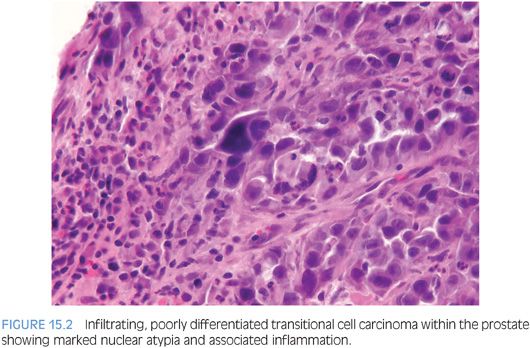
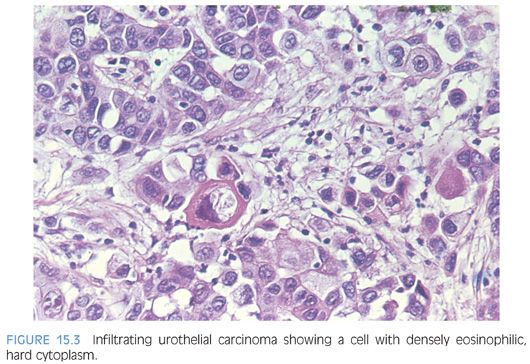
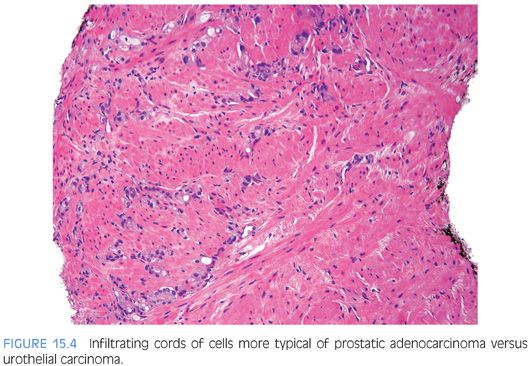
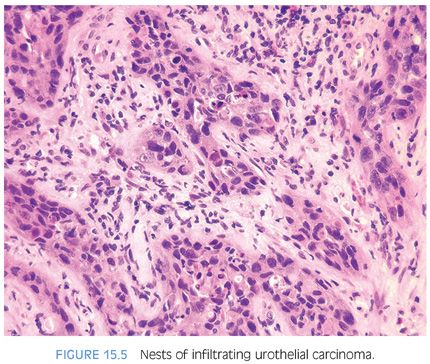
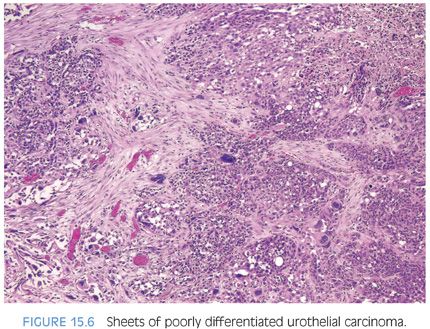
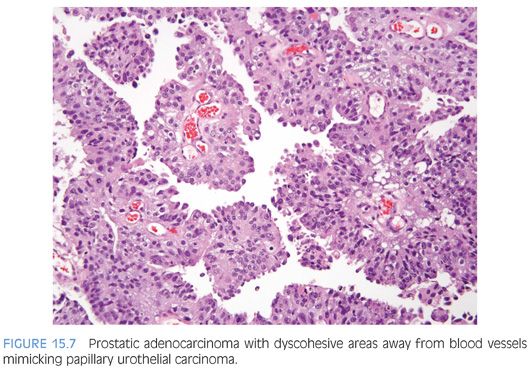
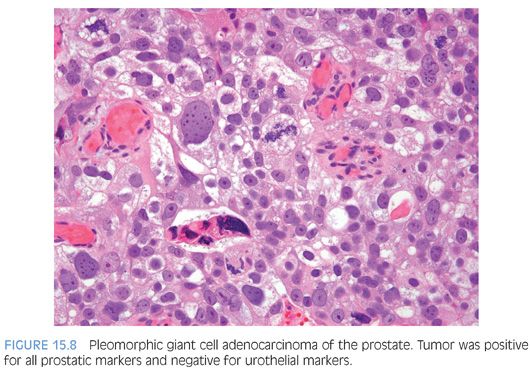
Although for most cases, the distinction between urothelial carcinoma and poorly differentiated prostatic adenocarcinoma can be made on morphologic grounds, there is overlap in a minority of cases. Whereas usual prostate adenocarcinoma has relatively bland cytology as opposed to the greater pleomorphism in urothelial carcinoma, there is a small subset of prostate adenocarcinomas with giant cell pleomorphic features indistinguishable from urothelial carcinoma3 (Fig. 15.8). Given the crucial difference in management and prognosis, resorting to immunostains is a must if the distinction between urothelial carcinoma and prostatic adenocarcinoma cannot be made with absolute certainty on morphologic features alone.
With only a few exceptions, immunoperoxidase staining for prostate-specific antigen (PSA) and prostate-specific acid phosphatase (PSAP) is very specific for prostatic tissue. Situations that can cause diagnostic difficulty include PSA and PSAP within periurethral glands as well as cystitis cystica and cystitis glandularis in both men and women.4,5 Other examples of cross-reactive staining include anal glands in men (PSA, PSAP) and urachal remnants (PSA).6,7 Some intestinal carcinoids and pancreatic islet cell tumors are strongly reactive with antibodies to PSAP, yet are negative with antibodies to PSA.8 Periurethral gland carcinomas in women and various salivary gland tumors may also be PSA and PSAP positive.9,10 Weak false-positive staining for PSAP has been reported in several breast and renal cell carcinomas, and we have seen some cases where PSA was focally and weakly positive though the patient was subsequently shown to have a nonprostatic tumor. This suggests that weak focal positive staining for either antigen should be interpreted with caution.
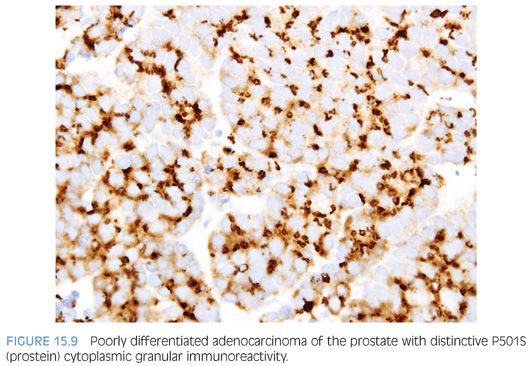
Although PSA and PSAP have proven to be useful in identifying prostate lineage, their sensitivity decreases in poorly differentiated prostate adenocarcinoma. In three studies addressing PSA and the latter issue, 35% to 70% and 25% to 50% of the cases showed less than 25% of the tumor cells staining with PSAP and PSA, respectively.11–13 The same studies found 5% to 13% of cases to be completely negative to PSAP or PSA. The significance of these figures is that given the, at times, limited amount of tissue sampled, up to 50% of the prostate adenocarcinoma may be interpreted as negative for PSA or PSAP, owing to only focal positivity that may not be sampled. Even when both PSA and PSAP are employed, the lack of immunoreactivity in a poorly differentiated tumor within the prostate, especially on limited amount of sample, does not exclude the diagnosis of a poorly differentiated prostatic adenocarcinoma. In such scenario, newer prostate lineage markers such as prostein (P501S), prostate-specific membrane antigen (PSMA), NKX3.1,14 HOXB13,15 and androgen receptor16 could be of added use. Of these markers, PSMA has lower specificity and androgen receptor can also be positive in urothelial carcinoma.17 HOXB13 has not been used in routine surgical pathology practice. P501S has the benefit of distinctive clumpy granular immunoreactivity and NKX3.1 is a very sensitive and specific nuclear antibody (Fig. 15.9). Combining some of the aforementioned markers with urothelial lineage markers will further facilitate resolving a urothelial versus prostatic carcinoma differential diagnosis (Table 15.1). Recent studies have documented high molecular weight cytokeratin (HMWCK) positivity in over 90% of urothelial carcinoma.14,18 HMWCK is only rarely and focally expressed in prostate carcinoma (8%).14 A cautionary note is warranted given that HMWCK labels squamous epithelia including areas of squamous differentiation in post-therapy recurrent prostate carcinoma lesions. HMWCK positivity that is restricted to areas of squamous differentiation does not exclude the diagnosis of adenocarcinoma of the prostate.19 P63 has a greater specificity albeit lower sensitivity for urothelial carcinoma compared to HMWCK (100% specificity and 83% sensitivity).14
Uroplakins are urothelium-specific transmembrane proteins expressed by the majority of noninvasive and up to two-thirds of advanced invasive and metastatic urothelial carcinomas as assessed by uroplakin III (UP III).20–24 Although highly specific for urothelial differentiation, UP III is only of moderate degree of sensitivity (as low as 40%) in high-grade urothelial carcinoma.25 Thrombomodulin is an endothelial cell–associated cofactor for thrombin-mediated activator of protein C. Its expression, predominantly as membranous staining, has been found in 69% to 100% of urothelial carcinoma.14,22,26,27 Thrombomodulin is only rarely positive in prostate adenocarcinoma.14,27 It is also expressed by nonurothelial tumors such as vascular tumors, mesotheliomas, and squamous cell carcinomas.27 Compared to UP III, thrombomodulin has a higher degree of sensitivity but lower specificity as a marker for urothelial carcinoma. In a recent study, we also found p63 to be superior to thrombomodulin as a urothelial marker in high-grade tumors.14
GATA3 (GATA binding protein 3 to DNA sequence [A/T]GATA[A/G]) is a member of a zinc finger transcription factor family. Several recent studies have confirmed its use as a marker of urothelial carcinoma.28–31 In the two largest studies, by Liu et al.30 and Miettinen et al.,31 86% and more than 90% of urothelial carcinomas were positive for GATA3, respectively. The nuclear staining is usually diffuse in more than 50% of cells. Less than 10% of prostatic adenocarcinomas were positive for GATA3.31 In our experience, GATA3 is specific in the differential diagnosis of urothelial carcinoma versus prostatic adenocarcinoma and is the marker of choice for identifying a poorly differentiated tumor in this region as being of urothelial origin14,32 (Table 15.1). Finally, in our experience, CK7 and CK20 are of limited use in this differential, given that they may be both positive in a subset of adenocarcinoma of the prostate.33,34
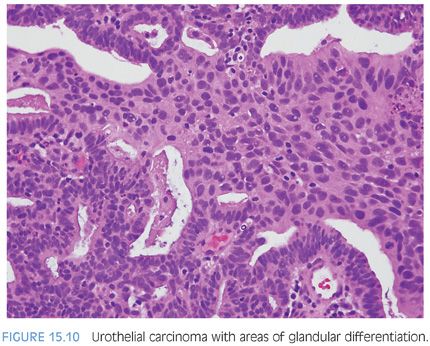
Distinguishing primary urothelial carcinoma with focal glandular differentiation (Fig. 15.10) and bladder adenocarcinoma extending to prostate is also of important clinical and management implications. In general, adenocarcinomas of the bladder resemble adenocarcinomas of the intestine, although signet ring and nonintestinal types also exist. Immunohistochemical markers of prostate lineage are again of great use in this regard. Adenocarcinomas of the bladder, whether as a pure tumor or with mixed urothelial carcinoma, have been reported to be occasionally positive for PSA or PSAP; however, there has yet to be a case reported positive for both.35,36
Although the specificity of newer prostate lineage markers has been tested against bladder urothelial carcinoma, the same could not be said about their pattern of reactivity in bladder adenocarcinoma. In a recent immunohistochemistry study evaluating 37 adenocarcinomas of bladder, our group demonstrated that a minority of bladder adenocarcinomas are positive for prostate antigens P501S and PSMA.37 P501S showed moderate diffuse cytoplasmic staining in 11% of cases including enteric-type and rare mucinous adenocarcinomas. The granular perinuclear staining pattern of P501S typically seen in prostatic adenocarcinoma was absent in all cases of bladder adenocarcinoma. In addition, PSMA showed diffuse cytoplasmic or membranous staining in 21% of bladder adenocarcinomas including signet ring, urachal, mucinous, and enteric-type variants. All cases were negative for PSA and PSAP. Therefore, immunoreactivity for P501S and PSMA should be interpreted with caution in such settings. The lack of granular perinuclear staining for P501S and the absence of membranous PSMA staining both favor a bladder adenocarcinoma. Membranous PSMA staining indistinguishable from that seen in prostate cancer can be seen in less than 10% of bladder adenocarcinoma. Our group has recently evaluated the expression of GATA-3 in primary bladder adenocarcinoma.38 Diffuse nuclear GATA-3 labeling was seen in 41% of signet ring adenocarcinoma of bladder but only in 7% of conventional adenocarcinomas, making the marker only helpful in the differential of tumors with signet ring features.
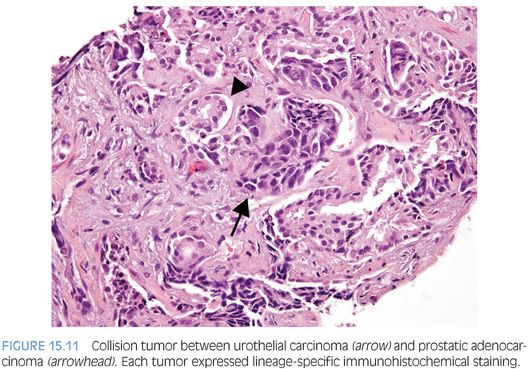
Almost 50% of cystoprostatectomy specimens performed for urothelial carcinoma also contain adenocarcinoma of the prostate.1,39–41 Therefore, the finding in a TURP of a small focus of well-differentiated adenocarcinoma of the prostate should not necessarily influence whether a separate focus of poorly differentiated tumor is urothelial carcinoma or adenocarcinoma of the prostate. We have even seen rare cases of collision tumors between prostatic adenocarcinoma and urothelial carcinoma (Fig. 15.11).
INTRADUCTAL UROTHELIAL CARCINOMA INVOLVING THE PROSTATE
Most commonly, urothelial carcinoma involves the prostate in a setting of a patient with bladder urothelial carcinoma. Although topical chemotherapy and immunotherapy for superficial bladder carcinomas appear to act by direct contact with neoplastic epithelium, it has become critical to identify those cases of bladder urothelial carcinomas with prostatic involvement, because conservative management will not treat these cases effectively. Currently, biopsies of the prostatic urethra and suburethral prostate tissue are often recommended as a staging procedure in patients undergoing conservative treatment for superficial bladder tumors. It is also important to evaluate the urothelium in routine TURP specimens, because we have seen several cases of carcinoma in situ (CIS) where no history of bladder cancer was present. Several studies have shown that by examining random sections of the prostate at the time of cystectomy for urothelial carcinoma of the bladder, between 12% and 20% of the cases will be shown to have prostatic involvement by urothelial carcinoma. If serial sections of the prostate in cystoprostatectomy specimens with bladder urothelial carcinoma are performed, involvement of the prostate by urothelial carcinoma may be found in 37% to 45% of the cases.1,42,43 If intraductal urothelial carcinoma is identified on TURP or transurethral biopsy, patients usually will be recommended for radical cystoprostatectomy. The finding of intraductal urothelial carcinoma also has been demonstrated to increase the risk of urethral recurrence following cystoprostatectomy, such that its identification may also result in prophylactic total urethrectomy.
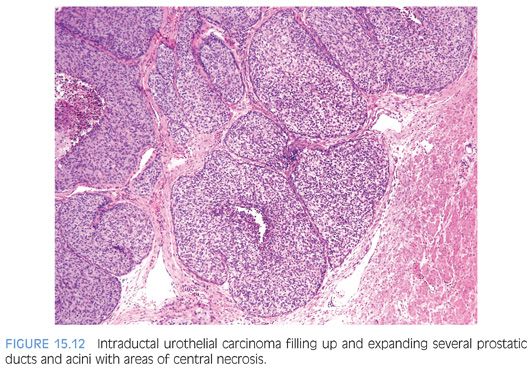
Intraductal urothelial carcinoma of the prostate is usually accompanied by CIS of the prostatic urethra. Involvement of the prostate appears to be by direct extension from the overlying urethra, because in the majority of cases, the more centrally located prostatic ducts are involved by urothelial neoplasia to a greater extent than the peripheral ducts and acini. Intraductal urothelial carcinoma of the prostatic ducts initially consists of malignant urothelial cells insinuating themselves between the basal cell layer and the columnar to cuboidal luminal epithelium of the prostatic ducts. More peripherally, urothelial carcinoma spreads in a pagetoid fashion within the ducts. Similar to that seen in the breast, large tumor cells with clear cytoplasm are seen in the midst of otherwise normal urothelium. With more extensive involvement, urothelial carcinoma fills and expands ducts and often develops central comedonecrosis (Fig. 15.12, eFigs. 15.4 to 15.17). Intraductal urothelial carcinoma of the prostatic ducts without prostatic stromal invasion tends to be seen in lower stage bladder urothelial carcinomas. Once resected by cystoprostatectomy, the noninvasive involvement of the prostate by urothelial carcinoma does not adversely affect survival; the prognosis is determined by the stage of the bladder tumor.1,2,44 In prostates with intraductal urothelial carcinoma and stromal invasion, the associated bladder tumors tend to be high-stage, in which case the already poor prognosis is not affected by the prostatic involvement.1 However, intraductal and infiltrating prostatic urothelial carcinoma may also be associated with low-stage bladder tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


