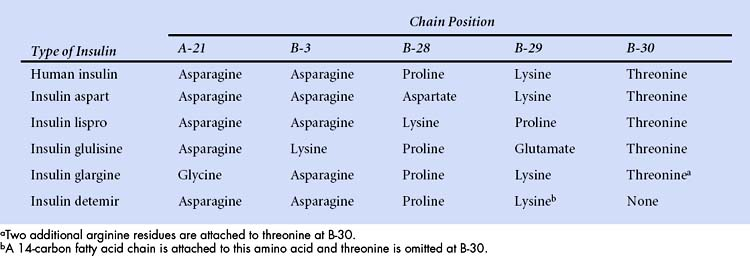
Differences in the amino acid composition of insulins are shown in the following table.
Secretion
Insulin secretion has meal-stimulated and basal components. Insulin release is activated by the rise in blood glucose concentration that follows the digestion and absorption of carbohydrates (Fig. 35–1A and 35–1B). Insulin is released at the rate of 1 Unit (U) per 10 g of dietary carbohydrate, and it usually peaks within 1 hour of eating. Insulin promotes the uptake and storage of glucose and other ingested nutrients, and the postprandial (post-meal) plasma concentrations of both insulin and glucose return to preprandial (pre-meal) levels within 2 hours. Basal secretion, which usually ranges from 0.5 to 1 U of insulin per hour, serves to retard hepatic glucose output during the postabsorptive state.
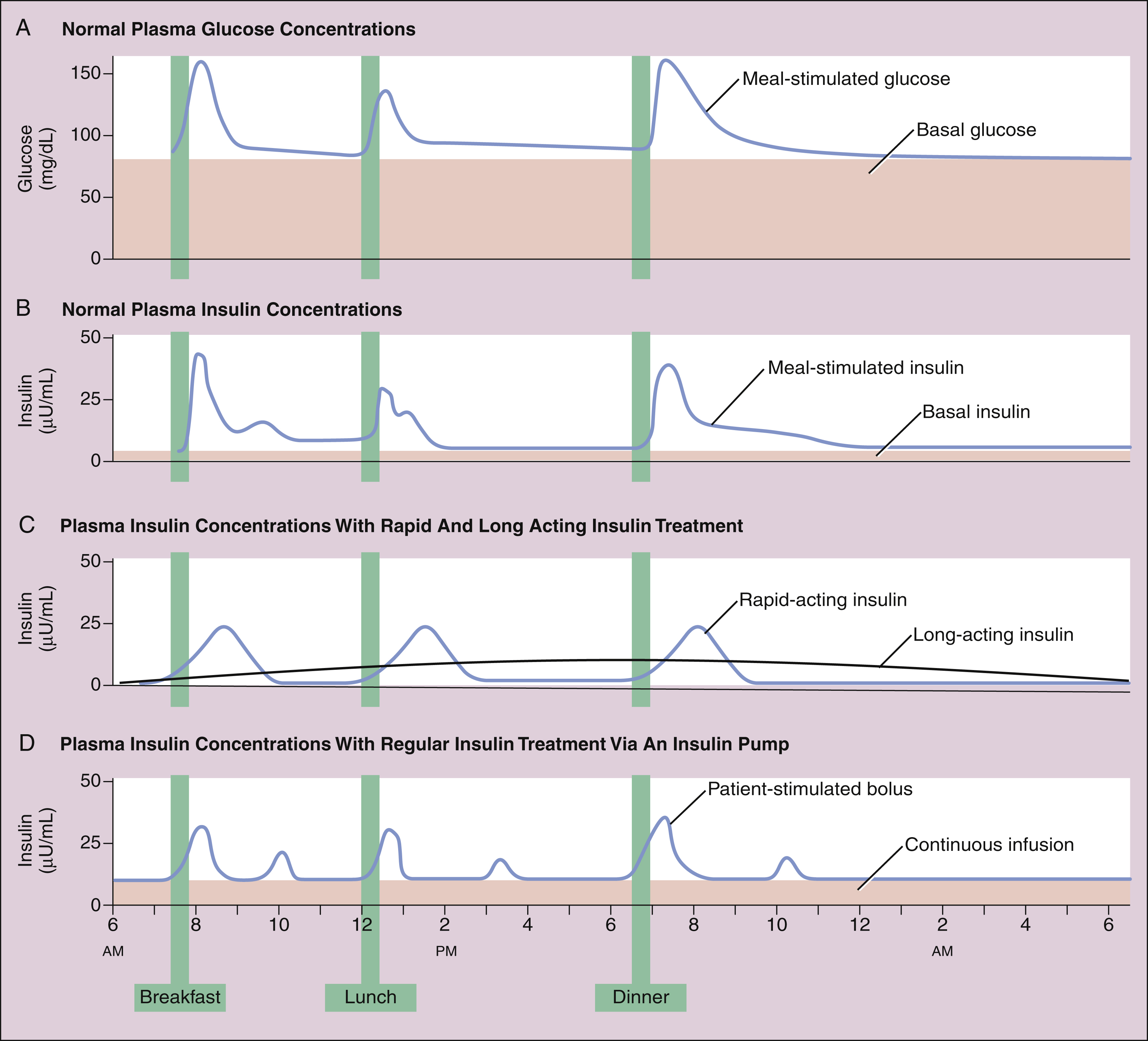
Figure 35–1 Time course of plasma glucose and insulin concentrations. (A) Plasma glucose concentrations result from hepatic glucose output in the fasting state and the digestion and absorption of carbohydrates after meals. (B) Plasma insulin levels result from a basal level of insulin secretion throughout the day and glucose-stimulated secretion after meals. (C) Insulin levels resulting from daily injections of long-acting insulin to provide the basal insulin requirement and pre-meal injections of rapid-acting insulin to control postprandial glycemia in type 1 diabetics. (D) Insulin levels obtained with an insulin pump. The pump delivers a constant infusion of regular insulin to fulfill the basal insulin requirement, and the patient activates small bolus injections of insulin before meals, snacks, and bedtime.
Physiologic Effects
Insulin is sometimes referred to as the “storage hormone” because it promotes formation of glycogen, triglycerides, and protein while inhibiting their breakdown.
Insulin has several important actions on the liver, the organ that normally serves as the major source of blood glucose to supply the brain in the fasting state. The liver provides blood glucose through the processes of gluconeogenesis (the formation of glucose from amino acids) and glycogenolysis (the breakdown of glycogen). Insulin stimulates enzymes involved in glycogen synthesis while inhibiting glycogenolytic and gluconeogenetic enzymes, thereby reducing glucose output by the liver.
Insulin promotes the uptake of glucose by skeletal muscle and adipose tissue by activating a glucose transporter in these tissues called GLUT 4. Skeletal muscle and adipose tissue are dependent on insulin for glucose uptake, whereas the brain can utilize blood glucose in the absence of insulin. By promoting glucose uptake, insulin facilitates the metabolism of glucose to provide energy for skeletal muscle contraction, and it stimulates glycogen synthesis. In adipose tissue, insulin increases the conversion of glucose to fatty acids for storage as triglyceride. It also promotes the uptake and esterification of fatty acids and inhibits lipolysis (the conversion of triglyceride to fatty acids). In skeletal muscle, insulin inhibits protein catabolism and amino acid output.
Mechanisms of Action
Insulin binds to insulin receptors located in the plasma membrane of target cells, which are primarily cells of the liver, skeletal muscle, and adipose tissue. Stimulation of insulin receptors activates a tyrosine kinase and leads to the phosphorylation of serine residues of target proteins. The phosphorylated proteins alter the synthesis or activity of enzymes involved in metabolic processes. Activation of tyrosine kinase also increases the insertion of glucose transporter molecules into cell membranes of muscle and fat tissue.
Glucagon
Glucagon is produced by alpha cells of the pancreas in response to decreased blood glucose concentrations. It activates glycogenolysis and gluconeogenesis and increases hepatic glucose production. Patients with diabetes continue to produce glucagon, and the imbalance between glucagon and insulin is one factor that contributes to the metabolic derangements of this disease. Glucagon is available in a formulation for subcutaneous injection that is used to counteract hypoglycemic reactions in patients with diabetes.
Diabetes Mellitus
Classification
Diabetes mellitus (diabetes) is characterized by elevated basal and postprandial blood glucose concentrations and affects about 20 million people in the United States. The two major forms of diabetes are type 1 and type 2, with the latter accounting for about 85% of cases of diabetes.
Type 1 diabetes usually has its onset before 30 years of age, with a median onset of 12 years of age. It is believed to be an autoimmune disease that is triggered by a viral infection or other environmental factor. The resulting destruction of pancreatic beta cells leads to severe insulin deficiency and excessive production of ketones, causing ketonemia and ketoacidosis. Persons with type 1 diabetes require exogenous insulin for survival.
Type 2 diabetes (Box 35-2) is a heterogeneous disease that usually has its onset after the patient reaches 30 years of age and is often associated with a significant degree of insulin resistance and obesity. Insulin resistance can be caused by the presence of insulin antibodies or by defects in insulin receptors and signal transduction mechanisms in target organs. Patients with type 2 diabetes are less susceptible to developing ketonemia and ketoacidosis than are type 1 patients. Most patients with type 2 diabetes have normal or elevated concentrations of insulin and do not require exogenous insulin for survival. Type 2 diabetes is usually treated with oral antidiabetic medications in combination with dietary modifications and exercise, but some patients benefit from insulin treatment.
BOX 35–2 A CASE OF POSTPRANDIAL HYPERGLYCEMIA
CASE PRESENTATION
A 52-year-old man with an 8-year history of type 2 diabetes is concerned that his diabetes is not well controlled. He has started a regular exercise program and has lost 12 pounds. His A1c level is now 8.0%, down from 8.4% at the previous determination. He is already taking maximal doses of metformin and glipizide and has been taking insulin glargine every evening for the past 3 months. His self-monitored blood glucose values show that his fasting blood glucose (FBG) values are fairly good, but his postprandial glucose (PPG) values are often high after breakfast and lunch, ranging from 158 to 230 mg/dL (normal < 140). His evening PPG values are usually acceptable. Based on these findings, his health care provider suggests that he reduce carbohydrate intake at breakfast and lunch or add a rapid-acting insulin preparation prior to these meals. After considering his diet and activity level, the man decides to use insulin aspart before breakfast and lunch and to adjust the dose based on PPG values.
CASE DISCUSSION
Self-monitored blood glucose (SMBG) is one of the most effective tools available to assist patients in achieving optimal glycemic control and target A1c levels. However, studies show that a large percentage of diabetics fail to follow recommended guidelines for SMBG. Obstacles to the effective use of SMBG include patient denial and unwillingness to adopt changes indicated by SMBG data, lack of patient confidence in their ability to use SMBG data, and the cost, inconvenience, and physical discomfort of SMBG. It is no surprise that patient education is the largest factor determining successful use of SMBG.
Blood glucose monitors enable patients to obtain a record of glucose levels on which to base dietary and treatment decisions. Pre- and post-meal glucose values can be used to guide the selection and adjustment of insulin therapy. Rapid-acting insulins such as insulin aspart and insulin lispro can help patients control PPG levels, while long-acting insulins such as insulin glargine and insulin detemir can improve FBG values and overall glycemic control.
Additional forms of diabetes include gestational diabetes, which has its onset during pregnancy, and secondary diabetes, which occurs in association with other endocrine disorders or with exposure to drugs or chemical agents that are toxic to the pancreas.
Pathophysiology
The early manifestations of diabetes are metabolic abnormalities resulting from lack of insulin, whereas the long-term complications of diabetes result in part from nonenzymatic glycosylation of proteins, primarily in the cardiovascular system, leading to endothelial and cardiac dysfunction, atherosclerosis, and other problems. The percentage of glycosylated hemoglobin (hemoglobin A1c) is used as a clinical marker of long-term control of glycemia in diabetics.
The acute metabolic abnormalities that occur in untreated diabetes result from decreased glucose uptake by muscle and adipose tissue, increased hepatic output of glucose, increased catabolism of proteins in muscle tissue, and increased lipolysis and release of fatty acids from adipose tissue. A reduction in glucose utilization combined with an increase in hepatic glucose production leads to hyperglycemia. Hyperglycemia can then cause glycosuria (glucose in the urine), osmotic diuresis, polyuria (excessive urine formation), and polydipsia (excessive water intake). These derangements lead to dehydration and the loss of calories and weight. For these reasons, diabetes has been described as “starvation in the midst of plenty.”
In patients with type 1 diabetes, the absence of insulin accelerates lipolysis, and this leads to increased production of ketones (acetoacetic acid, acetone, and β-hydroxybutyric acid) in the liver. When the body becomes unable to metabolize these ketones, the keto acids are excreted in the urine. These derangements ultimately lead to ketoacidosis, acetone breath, abnormal respiration, electrolyte depletion, vomiting, coma, and death. Insulin deficiency also leads to increased catabolism of proteins and increased loss of nitrogen in the urine.
The long-term complications of diabetes include microvascular complications, such as nephropathy and retinopathy; macrovascular complications, such as cerebrovascular disease, coronary artery disease, and peripheral vascular disease; and neuropathic complications, such as sensory, motor, and autonomic neuropathic disorders.
Although all of the complications of diabetes contribute significantly to morbidity, the most prevalent cause of death is coronary artery disease. Patients with diabetes often develop hypertension and dyslipidemia, characterized by a decrease in the high-density lipoprotein (HDL) cholesterol level and an increase in the triglyceride level. Furthermore, diabetes appears to be a risk factor for coronary artery disease that is independent of other risk factors such as smoking, hypertension, and dyslipidemia. For these reasons, patients with diabetes should exercise regularly, adhere closely to dietary guidelines, and comply with pharmacologic interventions to control hypertension and dyslipidemia and to achieve near-normal blood glucose concentrations.
INSULIN PREPARATIONS
Insulin preparations are used to treat all patients with type 1 diabetes and about one third of patients with type 2 diabetes. Insulin is also used to treat pregnant women with gestational diabetes.
For many years, therapeutic insulin was obtained from pork and beef pancreas. More recently, human insulin has been produced by expression of the human insulin gene in Escherichia coli or yeast using recombinant DNA technology. Human insulin is less likely than pork or beef insulin to elicit insulin antibodies leading to insulin resistance, and it is less likely to cause allergic reactions or lipodystrophy at injection sites. The latest innovation in insulin therapy has been the development of human insulin analogues that overcome some of the limitations of native human insulin as a therapeutic agent. These analogues have improved pharmacokinetic properties and result in more physiological insulin levels and better control of hyperglycemia without causing as much hypoglycemia as native human insulin preparations. The insulin analogues are discussed below.
The concentration of insulin preparations is expressed as the number of units of insulin per milliliter of solution or suspension. The United States Pharmacopeia (USP) defines 1 unit (U) as the amount of insulin needed to decrease the blood glucose concentration by a defined amount in a fasting rabbit. The insulin preparations used by most diabetics contain 100 U/mL. Regular insulin is also available in a concentrated preparation containing 500 U/mL for use by persons with insulin resistance who require more than 100 U as a single injection.
ADMINISTRATION AND ABSORPTION
Insulin is usually injected subcutaneously or is administered by continuous subcutaneous infusion with an insulin pump. Insulin preparations for inhalation are also available. Insulin absorption is most rapid from an abdominal injection site and is progressively slower from sites on the arm, thigh, and buttock. Because repeated injections at the same site can contribute to tissue reactions (lipodystrophy) that affect the rate of insulin absorption, patients should be taught to rotate injection sites within a particular anatomic area. Newer, silicone-covered needles are painless and have reduced patient aversion to insulin injections.
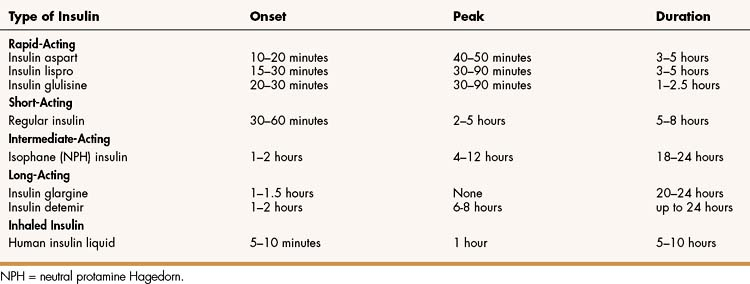
Based on their onset and duration of action, insulin preparations are classified as short-acting, rapid-acting, intermediate-acting, and long-acting (Table 35–1).
TABLE 35–1 Onset of Action, Peak Effect, and Duration of Action of Insulin Preparations Following Subcutaneous Administration or Inhalation
Rapid-Acting Insulin
Three rapid-acting preparations are now available to control postprandial glycemia. Insulin lispro, insulin aspart, and insulin glulisine are human insulin analogues with amino acid substitutions in the B chain as shown in Box 35-1. These modifications reduce aggregation of insulin molecules and enable more rapid absorption after subcutaneous injection compared to regular insulin. An ideal insulin for pre-meal administration would have an onset of action in 10 to 20 minutes, peak at 1 hour, and have a duration of action less than 3 hours. Hence, the rapid-acting insulin analogues are well suited or this purpose. Because the hypoglycemic effect of these insulins begins 10 to 20 minutes after subcutaneous injection, patients can easily coordinate insulin injections and mealtimes. These insulins are often used as part of a regimen that includes a long-acting insulin to provide basal insulin requirements.
Short-Acting Insulin
Regular insulin (insulin injection USP) has a slower onset and a longer duration of action than the rapid-acting insulin analogues after subcutaneous administration. It can also be given intravenously to treat diabetic ketoacidosis. Regular insulin consists of insulin hexamers crystallized around a zinc molecule. After subcutaneous injection, the hexamers dissociate into dimers and monomers that are absorbed into the circulation. Because of the time required for this process, the onset of action of regular insulin occurs 30 to 60 minutes after an injection and the duration of action is 5 to 8 hours. For this reason, regular insulin is not ideally suited to control postprandial glycemia. It is often absorbed too slowly to prevent postprandial hyperglycemia, while causing hypoglycemia later, thereby necessitating a snack and contributing to weight gain. Hence, a rapid-acting insulin preparation is usually more effective for pre-meal use and may contribute to a greater reduction in A1c levels than regular insulin.
Intermediate-Acting Insulin
NPH (neutral protamine Hagedorn) insulin is the only intermediate-acting insulin still available for human use, though a veterinary formulation of pork insulin zinc suspension recently became available to treat diabetic dogs. NPH insulin consists of particles of insulin combined with zinc and protamine that slowly dissolve after subcutaneous injection, enabling sustained absorption and blood levels. NPH is more prone to erratic absorption and intrapatient variability than the long-acting insulin analogues, and it must be gently rolled or inverted before each use to ensure uniform dosage. However, it offers a lower-cost alternative to insulin analogues to meet basal insulin requirements, especially in type 2 diabetics.
Long-Acting Insulins
Long-acting insulin preparations are used to provide basal levels of insulin and facilitate control of glycemia throughout the day. These preparations are formulated to slowly release insulin for absorption into the circulation following a subcutaneous injection. With the withdrawal of extended insulin zinc suspension (Ultralente) from the market, the only long-acting insulins currently available are insulin glargine and insulin detemir.
Insulin glargine contains amino acid substitutions in the A and B chains that enable it to be slowly released and absorbed after subcutaneous injection. It is formulated as a solution at a pH of 4, but it is less soluble at body pH and it forms microprecipitates after subcutaneous injection. These precipitates slowly release insulin glargine for absorption over a 24-hour period, providing a relatively constant hypoglycemic effect over this time period. Hence, it does not exhibit a peak effect (see Table 35–1). Insulin glargine is administered subcutaneously once or twice daily. It is often used in combination with a rapid-acting insulin given at mealtimes and is suitable for both type 1 and type 2 diabetics, with little risk of hypoglycemia.
Insulin detemir is a solution of recombinant human insulin that has been chemically modified by deletion of threonine at B-30 and the attachment of a 14-carbon fatty acid chain to the amino acid at B-29 (see Box 35-1). After subcutaneous injection, it reversibly binds to albumin in the extracellular and vascular spaces through its fatty acid chain, and then it is slowly released from albumin for absorption and distribution to target tissues. Insulin detemir provides a consistent duration of action of about 24 hours with little intrapatient variability. In clinical studies, insulin detemir improved glycemic control with little or no weight gain and a reduced risk of hypoglycemia compared to NPH insulin. Insulin detemir is administered once or twice daily to meet basal insulin requirements.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree