Figure 12–1 Pathophysiology and treatment of heart failure. ACE = angiotensin-converting enzyme.
The combination of edema-related hypoxemia and the heart’s failure to pump sufficient blood to adequately perfuse the tissues can lead to generalized tissue hypoxia and organ dysfunction. For this reason, patients with heart failure often experience symptoms of weakness and fatigue and have reduced exercise capacity.
In cases of right ventricular failure (right-sided heart failure), congestion in the peripheral veins leads to ankle edema in the ambulatory patient and to sacral edema in the bedridden patient. It also leads to hepatojugular reflux, characterized by an increase in jugular vein distention when pressure is applied over the liver. Ultimately, right-sided failure can lead to left-sided failure as the left ventricle is forced to work harder in an attempt to maintain cardiac output.
The reduction in cardiac output that occurs in heart failure triggers a cascade of compensatory neurohumoral responses. Although these responses attempt to restore cardiac output via the Frank-Starling mechanism (see Fig. 12–1), they are often maladaptive and counterproductive. The reduction in tissue perfusion activates both the sympathetic nervous system and the renin-angiotensin-aldosterone system, both of which in turn stimulate vasoconstriction. Arterial vasoconstriction increases aortic impedance to left ventricular ejection and thereby decreases cardiac output, especially in patients with a weak, dilated heart. When angiotensin II stimulates the secretion of aldosterone and antidiuretic hormone, this increases the amount of sodium and water retention, the plasma volume, and the venous pressure. In addition, angiotensin II and sympathetic activation lead to cardiac remodeling and ventricular wall thinning or fibrosis, which often reduce systolic and diastolic function. Hence, the net result of the neurohumoral responses is often a further reduction in cardiac output and an increase in circulatory congestion.
Mechanisms and Effects of Drugs for Heart Failure
The primary goals of drug therapy for heart failure are to improve symptoms, slow or reverse deterioration in myocardial function, and prolong survival. Drugs can also be used to treat underlying conditions, control arrhythmias, prevent thrombosis, and treat anemia.
The pharmacologic agents used to treat heart failure include drugs that (1) increase cardiac output, (2) reduce pulmonary and systemic congestion, and (3) slow or reverse cardiac remodeling. Cardiac output can be increased by positively inotropic drugs that increase cardiac contractility and by vasodilators that reduce cardiac afterload and the impedance to left ventricular ejection. Vasodilators also reduce venous pressure, circulatory congestion, and edema. Diuretics are used to mobilize edematous fluid and reduce plasma volume, thereby decreasing circulatory congestion. Angiotensin and sympathetic inhibitors have been shown to favorably influence cardiac remodeling and increase survival in persons with heart failure.
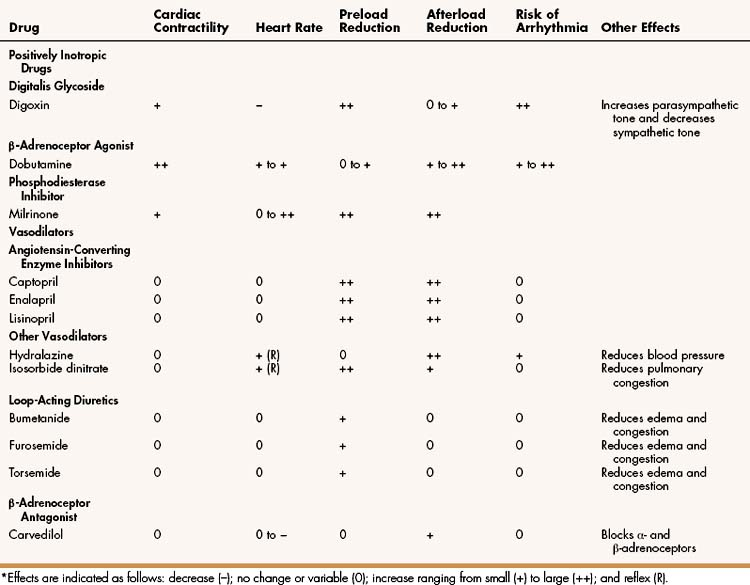
Table 12–1 compares the cardiovascular effects of drugs discussed in this chapter. Each of these drugs partly counteracts the loss of myocardial function and the maladaptive responses that occur during heart failure; however, none of the current therapies, either alone or in combination, has been completely satisfactory. Because heart failure has such a high incidence and poor prognosis, a much greater effort has been expended in the search for better means to treat it. The most significant development in recent decades has been the use of angiotensin inhibitors, β-adrenoceptor blockers, and other agents that attenuate cardiac remodeling and reduce the mortality rate in patients with heart failure. Ultimately, however, the successful treatment of patients with heart failure may require the development of novel drugs that activate genes capable of repairing or replacing myocardial tissue.
POSITIVELY INOTROPIC DRUGS
Derived from the Greek words for fiber (inos) and changing (tropikos), the term inotropic refers to a change in muscle contractility. Drugs that increase cardiac contractility are said to have a positive inotropic effect, whereas drugs that decrease contractility have a negative inotropic effect. Drugs with a positive effect include members of three groups: digitalis glycosides, β-adrenoceptor agonists, and phosphodiesterase inhibitors. The calcium-sensitizing agents represent a new class of inotropic drugs that are currently under investigation for the treatment of heart failure. These include levosimendan.
All of the currently available inotropic agents increase calcium influx into cardiac muscle cells, thereby increasing cardiac contractility. The β-receptor agonists and phosphodiesterase inhibitors increase calcium influx by increasing intracellular cyclic adenosine monophosphate (cAMP) levels. β-Agonists increase cAMP formation by activating adenylyl cyclase, whereas milrinone inhibits cAMP degradation. The mechanism by which digitalis glycosides increase calcium influx is described next.
Digitalis Glycosides
Despite the fact that the digitalis glycosides have been used to treat heart failure for more than 200 years, their effectiveness and place in therapy have been difficult to determine. Recent clinical trials indicate that digoxin provides a definite, yet limited, benefit to patients with heart failure caused by systolic dysfunction.
Drug Properties
Many digitalis glycosides have been isolated from plant and animal sources, including the leaves of Digitalis (foxglove) plants and the skin secretions of certain toads. Digoxin is the only digitalis glycoside that is extensively used today, having replaced digitoxin and crude digitalis leaf preparations in the treatment of heart failure and other cardiac disorders.
CHEMISTRY AND PHARMACOKINETICS
The digitalis glycosides are composed of a steroid nucleus, a lactone ring, and three sugar residues linked by glycosidic bonds. The stereochemical configuration of the steroid nucleus of digitalis glycosides is different than that of human steroids, and the digitalis glycosides lack most of the effects produced by gonadal or adrenal steroids.
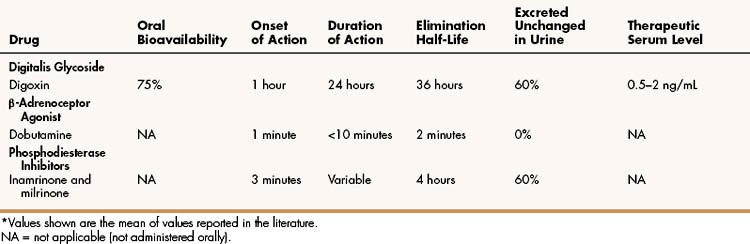
As shown in Table 12–2, digoxin is adequately absorbed from the gut and has a long half-life of about 36 hours. It is primarily eliminated by renal excretion of the parent compound. Because digoxin has a low therapeutic index, serum concentrations are useful in assessing the adequacy of the dosage and evaluating potential toxicity and should be in the range of 0.5 to 2 ng/mL.
MECHANISMS AND PHARMACOLOGIC EFFECTS
The direct and indirect actions of digoxin produce a unique constellation of effects on the cardiovascular system. It has a positive inotropic effect (an increase in the force of contraction), a negative chronotropic effect (a decrease in the heart rate), and a negative dromotropic effect (a decrease in conduction velocity). Among the various types of positively inotropic drugs, digoxin is unique in its ability to strengthen cardiac contraction while decreasing heart rate. Moreover, digoxin indirectly increases parasympathetic tone while reducing sympathetic tone. This contributes to its effects on heart rate and conduction velocity and may partly account for its important role in the treatment of heart failure and other cardiac disorders.
(1) POSITIVE INOTROPIC EFFECT. Digoxin produces a modest positive inotropic effect that is caused by an increase in intracellular calcium and is secondary to inhibition of the sodium pump (Na+,K+-ATPase) in the plasma membrane (sarcolemma). When the sodium pump is inhibited, the concentration of intracellular sodium is increased, thereby increasing the activity of the sodium-calcium exchanger and causing more calcium to enter the cardiac myocyte (Fig. 12–2). The increase in cytoplasmic calcium stimulates the release of additional calcium from the sarcoplasmic reticulum and leads to greater myofibril shortening (contraction).
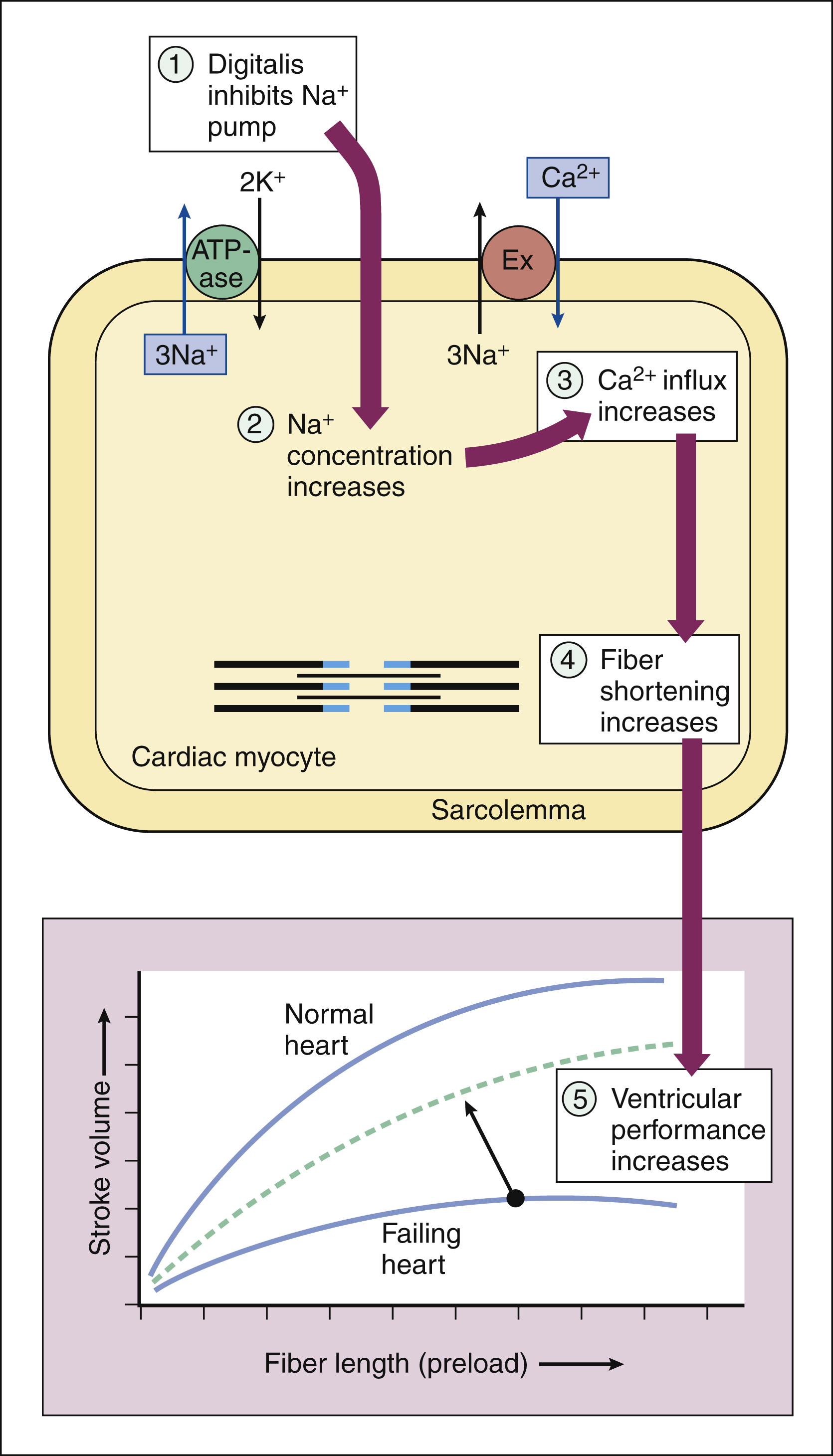
Figure 12–2 Mechanisms by which digoxin exerts its positive inotropic effect on the heart. Digoxin inhibits the sodium pump (ATPase) in the sarcolemma and increases the concentration of intracellular sodium. The high sodium concentration increases the activity of the sodium-calcium exchanger (Ex), thereby causing more calcium to enter the cardiac myocyte. Calcium activates muscle fiber shortening and increases cardiac contractility, which, in turn, increases stroke volume at any given fiber length (preload).
The positive inotropic effect of digoxin increases the velocity of cardiac muscle contraction and the peak systolic muscle tension. These actions increase stroke volume and cardiac output at any given diastolic fiber length (preload), as shown in Figure 12–3. The stroke volume is the amount of blood pumped by the ventricle during each systole, and the cardiac output is the amount of blood ejected from either ventricle of the heart per minute.
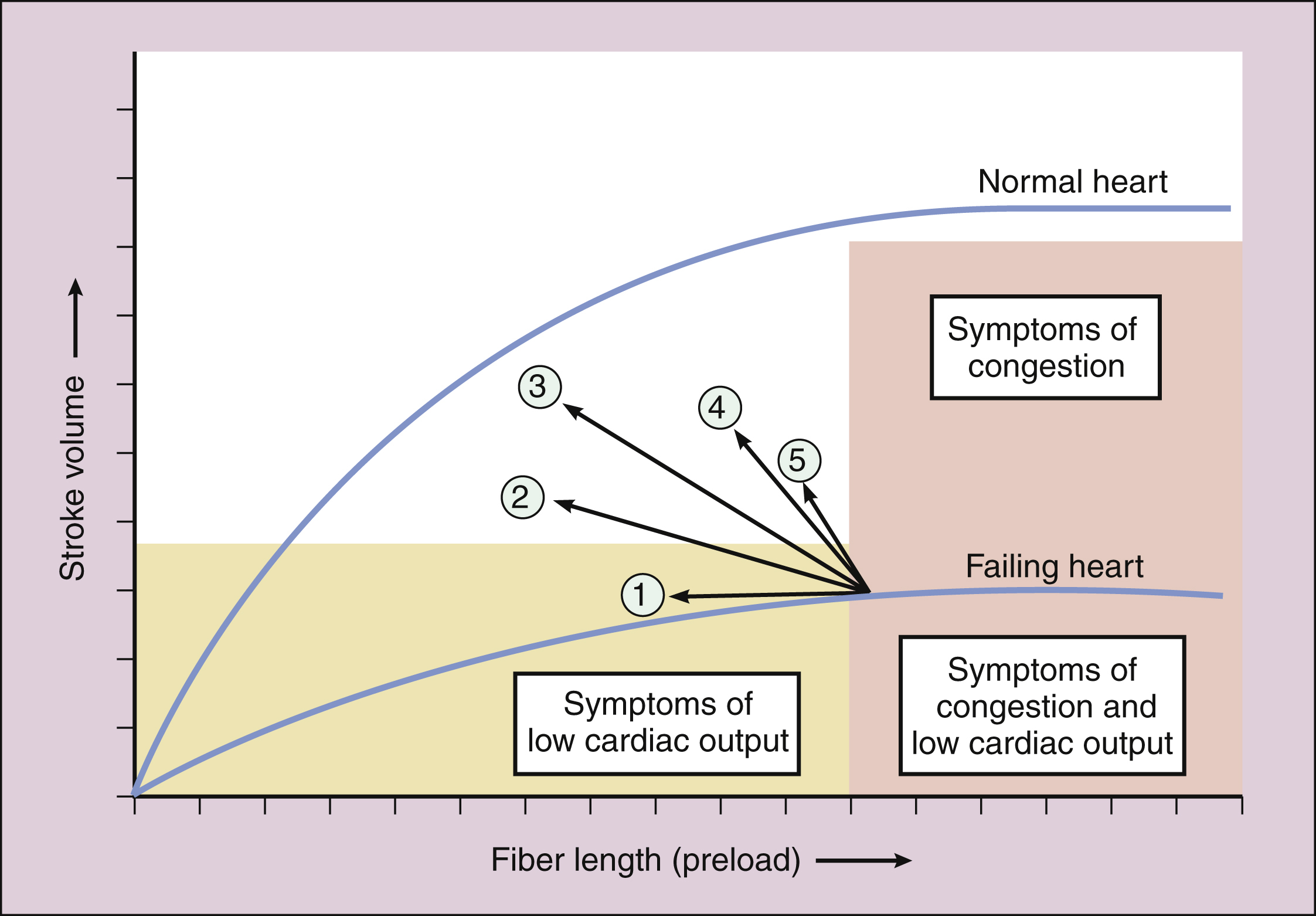
Figure 12–3 Effect of drug treatment on ventricular performance. Effects of the following drugs or drug combinations are shown: (1) a diuretic or a nitrate; (2) nitroprusside or an ACE inhibitor; (3) a positively inotropic drug plus a vasodilator; (4) dobutamine; and (5) digoxin. The positively inotropic drugs increase stroke volume at any given fiber length and thereby decrease venous pressure and preload. Some vasodilators (e.g., ACE inhibitors) decrease afterload and thereby increase stroke volume. Vasodilators can also decrease preload.
(2) ELECTROPHYSIOLOGIC AND ELECTROCARDIOGRAPHIC EFFECTS. Digoxin has several indirect effects on cardiac electrophysiology. It causes an increase in parasympathetic (vagal) tone, and this decreases the heart rate and atrioventricular (AV) node conduction velocity while increasing the AV node refractory period (Fig. 12–4). At the same time, it causes a decrease in sympathetic tone. In patients with heart failure, the reduction in sympathetic tone is caused partly by the withdrawal of reflex sympathetic stimulation secondary to the improved cardiac output produced by digoxin. The drug also appears to directly inhibit sympathetic nerve activity. The reduction in sympathetic tone augments the effect of vagal activation on the sinoatrial and AV nodes, and it also decreases sympathetic vasoconstriction and thereby counteracts the direct vasoconstrictive effect of digoxin. The effects of digoxin on the AV node account for its ability to slow the ventricular rate in patients with atrial fibrillation (see below).
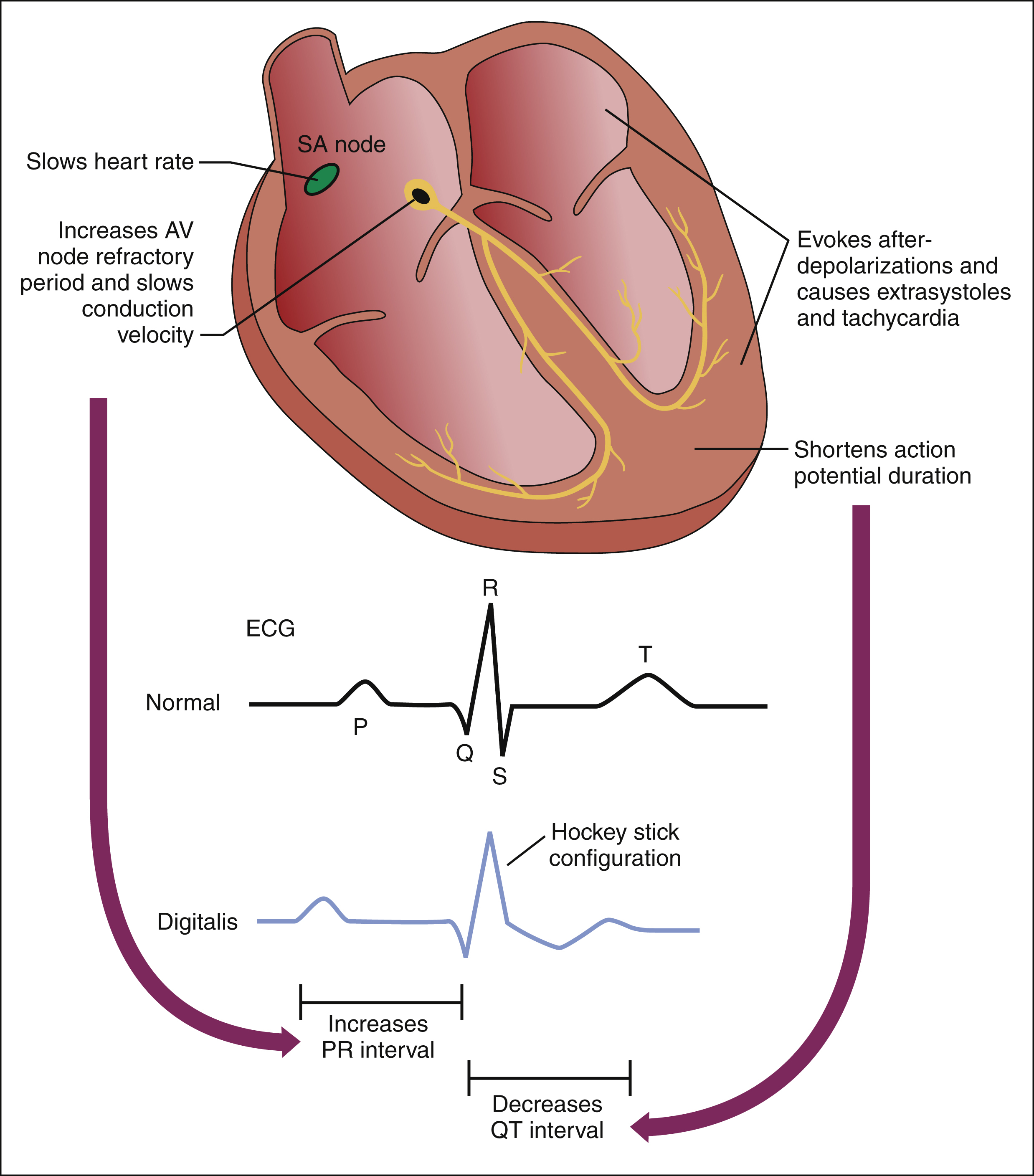
Figure 12–4 Electrophysiologic and electrocardiographic effects of digoxin. Digoxin causes an increase in parasympathetic (vagal) tone and a decrease in sympathetic tone. These actions slow the heart rate by decreasing sinoatrial (SA) node automaticity. The increased vagal tone and decreased sympathetic tone also slow the AV node conduction velocity while increasing the AV node refractory period. The reduced AV conduction velocity increases the PR interval on the electrocardiogram. In ventricular tissue, digoxin shortens the action potential duration, and this decreases the QT interval. Toxic concentrations of digoxin may evoke afterdepolarizations throughout the heart and thereby cause extra systoles and tachycardia. Digoxin also causes ST segment depression, which gives rise to the so-called hockey stick configuration on the electrocardiogram.
Digoxin also has direct effects on cardiac electrophysiology. Of particular importance is the drug’s ability to increase abnormal impulse formation by evoking spontaneous afterdepolarizations (see Fig. 12–4). These abnormal depolarizations occur during or immediately after normal cardiac repolarization and lead to extrasystoles (premature or coupled beats) and tachycardia (rapid beating of the heart). The afterdepolarizations appear to be caused by excessive calcium influx into cardiac cells, and they are more likely to occur after higher doses of digoxin are given.
Digoxin has several electrocardiographic effects (see Fig. 12–4). It shortens the ventricular action potential duration by accelerating repolarization, and this decreases the QT interval. It reduces the AV node conduction velocity and thereby increases the PR interval. Finally, it causes ST segment depression, which gives rise to the so-called hockey stick configuration of the ST segment.
ADVERSE EFFECTS
The most common adverse effects of digoxin are gastrointestinal, cardiac, and neurologic reactions. Frequently, the earliest signs of toxicity are anorexia, nausea, and vomiting. These reactions are often associated with elevated serum concentrations and may forewarn of more serious toxicity.
Arrhythmias, usually the most serious manifestation of digoxin toxicity, can include AV block and various tachyarrhythmias. Atrial tachycardia with 2:1 or 3:1 AV block is one of the most common types of digitalis-induced arrhythmia, but digoxin can also cause ventricular arrhythmias. Hypokalemia can precipitate arrhythmias in patients receiving digoxin, and the serum potassium level should be determined immediately if arrhythmias occur in these patients.
The neurologic effects of digoxin include blurred vision and yellow, green, or blue chromatopsia (a condition in which objects appear unnaturally colored). Severe digoxin toxicity can precipitate seizures.
Because digoxin has some estrogenic activity, it occasionally causes gynecomastia (excessive growth of male mammary glands).
INTERACTIONS
Several drugs can interact with digoxin and thereby affect its efficacy and toxicity (Table 12–3). Because antacids and cholestyramine can reduce the absorption of digoxin and decrease its therapeutic effects, their administration should be separated from the administration of digoxin by at least 2 hours. Diltiazem, quinidine, and verapamil reduce digoxin clearance and increase serum digoxin levels, which can cause digitalis toxicity. When digoxin is used concurrently with these drugs, only 50% of the usual dose of digoxin should be given, and serum digoxin levels should be monitored. Diuretics should be used cautiously with digoxin. Diuretic-induced hypokalemia can precipitate digitalis toxicity because the reduced serum potassium concentration increases digitalis binding to the sodium pump. Hypokalemia can also contribute directly to arrhythmias.
TABLE 12–3 Adverse Effects, Contraindications, and Drug Interactions of Positively Inotropic Drugs
| Drug | Common Adverse Effects | Common Drug Interactions |
|---|---|---|
| Digitalis Glycoside | ||
| Digoxin | Anorexia, nausea, and vomiting; arrhythmias; blurred vision; chromatopsia; gynecomastia; and seizures. | |
| β-Adrenoceptor Agonist | ||
| Dobutamine | Excessive vasoconstriction and tachyarrhythmias. | Activity affected by other agonists and antagonists. |
| Phosphodiesterase Inhibitor | ||
| Milrinone | Arrhythmias; hypotension; and thrombocytopenia. | Unknown. |
INDICATIONS
Digitalis glycosides have been used to treat heart failure for centuries, but their benefits have been uncertain, whereas their toxicity has been substantial. The improvement produced by digoxin in patients with systolic heart failure probably results from a combination of a modest positive inotropic effect and attenuation of the neurohumoral consequences of heart failure. As previously discussed, digoxin augments parasympathetic tone while reducing sympathetic tone, and these actions reduce heart rate, sympathetic vasoconstriction, and cardiac afterload. Digoxin is generally not used to treat diastolic heart failure because contractility is not impaired in this disorder.
In patients with heart failure, clinical trials have shown that although digoxin treatment does not prolong survival, it reduces symptoms and the need for hospitalization, and thereby improves the quality of life of persons with heart failure. Moreover, patients who have been withdrawn from digoxin exhibit worsening of heart failure. Hence, digitalis will probably continue to have a role in treating heart failure in combination with angiotensin inhibitors, diuretics, β-adrenoceptor antagonists, and other drugs.
The most certain indication for digoxin, however, appears to be the treatment of patients with both heart failure and atrial fibrillation. In these patients, the ventricular rate is often rapid and irregular, thereby causing palpitations and reducing cardiac output. By slowing the AV node conduction velocity and increasing the AV node refractory period, digoxin reduces the number of ectopic impulses that are transmitted from the atria to the ventricles and thereby slows the ventricular rate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree