolality of oral drug solutions and suspension. Am J Hosp Pharm 1988;45:832–834.
The exact osmolalities will vary with the exact formulations used and will often differ between various brands of a formulation.
There are some liquid preparations that are not suitable for administration by enteral feeding tubes. Some may be too viscous and may occlude the tubes. Syrups with pH values below 4 often produce incompatibility with the enteral nutrition (EN) formulations, which may result in clumping, increase in viscosity and clogging of the tubes. Not all syrups produce this effect.
Solid conventional-release dose forms can be crushed for administration with EN fluids. The finely ground tablet is added as a suspension in water (15–30 mL). The contents of liquid gelatin capsules are often viscous and it is not easy to remove all of the contents from a single capsule. Delayed-release pancreatic enzyme capsules that contain enteric-coated beads26 can be mixed with apple sauce or juice for administration via feeding tube. It has been suggested that the soft gelatin capsule can be dissolved in hot water and the whole contents administered. Extended-release tablets and capsules have to be treated as special cases: the contents of capsules containing coated pellets can be mixed with EN fluids, but there can be a tendency for these to clump and block narrow-bore feeding tubes.
Drug interactions with nutrient formulations
Certain drugs have been found to interact with nutrient solutions. It is not surprising, given the nature of these solutions, that drugs may bind to proteins or be affected by electrolytes. Phenytoin, carbamazepine, warfarin and some fluoroquinolones have been reported to have lowered bioavailability because of various interactions. Phenytoin absorption has been reported to be reduced by up to 70% on co-administration with enteral feeds.27,28
Summary
This chapter has covered many of the areas in which drug formulation is important clinically for specific age groups. Special formulations for paediatric and geriatric patients form a group of products that are key to optimising therapy in these vulnerable age groups. The fact that both groups are themselves diverse emphasises the importance of the appropriate choice of medication and delivery system and hence the need for closer attention to the individual as a patient. Some wider issues are also discussed, including enteral feeding, interactions of drugs with nutritional fluids and the osmolalities of liquid formulations and there is renewed emphasis to devise and produce new administration systems and formulations to advance personalised medicine through personalised medicines.
References
1. Johnson TN. The development of drug metabolising enzymes and their influence on the susceptibility to adverse drug reactions in children. Toxicology 2003;192:37–48.
2. Strassburg CP et al. Developmental aspects of human hepatic drug glucoronidation in young children and adults. Gut 2002;50:259–265.
3. Ernest TB et al. Developing paediatric medicines: identifying the needs and recognizing the challenges. J Pharm Pharmacol 2007;59:1043–1055.
4. Lowey AR, Jackson MN. A survey of extemporaneous preparation in NHS trusts in Yorkshire, the North East and London. Hosp Pharm 2008;15:217–219.
5. Berger-Gryllaki M et al. The development of a stable oral solution of captopril for paediatric patients. Eur J Hosp Pharm Sci 2007;13:67–72.
6. Mulla H et al. Variations in captopril formulations used to treat children with heart failure: a survey in the United Kingdom. Arch Dis Child 2007;92:409–411.
7. Thompson JE, Davidson LW. Vehicles for oral delivery. In: A Practical Guide to Contemporary Pharmacy Practice, 3rd edn. Philadelphia, PA: Wolter-Kluwer.
8. American Academy of Pediatrics. Ethanol in liquid preparations intended for children. Pediatrics 1984; 73:405–407.
9. Casella SJ et al. Accuracy and precision of low-dose insulin administration. Pediatrics 1993;91:1155–1157.
10. Gnanalingham MG et al. Accuracy and reproducibility of low dose insulin administration using pen-injectors and syringes. Arch Dis Child 1998;79:59–62.
11. Thomson SA et al. Minitablets: new modality to deliver medicines to preschool-aged children. Pediatrics 2009;123:e235–e238.
12. Barennes H et al. Sublingual sugar administration as an alternative to intravenous dextrose administration to correct hypoglycemia among children in the tropics. Pediatrics 2005;116:e648–e653.
13. Harris DI et al. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies study): a randomised, double-blind, placebo-controlled trial. Lancet 2013;382:2077–2083.
14. Marran K, Segal D. SKINNY – Skin thickness and Needles in the Young. South Afr J Child Health 2014;8:92–95.
15. Delgado-Charroe MB, Guy RH. Effective use of transdermal drug delivery in children. Adv Drug Del Rev 2014;73:63–82.
16. Coulter RA. Pediatric use of topical ophthalmic drugs. Optometry 2004;75:419–429.
17. Wildhaber JH et al. Inhalation therapy in asthma: nebulizer or pressurized metered-dose inhaler with holding chamber? In vivo comparison of lung deposition in children. J Pediatr 1999;135:28–33.
18. Wooten JM. Pharmacotherapy considerations in elderly adults. South Med J 2012;105:437–445.
19. Gidal BE. Drug absorption in the elderly: biopharmaceutical considerations for the antiepileptic drugs. Epilepsy Res 2006;68S:S65–S69.
20. Perkins AC et al. The use of scintigraphy to demonstrate the rapid esophageal transit of the oval film-coated placebo risedronate tablet compared to a round uncoated placebo tablet when administered with minimal volumes of water. Int J Pharm 2001;222:295–239.
21. Schindler JS, Kelly JH. Swallowing disorders in the elderly. Laryngoscope 2002;112:589–602.
22. Wahlich J et al. Medicines for older adults. Learning from practice to develop patient centric drug products. Int J Pharm 2013;456:252–257.
23. Kaestli L-Z et al. The use of transdermal formulations in the elderly. Drugs Ageing 2008;25:269–280.
24. Russell TL et al. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm Res 1993;10:187–196.
25. Williams NT. Medication administration through enteral feeding tubes. Am J Health-Syst Pharm 2009;65:2347–2357.
26. Ferrone M et al. Pancreatic enzyme pharmacotherapy. Pharmacotherapy 2007;27:910–922.
27. Gilbert S et al. How to minimise interaction between phenytoin and enteral feedings. Two approaches. Nutr Clin Pract 1996;11:28–31.
28. Doak KK et al. Bioavailability of phenytoin acid and phenytoin sodium with enteral feedings. Pharmacotherapy 1998;18:637–6645.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree