Figure 34–1 Biosynthesis of sex steroids. In the ovary, pregnenolone is converted to androstenedione and testosterone in thecal cells. These steroids are then converted to estrone and estradiol in granulosa cells. Enzymes involved in these steps include the cholesterol side-chain cleavage enzyme (P450scc), steroid 17α-hydroxylase (P45017α), 3β-hydroxysteroid dehydrogenase (3β-HSD), 17β-hydroxysteroid dehydrogenase (17β-HSD), and aromatase. Estrone and estradiol are partly converted to estriol by other enzymes. After ovulation, the major product of thecal cells is progesterone, owing to the development of a relative deficiency of 17α-hydroxylase activity.
In females, ovarian thecal cells secrete small quantities of testosterone. In males, about 95% of testosterone is produced by Leydig’s cells in the testes, and the remainder is derived from the adrenal cortex. Testosterone is synthesized in the testes by the same pathways as in the ovaries. Testosterone is subsequently converted to dihydrotestosterone (DHT) by 5α-reductase in the prostate, hair follicles, and skin. In the plasma, testosterone is primarily bound to sex steroid–binding globulin. In the liver, it is converted to androstenedione and other metabolites, including sulfate and glucuronide conjugates. About 90% of these metabolites are excreted in the urine.
Although both testosterone and DHT activate androgen receptors, DHT has greater receptor affinity and forms a more stable receptor-ligand complex than testosterone. If DHT formation is inhibited, this significantly reduces androgenic stimulation of the prostate gland and hair follicles. The androgen receptor located in target cells interacts with response elements in target genes and thereby stimulates protein synthesis in the same manner as other gonadal steroids.
Physiologic Actions of Estrogens and Progesterone
In females, estrogens and progesterone have multiple actions and interactions that are necessary for reproductive activity. Estrogens are formed in the granulosa cells of the ovary, whereas progesterone is primarily produced by the corpus luteum in response to LH secretion. Estrogens promote the development and growth of the fallopian tubes, uterus, and vagina, as well as secondary sex characteristics such as breast development, skeletal growth, and axillary and pubic hair patterns.
The pattern of hormonal changes occurring during the menstrual cycle is depicted in Figure 34–2. During the follicular phase of the cycle, ovarian follicles are recruited and a dominant estrogen-secreting follicle develops. Estrogen levels gradually increase, whereas progesterone levels remain very low. A surge of LH is released at mid-cycle in response to positive estrogen feedback to the pituitary gland, and this LH surge triggers ovulation. During the luteal phase of the cycle, the follicle becomes the corpus luteum (literally “yellow body”) that secretes both estrogen and progesterone in response to LH. Together, these hormones prepare the uterus for implantation of a fertilized egg as the endometrium becomes more vascular and secretory. If pregnancy does not occur, the corpus luteum ceases to produce estrogen and progesterone, resulting in menstruation. If pregnancy occurs, the placenta produces human chorionic gonadotropin, which maintains the production of progesterone by the corpus luteum. After about 3 months, the placenta becomes the predominant source of progesterone. This hormone serves to maintain pregnancy and prevents endometrial sloughing and miscarriage.
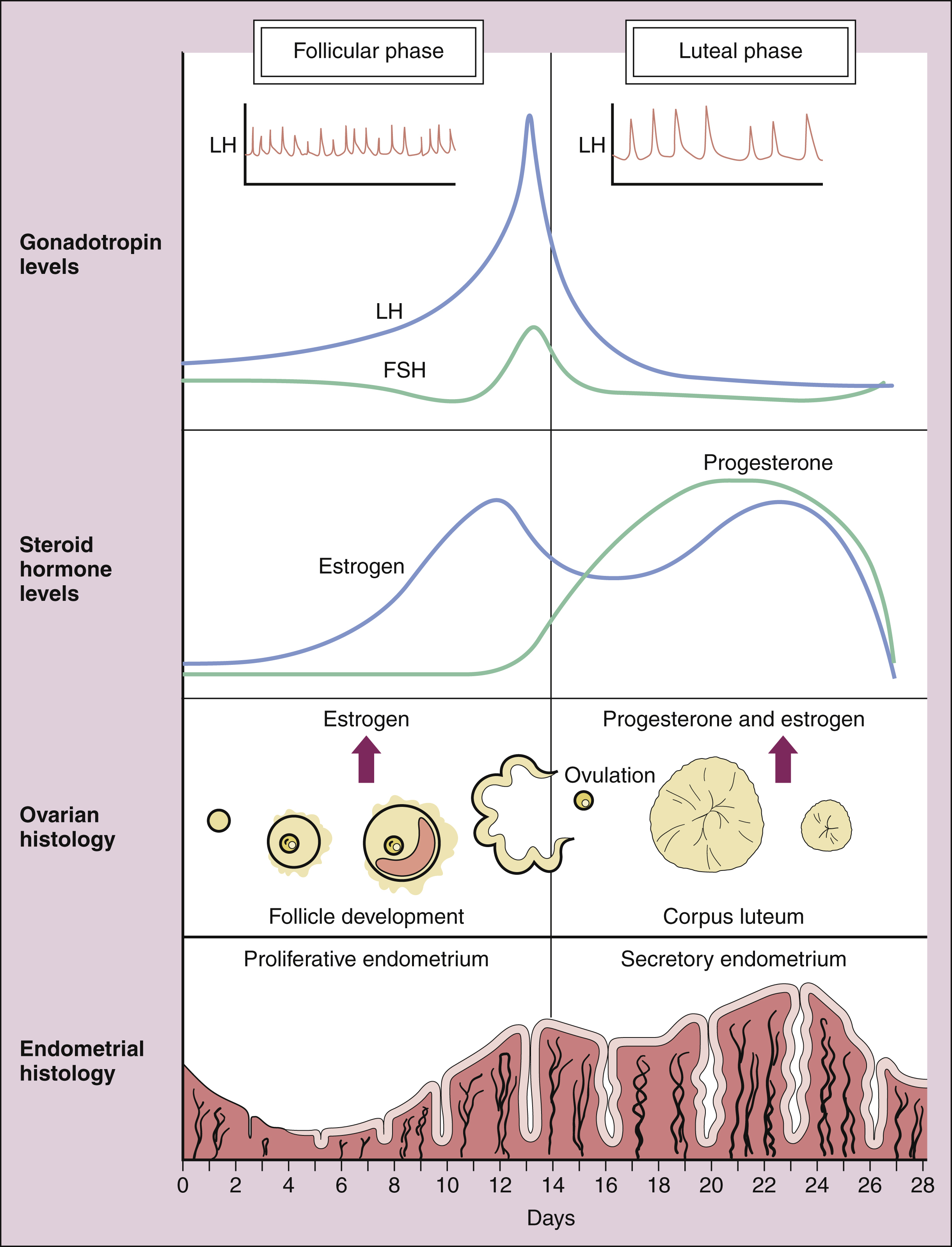
Figure 34–2 Hormone secretion during the human menstrual cycle. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are released in a pulsatile manner from the pituitary gland in response to the pulsatile secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus. FSH stimulates ovarian follicle development and the secretion of estrogen during the follicular phase of the cycle. After ovulation, the corpus luteum produces both estrogen and progesterone during the luteal phase. Estrogen decreases FSH and LH secretion during most of the cycle (feedback inhibition) while provoking the mid-cycle LH and FSH surge that triggers ovulation. Progesterone decreases the frequency of hypothalamic GnRH pulses and the frequency of pulsatile LH and FSH secretion (inset in top panel). During the luteal phase, progesterone increases the amount of LH released (pulse amplitude). Estrogen stimulates the proliferation of the endometrium during the follicular phase, whereas progesterone causes the endometrium to become more vascular and secretory during the luteal phase.
Estrogens have a number of other actions that are important in reproduction and other bodily functions. They sensitize the myometrium to oxytocin at parturition, and this facilitates labor. They stimulate protein synthesis in the brain and may thereby affect mood and emotions. Estrogens influence the distribution of body fat and thereby contribute to the development of feminine body contours. They enhance blood coagulation by increasing the synthesis of clotting factors, and they prevent osteoporosis by inhibiting bone resorption. In males and females, estrogens are responsible for epiphyseal closure, which halts linear bone growth.
Estrogens and progestins have different effects on serum lipoprotein levels. Estrogens decrease the levels of low-density lipoprotein (LDL) cholesterol and lipoprotein(a) while increasing the levels of high-density lipoprotein (HDL) cholesterol. In contrast, progestins produce a dose-related increase in LDL levels and a decrease in HDL levels.
Progesterone and other progestins increase basal body temperature by 0.5° C to 0.8° C (1.0° F to 1.5° F) at ovulation and throughout the luteal phase. They also affect the emotional state and have mild mineralocorticoid (salt-retaining) properties. Some of the synthetic progestins have other effects that are attributed to their androgenic activity (see below).
Physiologic Actions of Testosterone
LH stimulates testosterone synthesis in Leydig’s cells, whereas FSH promotes spermatogenesis by Sertoli’s cells in the seminiferous tubules. These cells provide an environment rich in testosterone, which is necessary for germ cell development. Sertoli’s cells also produce a protein called inhibin, which acts in concert with DHT as a feedback regulator of FSH secretion by the pituitary.
Testosterone is responsible for the development of secondary sex characteristics in males during puberty. These include growth of the larynx, thickening of the vocal cords, and growth of facial, axillary, and pubic hair. In addition to stimulating growth of the penis, scrotum, seminal vesicles, and prostate gland, testosterone stimulates and maintains sexual function in males. Testosterone and other androgens also do the following: increase lean body mass; stimulate skeletal growth; accelerate epiphyseal closure; increase sebaceous gland activity and sebum production, thereby contributing to the development of acne in both sexes; increase the production of erythropoietin in the kidneys; and decrease the levels of HDL cholesterol.
ESTROGENS AND PROGESTINS
An estrogen or progestin preparation can be used alone for the treatment of various disorders, or the two preparations can be used in combination for hormone replacement therapy (HRT) in postmenopausal women or for contraception in women of childbearing age.
Estrogens
Drug Properties
CHEMISTRY
The natural estrogens include estradiol and conjugated estrogens. Estradiol is an 18-carbon steroid with an aromatic A-ring (see Fig. 34–1). Conjugated equine estrogens are sulfate esters of estrone and equilin and can be obtained from the urine of pregnant mares. Ethinyl estradiol and mestranol are synthetic derivatives of estradiol.
PREPARATIONS
Micronized estradiol is an orally administered estradiol preparation that has good bioavailability. Also available are vaginal estradiol tablets and a vaginal ring that slowly releases estradiol.
Ethinyl estradiol and mestranol are modified by the addition of an ethinyl group to estradiol, which reduces first-pass metabolism, increases the half-life to about 20 hours, and results in greater oral potency compared with native estradiol. These agents are primarily used in estrogen-progestin contraceptives. Conjugated equine estrogens are hydrolyzed to estrone and equilin before absorption from the gut. They undergo relatively little first-pass metabolism and are converted in the liver to sulfate and glucuronide conjugates that are excreted in the urine. Estrone is also available in a rapidly absorbed formulation for intramuscular administration.
Several long-acting formulations of estradiol are available for transdermal or intramuscular administration. Transdermal estradiol systems slowly release the drug for absorption through the skin. These preparations are formulated for twice-weekly or weekly application. Estradiol cypionate and estradiol valerate are long-acting esters of estradiol that are slowly absorbed following intramuscular administration and provide effective plasma concentrations of estradiol for several weeks.
Following their absorption, the natural estrogens are highly bound to sex steroid–binding globulin, are widely distributed, and are concentrated in fat. They undergo enterohepatic cycling, in which conjugated metabolites are excreted in the bile and converted to free estrogens by intestinal bacteria. The free estrogens are then reabsorbed into the circulation. As with estrone and equilin, estradiol is metabolized in the liver to sulfate and glucuronide conjugates, and these conjugates are primarily excreted in the urine, with small amounts excreted in the feces.
INDICATIONS
Estrogen preparations are used in the treatment of primary hypogonadism, including cases caused by surgical oophorectomy, menopause, and other causes. Micronized estradiol, transdermal estradiol, and conjugated equine estrogens are primarily used for HRT (see below) in postmenopausal women. New low-dose and ultra-low-dose preparations are available containing 0.3 mg conjugated estrogens or 0.5 mg estradiol, respectively. Combination estrogen-progestin preparations contain ethinyl estradiol or mestranol. These preparations are often used for oral contraception and for the treatment of acne vulgaris and dysmenorrhea.
ADVERSE EFFECTS
Estrogens occasionally cause breast tenderness, headache, edema, nausea, vomiting, anorexia, and changes in libido. These effects are less likely to occur in women using the lower-dose preparations now recommended for HRT.
The more serious adverse effects of estrogens include hypertension, thromboembolic disorders, and gallbladder disease. The hypertensive effect of estrogens has been partly attributed to increased angiotensinogen synthesis and formation of angiotensin II, whereas thromboembolic complications result from increased hepatic synthesis of clotting factors. Estrogens increase cholesterol excretion in the bile, accounting for their tendency to cause gallstones.
Estrogens are contraindicated during pregnancy and should be avoided in women with uterine fibroids. Estrogens should be used with great caution in women with hepatic diseases, endometriosis, thromboembolic diseases, or hypercalcemia.
Progesterone and Its Derivatives
Progesterone is the primary natural progestin in mammals. Progesterone undergoes extensive first-pass metabolism following oral administration and has a short plasma half-life. To extend the oral bioavailability and half-life, esters of progesterone have been developed. These include megestrol, hydroxyprogesterone caproate, and medroxyprogesterone acetate. Megestrol is administered orally, whereas hydroxyprogesterone caproate is administered as a long-acting intramuscular preparation. Medroxyprogesterone acetate can be given either orally or intramuscularly. Following their absorption, the progesterone esters are bound to albumin in the circulation. The esters are converted to several hydroxylated metabolites and to pregnanediol glucuronide in the liver, and these metabolites are excreted in the urine.
Progesterone esters are used to suppress ovarian function in the treatment of dysmenorrhea, endometriosis, and uterine bleeding. In this setting, the progesterone derivatives produce feedback inhibition of gonadotropin secretion by the pituitary gland. In HRT (see below), the progesterone esters are used in combination with estrogens to decrease the incidence of estrogen-induced irregular bleeding and to prevent uterine hyperplasia and endometrial cancer.
Synthetic Progestins
Synthetic progestins are primarily used as oral contraceptives (see below), but they are also used to treat dysmenorrhea, endometriosis, and uterine bleeding in the same manner as the progesterone esters.
Most of the synthetic progestins are derivatives of 19-nortestosterone (testosterone without a methyl group on carbon-19) and have varying degrees of estrogenic, antiestrogenic, and androgenic activity. The drugs contain substitutions that increase their oral bioavailability and duration of action. Their half-lives range from 7 to 24 hours, whereas the half-life of progesterone is only about 5 minutes.
Two classes of 19-norprogestins have been developed: estranes and gonanes. The estranes include norethindrone and norethynodrel, whereas the gonanes include levonorgestrel, desogestrel, and norgestimate. Desogestrel and norgestimate have improved progestational selectivity and lessened androgenic activity. Drospirenone is a spironolactone derivative with antiandrogenic effects (see below).
Hormone Replacement Therapy
Menopause refers to the cessation of menstruation that occurs in most women between the ages of 45 and 55. Before menopause, the supply of eggs in a woman’s ovaries declines and ovulation becomes irregular. The ovarian follicles fail to develop and secrete normal amounts of estrogen. When estrogen levels are no longer sufficient to suppress FSH secretion by the pituitary gland, FSH levels rise. When FSH levels are above 40 mIU/mL, a woman is said to be in menopause.
Although estrogens can be used alone for HRT in women who have had a hysterectomy, estrogen should be used in combination with a progestin for women with a uterus. This is because giving estrogen alone increases the risk of endometrial cancer in these women.
Therapeutic Effects
Studies consistently show that estrogens relieve symptoms of menopause in up to 90% of women. These symptoms include hot flashes or flushes that consist of alternating chills and sweating accompanied by nausea, dizziness, headache, tachycardia, and palpitations (Box 34-1). Episodes of these symptoms often occur several times a day, but night sweats are particularly common. These symptoms occur in association with surges in GnRH and gonadotropins that result from the lack of estrogen feedback inhibition. The gonadotropin surges alter hypothalamic thermoregulatory centers, leading to the symptoms described earlier in this paragraph.
BOX 34–1 A CASE OF HOT FLASHES AND LOSS OF SLEEP
CASE PRESENTATION
A 50-year-old woman complains to her health care provider of episodes of hot flashes that occur mostly at night accompanied by sweating and interrupted sleep. She has also noticed vaginal dryness and feeling slightly depressed. Her periods have been increasingly infrequent over the past year. She suspects that she is entering menopause and asks about HRT. The woman has been healthy throughout her adult life and adheres to a good diet and a regular exercise program. Her mother developed osteoporosis after menopause and the patient is concerned about maintaining healthy bones. She does not have a family history of premature cardiovascular disease or of reproductive tract cancer. Her physical exam and laboratory tests are normal and she is scheduled for a bone density determination. She is started on a low dose of oral estrogen and a vaginal estrogen cream along with cyclic medroxyprogesterone. Her bone density will be monitored and appropriate therapy provided. She is encouraged to maintain a high level of calcium intake and to increase her vitamin D supplementation. This case is continued in Chapter 36.
CASE DISCUSSION
Menopause is defined as the absence of menstruation for 12 consecutive months and is caused by cessation of estrogen production by the ovaries. Common symptoms of menopause include hot flashes, mood swings, sleeplessness, vaginal dryness, and urinary incontinence. Many women experience irregular periods and other symptoms of menopause for several months preceding menopause. Tachycardia, depression, and other symptoms of estrogen withdrawal may also occur. Low doses of estrogens control most menopausal symptoms, and vaginal estrogen preparations effectively relieve vaginal atrophy. Clinical trials have been more confusing than helpful with respect to HRT and the risk of cardiovascular disease and cancer. In some cases, trials involving older women with heart disease have been erroneously extrapolated to healthy younger women. The latest analysis of trials suggests that hormonal replacement may protect against long-term cardiovascular disease, but may increase short-term risk in some women. For most healthy menopausal women, short-term HRT is a relatively safe and effective method of controlling menopausal symptoms and may provide some long-term cardiovascular benefits.
Estrogens also relieve other menopausal symptoms, including urogenital, vulvar, and vaginal atrophy, and they protect against osteoporosis. Estrogens may improve mood and reduce cognitive difficulties, possibly secondary to improved sleep.
Although some epidemiologic studies suggest that HRT reduces the risk of cardiovascular disease, some recent clinical trials of estrogen replacement in postmenopausal women have reached the opposite conclusion. The Nurses’ Health Study was an observational study of a cohort of women that obtained information about the relationship between cardiovascular disease and lifestyle, lipid levels, and HRT. This study concluded that HRT decreased the risk of coronary artery disease and that women with the highest risk of cardiovascular disease obtained the greatest benefit from HRT. This study has the same limitations as all cohort observational studies, and it has been postulated that the women who sought HRT were also more likely to adopt a healthy lifestyle that reduced their risk of heart disease.
The Postmenopausal Estrogen/Progestin Interventions study was a randomized, prospective trial of HRT in 875 women between the ages of 45 and 64. It demonstrated beneficial effects of HRT on risk factors for heart disease by showing that HRT reduced cholesterol and fibrinogen levels. The relatively short duration of the trial (3 years) was not sufficient to determine long-term effects of HRT on morbidity and mortality.
The Heart and Estrogen-Progestin Replacement Study was the first trial to investigate the effects of HRT in older postmenopausal women with existing heart disease. This study found a lack of benefit of HRT on fatal or nonfatal myocardial infarction. In fact, the study found that women were at an increased risk of myocardial infarction during the first year of HRT, although the risk of myocardial infarction decreased in subsequent years. After an additional 3 years of study, no significant differences were noted in cardiovascular outcomes between women on HRT and those not receiving HRT. This trial has been criticized because women in the study were already receiving cardioprotective medications and were allowed to begin or change statin therapy during the study. Also, the average age of women in the study was 67, which is well above the age when women enter menopause.
The Women’s Health Initiative included a randomized trial of more than 16,000 women who received conjugated equine estrogen plus medroxyprogesterone acetate or a placebo. After 5 years, the study was halted because the data showed that women receiving HRT had increased risk of coronary artery disease, stroke, and pulmonary embolism. HRT, however, reduced the risk of colorectal cancer and hip fractures. Another arm of this study looked at the effect of estrogen by itself in women without a uterus. This study was stopped when it was reported that estrogen increased the risk of stroke but did not affect the incidence of coronary artery disease in these women. The Women’s Health Initiative study has been criticized for the older age of the participants.
More recently, a trial conducted by the Women’s Health Initiative in younger and generally healthier 50- to 59-year-old women found that that both estrogen alone and estrogen and progestin in combination provided cardioprotective effects with a reduction in coronary events and total mortality. Considering that neither aspirin nor statins have shown a statistically significant reduction in coronary heart disease in younger menopausal women, it is remarkable that estrogen may in fact reduce the risk of heart disease in these women (see Lobo, 2007).
In summary, low-dose estrogen preparations are still a very useful treatment for relieving menopausal symptoms in younger menopausal women. Estrogens also protect against osteoporosis and certain cancers and may reduce the risk of cardiovascular disease in younger women. The role of estrogens in older women remains uncertain. Older women with existing heart disease should not be placed on estrogen therapy. Other medications are available to prevent osteoporosis in menopausal women.
Treatment Considerations
Most women with an intact uterus should begin HRT with a combination of a progestin and a low-dose estrogen. Doses should be titrated to provide symptom relief without causing side effects such as headache, nausea, weight gain, breast tenderness, or vaginal bleeding. After 2 or 3 years of therapy, the dose of estrogen can be gradually reduced to the lowest level required to prevent hot flashes and other symptoms.
Estrogens can be administered using a wide variety of dosage forms and routes of administration, enabling women to try alternative preparations and determine which one is most effective and tolerable for them. Oral tablets are convenient and relatively inexpensive, whereas transdermal skin patches require less frequent administration. Women who suffer mainly vulvovaginal symptoms may prefer a vaginal cream, tablet, or ring. Other options include intramuscular injections and subcutaneously implanted pellets.
Hormone replacement therapy can be given as cyclic or continuous hormonal therapy. In cyclic therapy, estrogen is given for 25 days, a progestin (e.g., medroxyprogesterone) is given for the last 10 to 13 days of estrogen treatment, and then no therapy is given for 5 to 6 days. In continuous therapy, estrogen is given every day, and a progestin is added for the first 10 to 13 days of each month. The addition of a progestin suppresses endometrial hyperplasia and diminishes the risk of endometrial cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


