Chapter 23
Structure and Function of the Reproductive Systems
Gillian Tufts, George Rodway, Sue E. Huether and Angela Deneris∗
The male and female reproductive systems have several anatomic and physiologic features in common. Most obvious is their major function, reproduction, through which a 23-chromosome female gamete, the ovum, and a 23-chromosome male gamete, the spermatozoon (sperm cell), unite to form a 46-chromosome zygote that is capable of developing into a new individual. The male reproductive system produces sperm and delivers them to the female reproductive tract. The female reproductive system produces the ovum and, if it is fertilized, can nurture and protect it (at that point called the embryo and developing fetus) and expel it at birth. These functions are determined not only by anatomic structures but also by complex hormonal, neurologic, and psychogenic factors.1
Development of the Reproductive Systems
Sexual Differentiation and Hormone Production In Utero
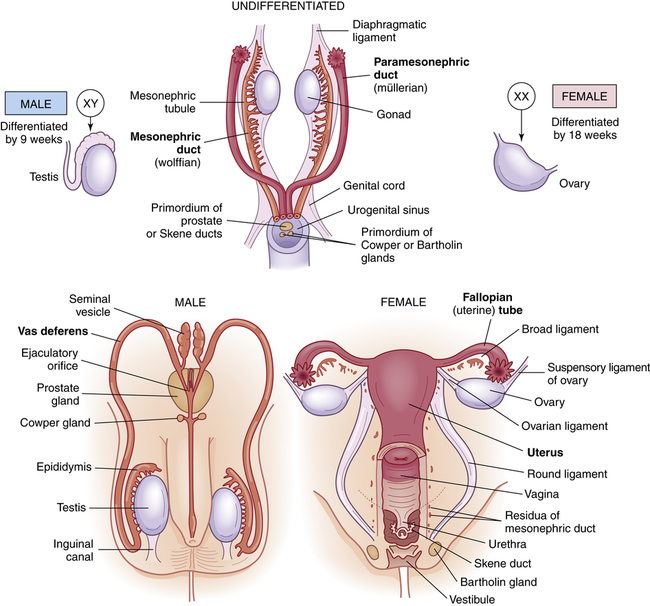
Initially in embryonic development, the reproductive structures of male and female embryos are homologous (the same) and consist of one pair of primary sex organs, or gonads, and two pairs of ducts, the mesonephric ducts (wolffian ducts) and the paramesonephric ducts (müllerian ducts) (Figure 23-1). Both pairs of ducts empty into an opening called the urogenital sinus.
Embryonic and fetal development of the internal genitalia.
Female gonadal development occurs in the absence of SRY expression and with the expression of other genes.2 The presence of estrogen and the absence of testosterone cause regression of the wolffian system and, at 6 to 8 weeks’ gestation, the two female gonads develop into ovaries, which will produce ova. By the tenth week, the loss of wolffian ducts allows the müllerian ducts to join and become the uterus, fallopian tubes, cervix, and upper two thirds of the vagina. The fallopian tubes carry ova from the ovaries to the uterus during a woman’s reproductive years.
Like the internal reproductive structures, the external structures develop from homologous embryonic tissues. During the first 7 to 8 weeks of gestation, both male and female embryos develop an elevated structure called the genital tubercle (Figure 23-2). Testosterone is necessary for the genital tubercle to differentiate into male genitalia; otherwise, female genitalia develop, which may occur even in the absence of ovaries possibly because of the presence of placental estrogens.
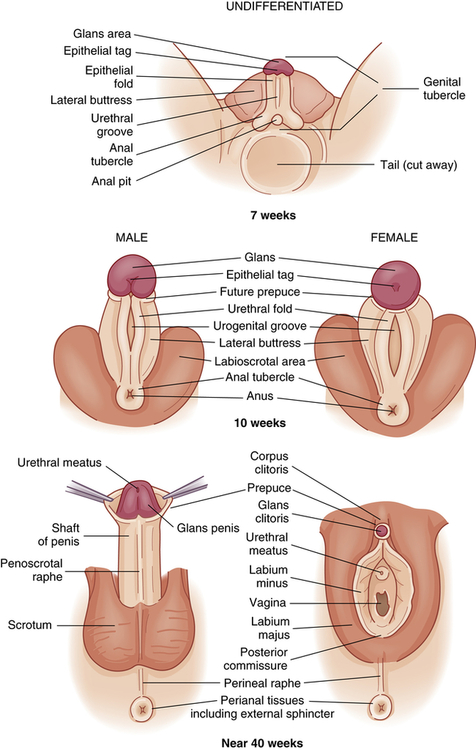
Embryonic and fetal development of the external genitalia.
Anterior pituitary gland development starts between the 4th and 6th weeks of fetal life, and the vascular connection between the hypothalamus and the pituitary is established by the 12th week. Gonadotropin-releasing hormone (GnRH) is produced in the hypothalamus by 10 weeks’ gestation and controls the production of two gonadotropins by the anterior pituitary gland, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In the female fetus, high levels of FSH and LH are excreted. FSH and LH stimulate the production of estrogen and progesterone by the ovary. The production of FSH and LH rises until about 28 weeks’ gestation, until the production of estrogen and progesterone by the ovaries and placenta is high enough to result in the decline of gonadotropin production.1 Production of primitive female gametes (ova) occurs solely during fetal life. From puberty to menopause, one female gamete matures per menstrual cycle. Production of the male gametes (sperm) begins at puberty; after that millions are produced daily, usually for life.
Puberty and Reproductive Maturation
Puberty is the onset of sexual maturation and differs from adolescence. Adolescence is the stage of human development between childhood and adulthood and includes social, psychologic, and biologic changes. In girls, puberty begins at about ages 8 to 9 years with thelarche (breast development). In boys, it begins later—at about age 11 years. Genetics, environment, ethnicity, general health, and nutrition can influence the timing of puberty. Girls who are obese mature earlier, perhaps from higher estrogen levels related to leptin and gonadotropin secretion,3 and girls who have low body fat, reduced body weight, and intense exercise may experience delayed maturation.4 Although leptin is not the trigger for puberty onset, it plays an important permissive role.
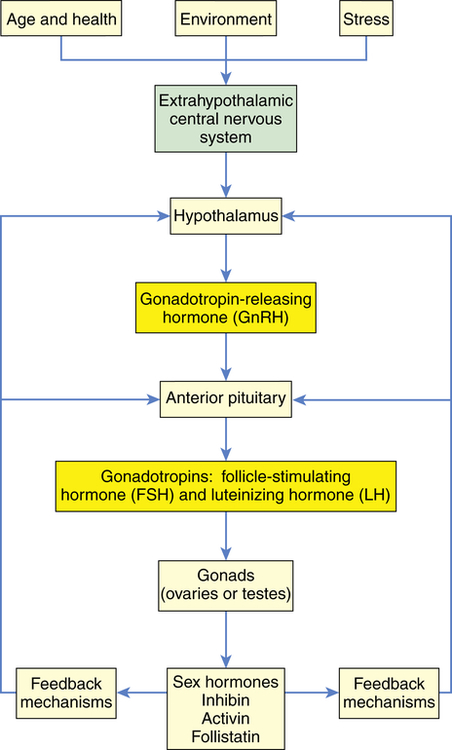
Reproductive maturation involves the hypothalamic-pituitary-gonadal (HPG) axis, the central nervous system, and the endocrine system (Figure 23-3). A sequential series of hormonal events promotes sexual maturation as puberty approaches. About 1 year before puberty in girls, there is an increase in frequency and amplitude of nocturnal pulses of gonadotropin secretion, LH, and FSH and an increased response in the pituitary to GnRH. This, in turn, stimulates gonadal maturation (gonadarche) with estradiol secretion in girls and testosterone secretion in boys. Estradiol causes breast development (thelarche), maturation of the reproductive organs (vagina, uterus, ovaries), and fat deposit in hips in girls. Estrogen and increased production of growth factors cause rapid skeletal growth in both boys and girls. Testosterone causes growth of the testes, scrotum, and penis. A positive feedback loop is created with gonadotropins stimulating the gonads to produce more sex hormones. The most important hormonal effects occur in the gonads. In males the testes begin to produce mature sperm that are capable of fertilizing an ovum. Male puberty is complete with the first ejaculation that contains mature sperm. In females, the ovaries begin to release mature ova. Female puberty is complete with the first ovulatory menstrual period; however, this can take up to 1 to 2 years after menarche. Adrenarche is the increased production of adrenal androgens (dehydroepiandrosterone and androstenedione, which is converted to testosterone and estrogen) prior to puberty, which occurs in both sexes, and is exhibited by axillary and pubic hair growth and body odor. Puberty is complete when an individual is capable of reproduction.
The Female Reproductive System
The function of the reproductive system is to produce mature ova and, when they are fertilized, protect and nourish them through embryonic and fetal life and expel them at birth. In females the most important internal reproductive organs in females are the ovaries, fallopian tubes, uterus, and vagina. The external genitalia protect body openings and play an important role in sexual functioning.1,5,6
External Genitalia
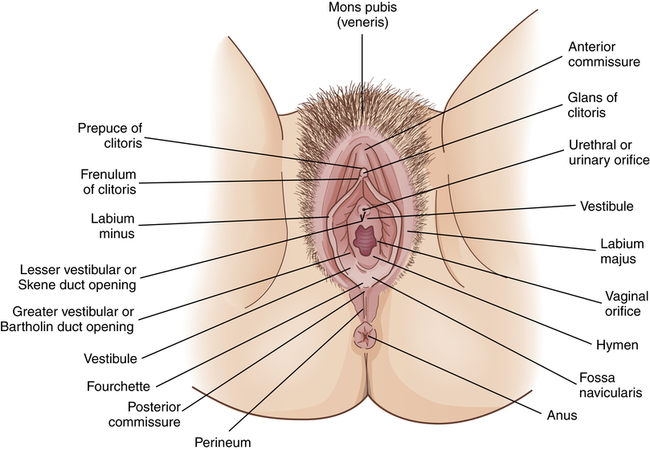
Figure 23-4 shows the external female genitalia, which are known collectively as the vulva, or pudendum. The major structures are as follows:
• Mons pubis (mons veneris). A fatty layer of tissue over the pubic symphysis (joint of the pubic bones). During puberty the mons pubis becomes covered with pubic hair, and sebaceous and sweat glands become more active. Estrogen causes fat to be deposited under the skin, giving the mons pubis a moundlike shape. This cushion of tissue protects the pubic symphysis during sexual intercourse.
• Labia majora (singular, labium majus). Two folds of skin that arise at the mons pubis and extend back to the fourchette, forming a cleft. Like the mons pubis, the labia majora undergo changes at puberty: the amount of fatty tissue increases, pubic hair grows on the lateral surfaces, and sebaceous glands on the hairless medial surfaces begin to secrete lubricants. Because of an extensive network of nerve endings, the labia majora are highly sensitive to temperature, touch, pressure, and pain and are homologous to the male scrotum (see Figures 23-2 and 23-15). The principal function of the labia majora is to protect the inner structures of the vulva.
• Labia minora (singular, labium minus). Two smaller, thinner folds of skin lie within the labia majora. Anteriorly they form the clitoral hood, or prepuce, and frenulum then split to enclose the vestibule and converge near the anus, forming the fourchette. The labia minora are hairless, pink, and moist and are well supplied with nerves, blood vessels, and sebaceous glands. These glands secrete a bactericidal fluid that has a distinctive odor and lubricates and waterproofs the vulvar skin. During sexual arousal the labia minora become swollen with blood.
• Clitoris. A richly innervated, erectile organ that lies anterior between the labia minora. It is a small, cylindric structure having a glans that is visible and a shaft that lies beneath the skin (see Figure 23-4). The clitoris is homologous to the male penis. Like the penis the clitoris is a major site of sexual stimulation and orgasm. With sexual arousal erectile tissues in the clitoris fill with blood, causing it to enlarge somewhat. Similar to other vulvar glands, the clitoris secretes a fluid, called smegma, which has a unique odor and may be erotically stimulating to the male.
• Vestibule. An area protected by the labia minora and contains the external opening of the vagina, called the introitus, or vaginal orifice. A thin, perforated membrane called the hymen may cover the introitus. The vestibule also contains the opening of the urethra, or urinary meatus (orifice). These structures are lubricated by two pairs of glands: Skene glands and Bartholin glands. The ducts of Skene glands (also called the lesser vestibular or paraurethral glands) open on both sides of the urinary meatus. The ducts of Bartholin glands (greater vestibular or vulvovaginal glands) open on either side of the introitus. In response to sexual stimulation, Bartholin glands secrete mucus that lubricates the inner labial surfaces, as well as enhances the viability and motility of sperm. Skene glands help lubricate the urinary meatus and the vestibule. Secretions from both sets of glands facilitate coitus. Also, in response to sexual excitement, the highly vascular tissue just beneath the vestibule fills with blood and becomes engorged.
• Perineum. An area with less hair, skin, and the subcutaneous tissue lying between the vaginal orifice and anus. Unlike the rest of the vulva, this area has little subcutaneous fat so that the skin is close to the underlying muscles. The perineum covers the muscular perineal body, a fibrous structure that comprises elastic fiber, connective tissue, and the common attachment of the bulbocavernosus, the external anal sphincter, and the levator ani muscles (see Figure 23-4). The perineum varies in length from 2 to 5 cm or more and stretches remarkably. The length of the perineum and the elasticity of the perineal body influence tissue resistance and injury during childbirth.
Internal Genitalia
Vagina
The vagina is an elastic fibromuscular canal, 9 to 10 cm long in a reproductive-aged female, which extends up and back from the introitus to the lower portion of the uterus. As Figure 23-5 shows, it lies between the urethra (and part of the bladder) and the rectum. Mucosal secretions from the upper genital organs, menstrual fluids, and products of conception leave the body through the vagina, which also receives the penis during coitus. During sexual excitement the vagina lengthens and widens and the lower third becomes congested with blood.
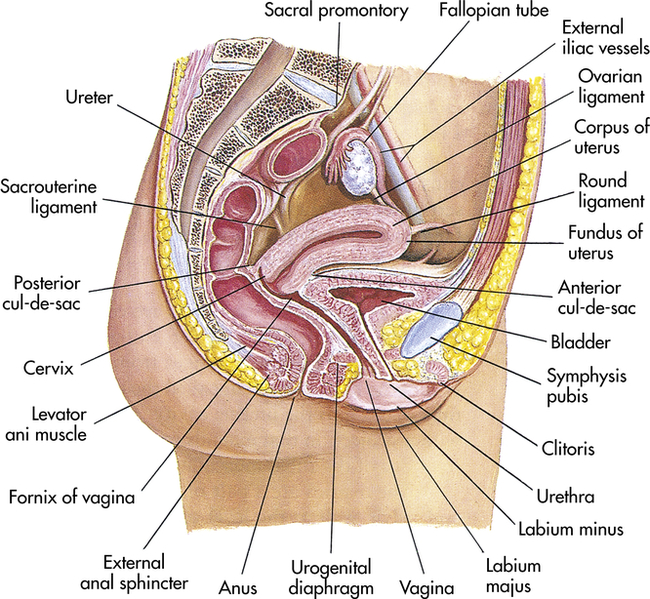
Midsagittal view. (Modified from Seidel HM et al: Mosby’s guide to physical examination, ed 7, St Louis, 2011, Mosby.)
The vaginal wall is composed of four layers:
1. Its lining is a mucous membrane of squamous epithelial cells. (Types of epithelium are described and illustrated in Chapter 1, Table 1-7.) This layer thickens and thins in response to hormones, particularly estrogen. The squamous epithelial membrane is continuous with the membrane that covers the lower part of the uterus. In women of reproductive age, the mucosal layer is arranged in transverse wrinkles, or folds, called rugae (singular, ruga) that permit stretching during coitus and childbirth.
2. Fibrous connective tissue containing numerous blood and lymphatic vessels
The upper part of the vagina surrounds the cervix, the lower end of the uterus (see Figure 23-5). The recessed space around the cervix is called the fornix of the vagina. The posterior fornix is “deeper” than the anterior fornix because of the angle at which the cervix meets the vaginal canal. In most women this angle is about 90 degrees. A pouch called the cul-de-sac separates the posterior fornix and the rectum.
Uterus
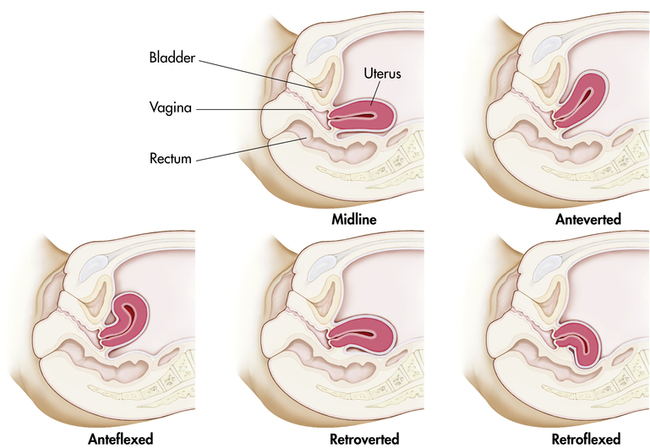
At puberty the uterus attains its adult size and proportions and descends from the abdomen to the lower pelvis, between the bladder and the rectum (see Figure 23-5). The uterus of a mature, nonpregnant female is approximately 7 to 9 cm long and 6.5 cm wide, with muscular walls 3.5 cm thick. It is held loosely in position by ligaments, peritoneal tissue folds, and pressure of adjacent organs, especially the urinary bladder, sigmoid colon, and rectum. In most women the uterus is anteverted; that is, it is tipped forward so that it rests on the urinary bladder. However, it may be retroverted, or tipped backward. Various degrees of flexion are normal (Figure 23-6).
Figure 23-7 shows a cross section of the uterus. The uterus has two major parts: the body, or corpus, and the cervix. The top of the corpus, above the insertion of the fallopian tubes, is called the fundus. The diameter of the uterine cavity is widest at the fundus and narrowest at the isthmus, which is the narrowed part of the corpus just above the cervix. The cervix, or neck of the uterus, extends from the isthmus to the vagina. The passageway between the cervix’s upper opening (the internal os) and its lower opening (the external os) is called the endocervical canal. The entire uterus, like the upper vagina, is innervated exclusively by motor and sensory fibers of the autonomic nervous system.
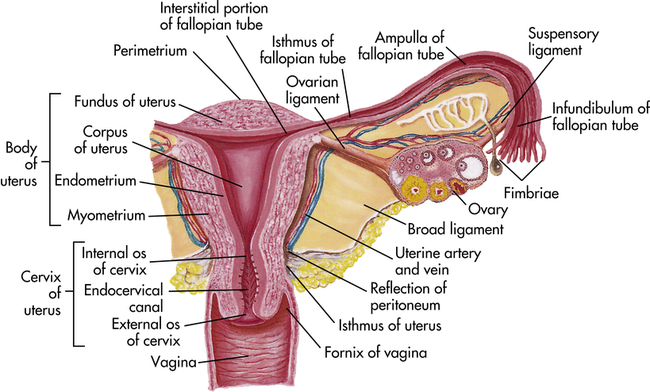
The uterine wall is composed of three layers: the perimetrium, the myometrium, and the endometrium (see Figure 23-7). The perimetrium (parietal peritoneum) is the outer serous membrane that covers the uterus. The myometrium is the thick muscular middle layer. The myometrium is thickest at the fundus, apparently to facilitate birth. The endometrium, or uterine lining, is composed of a functional layer (superficial compact layer and spongy middle layer) and a basal layer. The functional layer of the endometrium is responsive to sex hormones. Between puberty and menopause this layer proliferates and sloughs off monthly. The basal layer, which is attached to the myometrium, regenerates the functional layer after it sloughs (menstruation).
The endocervical canal does not have an endometrial layer. Rather, it is lined with columnar epithelial cells (see Table 1-7). The endocervical lining is continuous with that of the outer cervix and vagina, but it is not made up of the same type of epithelial cells. The point at which the columnar epithelium of the cervix meets the squamous epithelium of the vagina is called the transformation zone, or the squamous-columnar junction. The transformation zone is especially susceptible to the oncogenic human papillomavirus (HPV), especially HPV types 16 and 18, which can lead to cervical dysplasia and, ultimately, cervical cancer (see What’s New? Human Papillomavirus (HPV) Vaccine and Cancer Prevention in Women and Men); these are the cells sampled during the Papanicolaou test (Pap test).7
The cervix acts as a mechanical barrier to infectious microorganisms that may be present in the vagina. The external cervical os is a very small opening that contains thick, sticky mucus (the mucous plug) during the luteal phase of the menstrual cycle and all of pregnancy. During ovulation the mucus changes under the influence of estrogen and forms watery strands, or spinnbarkeit mucus, to facilitate the transport of sperm into the uterus. In addition, the downward flow of cervical secretions moves microorganisms away from the cervix and uterus. In women of reproductive age, the pH of these secretions is inhospitable to most bacteria. Furthermore, mucosal secretions contain enzymes and antibodies (mostly immunoglobulin A) of the secretory (humoral) immune system (see Chapter 8). These defenses do not always prevent infection, even if they are intact. Besides infection, uterine pathophysiology includes displacement of the uterus within the pelvis, benign growths (fibroids) of the uterine wall, hyperplasia of the endometrium, endometriosis, and cancer.
Fallopian Tubes
The two fallopian tubes (oviducts, uterine tubes) enter the uterus bilaterally just beneath the fundus (see Figure 23-7). Their function is to conduct the ova from the spaces around the ovaries to the uterus. From the uterus the fallopian tubes curve up and over the two ovaries. Each tube is 8 to 12 cm long and about 1 cm in diameter, except at its ovarian end, which flares out like the bell of a trumpet. This widened end, called the infundibulum, is fringed or fimbriated. The fimbriae (singular, fimbria) (fringes) move, creating a current that draws the ovum into the infundibulum. Once the ovum has entered the fallopian tube, cilia and peristalsis (muscle contractions) keep it moving toward the uterus.
The ampulla, or distal third, of the fallopian tube is the usual site of fertilization (see Figure 23-7). Sperm released into the vagina travel upward through the endocervical canal and uterine cavity and enter the fallopian tubes. If an ovum is present in either tube, fertilization can occur. Whether or not the ovum encounters sperm, it continues to travel through the fallopian tube to the uterus. If fertilized, the ovum (then called a blastocyst) implants itself in the endometrial layer of the uterine wall. If not fertilized, the ovum breaks down within 12 to 24 hours.
Disorders that affect the fallopian tubes (e.g., congenital malformations, infection, and inflammation) can block the path of sperm and ovum and cause infertility or ectopic (tubal) pregnancy.
Ovaries
The almond-shaped ovaries are located on both sides of the uterus and are suspended and supported by the mesovarian portions of the broad ligament, ovarian ligaments, and suspensory ligaments (see Figure 23-7). The ovaries are smaller than their male homologs, the testes. In women of reproductive age, each ovary is 3 to 5 cm long, 2.5 cm wide, and 2 cm thick and weighs 4 to 8 g. Size and weight vary somewhat from phase to phase of the menstrual cycle (see p. 778).
Figure 23-8, A shows a cross section of an ovary. The central part, or medulla, is composed of connective tissue and contains many small arteries, veins, and lymphatics that enter at the hilum. Surrounding the medulla is the cortex. At birth the cortex of each ovary contains approximately 2 million ova within primordial (immature) ovarian follicles. Follicles grow and undergo atresia continuously and irrevocably throughout a woman’s life. By puberty the number ranges between 300,000 and 500,000 ova. Between puberty and menopause the ovarian cortex always contains follicles and ova in various stages of development, including the primary and secondary follicles (see Figure 23-8, A). Once every menstrual cycle (about every 28 days), usually only one of the follicles reaches maturation (see Figure 23-8, B and C) and discharges its ovum through the ovary’s outer covering, the germinal epithelium. During the reproductive years, 400 to 500 ovarian follicles mature completely and release an ovum (ovulation). The rest either fail to develop at all or degenerate without maturing completely and are known as atretic follicles1 (see Figure 23-8).
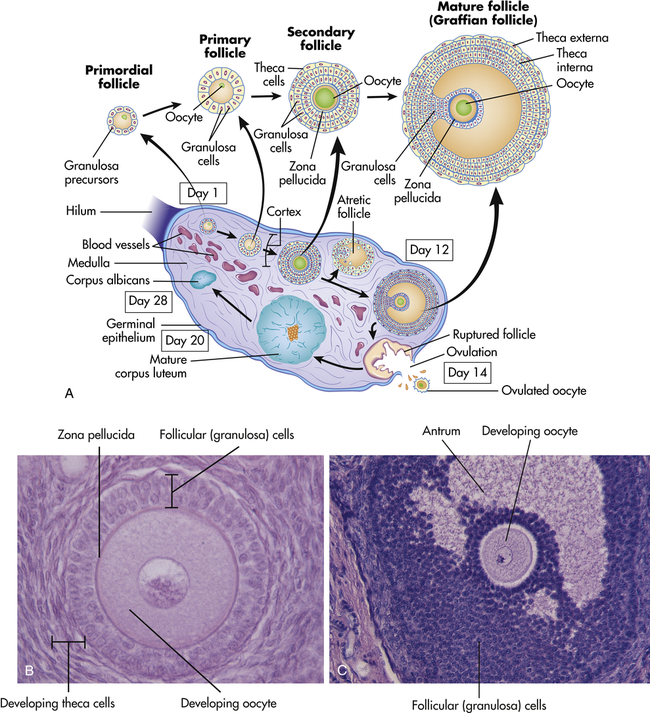
A, Schematic representation (not to scale) of the structure of the ovary, showing the various stages in the development of the follicle and its successor structure, the corpus luteum. B, A developing oocyte surrounded by hormone-secreting follicular (granulosa) cells. C, A more mature ovarian follicle has a fluid-filled cavity called the antrum. (A adapted from Berne RM, Levy MN, editors: Physiology, ed 5, St Louis, 2003, Mosby. B and C from Patton KT, Thibodeau GA: Anatomy & physiology, ed 8, St Louis, 2013, Mosby.)
After release of the mature ovum (ovulation), the follicle develops into another structure, the corpus luteum (see Figure 23-8). The immediate fate of the corpus luteum depends on whether the ejected ovum is fertilized. If fertilization occurs, the corpus luteum enlarges and begins to secrete hormones that maintain and support pregnancy. If fertilization does not occur, the corpus luteum secretes these hormones for approximately 14 days and then degenerates, which triggers the maturation of another follicle. The ovarian cycle—the process of follicular maturation, ovulation, corpus luteum development, and corpus luteum degeneration—is continuous from puberty to menopause, except during pregnancy or hormonal contraceptive use. At menopause this process ceases and the ovaries atrophy to the point that they cannot be felt during pelvic examination.
Sex hormones are secreted by cells within the ovarian cortex including two types of cells in the ovarian follicle (theca cells and granulosa cells) and cells of the corpus luteum (see Figure 23-8). These cells all contain receptors for the gonadotropins (LH, FSH) or for the sex hormones, which are discussed in the next section.
Female Sex Hormones
The sex hormones are all steroid hormones and are synthesized from cholesterol (see Chapter 21). Male and female sex hormones are present in all adults. However, the female body contains low levels of testosterone and other androgens, and the male body contains low levels of estrogen. Individual effects of sex hormones depend on their amount and concentration in the blood.
Estrogens and Androgens
Estrogen has numerous biologic effects, many of which involve interactions with other hormones, and is needed for maturation of reproductive organs, development of secondary sex characteristics (differentiating male and female physical characteristics that are not directly related to reproduction), closure of long bones after the pubertal growth spurt, regulation of the menstrual cycle, and endometrial regeneration after menstruation. Estrogen also has metabolic effects on the bones, liver, blood vessels, brain and central nervous system, kidneys, and skin. After menopause ovarian production of estradiol and estrone is markedly diminished (see Menopause, p. 791). At this time the majority of estrogen is derived from extraovarian and extraglandular production of estrones.1
Like other steroid hormones, estrogens are derived from cholesterol in a complex, enzyme-mediated series of reactions. (Mechanisms of hormone synthesis and action are described in Chapter 21.) The hypothalamus secretes GnRH in a pulsatile manner that stimulates gonadotropin (LH and FSH) release from the anterior pituitary. Gonadotropins trigger ovarian production of estrogen. The primary function of LH is to stimulate theca cells of the ovarian follicle to produce androgens, mainly androstenedione. (Androgens are discussed further on p. 789 and in the section on male reproductive function.) Some of these androgens are converted to estrogen by the theca cells themselves, and others diffuse into the granulosa cells. Within the granulosa layer, FSH induces conversion (aromatization) of androgens to estrogens. Estrogens are then released into the bloodstream. Estrogen and FSH together increase FSH receptors in the follicle, stimulating additional granulosa cells until a dominant follicle is determined.
Progesterone
Luteinizing hormone (LH) from the anterior pituitary stimulates the corpus luteum to secrete progesterone, the second major female sex hormone. LH surge occurs when there is a peak level of estrogen, about 24 to 36 hours before ovulation. LH promotes luteinization of the granulosa in the dominant follicle and results in progesterone production and the development of blood vessels and connective tissue. A rising level of progesterone can be detected from the preovulatory follicle as early as day 10 of the menstrual cycle. Small amounts of progesterone also are secreted steadily by the adrenal cortex. Before ovulation the ovary and the adrenal glands each contribute approximately 50% of total progesterone production. Conversely, large amounts are secreted from the ovary while the corpus luteum is active for about 9 to 13 days after ovulation. Progesterone secreted by the corpus luteum stimulates the thickened endometrium to become more complex in preparation for implantation of a blastocyst. If conception and implantation do occur, the corpus luteum persists and secretes progesterone (and estrogen) until the placenta is well established at approximately 8 to 10 weeks’ gestation. Together, estrogen and progesterone control the menstrual cycle. The opposing and complementary effects of progesterone and estrogen are listed in Table 23-1.
TABLE 23-1
COMPLEMENTARY AND OPPOSING EFFECTS OF ESTROGEN AND PROGESTERONE
| STRUCTURE | EFFECT OF ESTROGEN | EFFECT OF PROGESTERONE |
| Vaginal mucosa | Proliferation of squamous epithelium; increase in glycogen content of cells; layering (cornification) of cells | Thinning of squamous epithelium; decornification |
| Cervical mucosa | Production of abundant fluid secretions that favor survival and enhance motility of sperm | Production of thick, sticky secretions that tend to “plug” the cervical os |
| Fallopian tube | Increase of motility and ciliary action | Decrease of motility and ciliary action |
| Uterine muscle | Increase of blood flow; increase of contractile proteins and uterine muscle and myometrial excitability and action potential; increase of sensitization to oxytocin | Relaxation of myometrium; decrease of sensitization to oxytocin |
| Endometrium | Stimulation of growth; increase in number of progesterone receptors | Activation of glands and blood vessels; accumulation of glycogen and enzymes; decrease in number of estrogen receptors |
| Breasts | Growth of ducts; promotion of prolactin effects | Growth of lobules and alveoli; inhibition of prolactin effects |
Progesterone is sometimes called the hormone of pregnancy. Progesterone’s effects in pregnancy include: (1) maintaining the thickened endometrium; (2) relaxing smooth muscle in the myometrium, which prevents premature contractions and helps the uterus expand; (3) thickening (hypertrophy) of the myometrium, which prepares it for the muscular work of labor; (4) promoting growth of lobules and alveoli in the breast in preparation for lactation but prevents lactation until the fetus is born; (5) preventing additional maturation of ova by way of suppressing FSH and LH, thereby stopping the menstrual cycle; (6) providing immune modulation allowing tolerance against fetal antigens (the mother’s immune system does not attack the fetus); and preventing preterm birth.8–10
The Menstrual (Ovarian) Cycle
In addition to pregnancy the obvious manifestation of female reproductive functioning is menstrual bleeding (the menses), which starts with menarche (first menstruation) and ends with menopause (cessation of menstrual flow for 1 year). In the United States the average age of first menstruation is 12 years in black females, 12.5 years in Hispanic females, and 12.6 years in white females, with a range from 9 to 17 years.11 Menarche appears to be related to body weight, especially percentage of body fat (ratio of fat to lean tissue), which may trigger a change in the metabolic rate and lead to hormonal changes associated with early menarche.12 There is an increased sensitivity to leptin (a regulatory hormone of appetite and energy metabolism) during puberty and, in theory, the adolescent consumes more calories to meet caloric needs of the pubertal growth spurt.13 The percent of body fat and leptin levels in girls continue to increase, whereas muscle mass increases in boys.14
At first, cycles are anovulatory and may vary in length from 10 to 60 days or more. As adolescence proceeds into adulthood, regular patterns of menstruation and ovulation are established at intervals ranging from 25 to 35 days. The length of the menstrual cycle varies considerably among women. The commonly accepted cycle average is 28 (27 to 30) days, with rhythmic intervals of 21 to 35 days considered normal. Approximately 2 to 8 years before menopause, cycles begin to lengthen again. Menstrual cyclicity and regular ovulation are dependent on (1) the activity of the gonadostat (GnRH pulse generator); (2) the initial pituitary secretion of the gonadotropin FSH; and (3) estrogen (estradiol) positive feedback for the preovulatory FSH and LH surge, oocyte maturation, and corpus luteum formation and production of progesterone.15
Phases of the Menstrual Cycle
The menstrual (ovarian) cycle (Figure 23-9) consists of ovulation, which occurs in two phases: the follicular/proliferative and the luteal/secretory phase and each lasts about 14 days. Ovulation occurs between the follicular and luteal phases. If implantation of the blastocyst does not occur in the late luteal phase, menstruation (menses) occurs, also known as the ischemic/menstrual phase.16
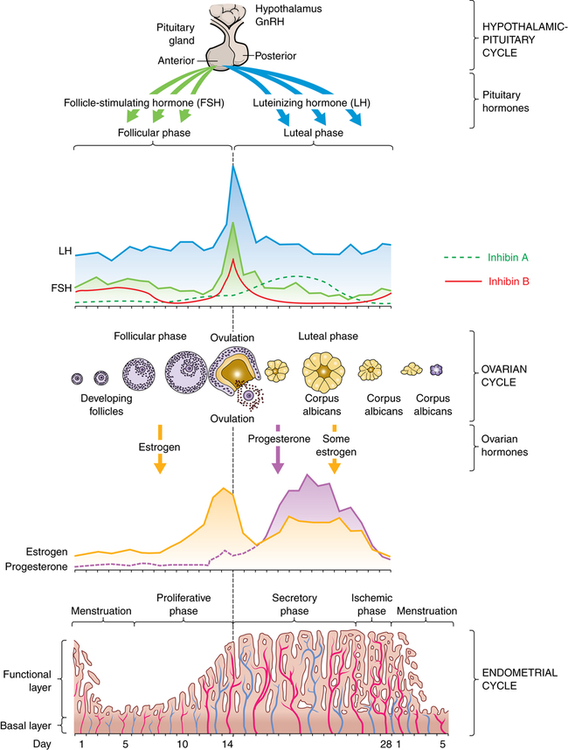
GnRh, Gonadotropin-replacing hormone. (Adapted from Lowdermilk DL et al: Maternity and women’s health care, ed 10, St Louis, 2012, Mosby.)
During the follicular/proliferative phase GnRH and a balance between activin and inhibin from the granulosa cells contribute to the rise of FSH, which stimulates a number of follicles. The pulsatile secretion of FSH from the anterior pituitary gland rescues a dominant ovarian follicle from apoptosis by day 5 to 7 of the cycle. Together estrogen and FSH increase FSH receptors in the granulosa cells of the primary follicle, making them more sensitive to FSH. FSH and estrogen combine to induce production of LH receptors on the granulosa cells, thus promoting LH stimulation to combine with FSH stimulation, causing a more rapid secretion of follicular estrogen. As estrogen increases, FSH levels drop because of an increase in inhibin-B secreted by the granulosa cells in the dominant follicle. This drop in FSH decreases the growth of less-developed follicles (see Figure 23-9). Estrogen causes cells of the endometrium to proliferate and stimulates production of LH. A surge in both FSH and LH are required for final follicular growth and ovulation.
Increase in stromal tissue in the late follicular phase is associated with a rise in androgen levels. Androgen production enhances the process of follicle atresia and may stimulate libido at the point of ovulation.1
Ovulation marks the beginning of the luteal/secretory phase of the menstrual cycle. The ovarian follicle begins its transformation into a corpus luteum (see Figure 23-8, A), hence the name luteal phase. Pulsatile secretion of LH from the anterior pituitary stimulates the corpus luteum to secrete progesterone, which in turn initiates the secretory phase of endometrial development. Glands and blood vessels in the endometrium branch and curl throughout the functional layer, and the glands begin to secrete a thin glycogen-containing fluid, the secretory phase. If conception occurs, the nutrient-laden endometrium is ready for implantation. Human chorionic gonadotropin (HCG) is secreted 3 days after fertilization by the blastocytes and maintains the corpus luteum once implantation occurs at about day 6 or 7. HCG can be detected in maternal blood and urine 8 to 10 days after ovulation. The production of estrogen and progesterone continues until the placenta can adequately maintain hormonal production. If conception and implantation do not occur, the corpus luteum degenerates and ceases production of progesterone and estrogen. Without progesterone or estrogen to maintain it, the endometrium becomes ischemic (blood-starved) and disintegrates. Menstruation then occurs—the ischemic/menstrual phase—marking the beginning of another cycle.
Ovarian cycles appear to have a minimum length of 24 to 26.5 days: the primary ovarian follicle requires 10 to 12.5 days to develop, and the luteal phase appears relatively fixed at 14 days (±3 days). Menstrual blood flow usually lasts 3 to 7 days, but it may last as long as 8 days or stop after 1 to 2 days and still be considered within normal limits. Bleeding is consistently scant to heavy and varies from 30 to 80 ml, with most blood loss occurring during the first 3 days of menses. Menstrual discharge consists of blood, mucus, and desquamated endometrial tissue and does not clot under normal circumstances. It is usually dark and produces a characteristic musty odor on oxidation. Environmental factors such as severe emotional stress, illness, malnutrition, obesity, and seasonal variation may affect the length of the menstrual cycle.1,17–19
Hormonal Controls
Hormonal control of the menstrual cycle depends on complex interactions among the hypothalamus, the anterior pituitary, and the ovaries (or hypothalamic-pituitary-ovarian [HPO] axis)20 (Table 23-2). Hormonal control is dependent on negative and positive ovarian feedback mechanisms. GnRH controls the gonadotropin production of FSH and LH, and the constant and pulsatile release of GnRH is critical to the timing of the menstrual cycle. GnRH is secreted by the hypothalamus into the hypophyseal portal system and travels to the anterior pituitary, where it stimulates the secretion of LH and FSH. FSH and LH are released from the anterior pituitary in pulses that correspond to the pulsatile secretion of GnRH.
TABLE 23-2
HORMONAL FEEDBACK MECHANISM IN THE MENSTRUAL CYCLE
| PHASE OF CYCLE AND OVARIAN HORMONE LEVELS | FEEDBACK TO HYPOTHALAMUS AND ANTERIOR PITUITARY | RESULTANT GNRH, FSH, AND LH LEVELS | OVARIAN AND MENSTRUAL EVENTS |
| Early follicular phase: estrogen levels low; minute amount of progesterone secreted | Negative and inhibitory | All low | Ovarian follicle develops; endometrium proliferates |
| Late follicular (preovulatory) phase: estrogen levels high; progesterone increases with small surge before ovulation | Positive and stimulatory | All surge; LH dominates | Process of ovulation begins; endometrial proliferation complete |
| Ovulatory phase: estrogen levels dip; progesterone levels begin to rise | Negative and inhibitory | All fall sharply | Corpus luteum begins to develop; endometrium enters secretory phase |
| Early luteal phase: estrogen and progesterone levels high; progesterone dominates | Negative and inhibitory | All continue to decline, but gradually | Corpus luteum fully developed; endometrium ready for implantation |
| Late luteal phase: estrogen and progesterone levels fall sharply | Negative and inhibitory; feedback lessens slightly | All rise slightly | Corpus luteum regresses; endometrium breaks down; menstruation begins |
| Menstrual phase: estrogens levels low; minute amount of progesterone secreted | Negative and inhibitory | All low | More ovarian follicles begin to develop; functional layer of endometrium is shed |
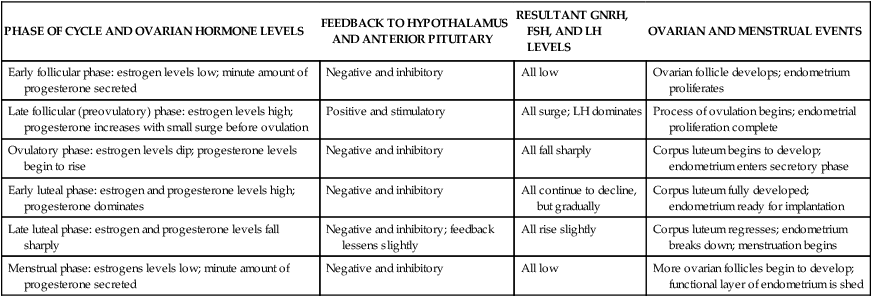
FSH, Follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
During the early follicular phase, estrogen levels rise steadily and, through negative feedback, suppress FSH and positively increase the production of LH. During the late follicular phase, the preovulatory rise in progesterone facilitates the positive feedback of estrogen; estrogen levels begin to increase, stimulating a surge of LH secretion from the anterior pituitary. The midcycle surge of LH and FSH induces ovulation. A nonsteroidal ovarian factor, gonadotropin surge-attenuating factor (GnSAF), may antagonize the effect of estrogen on the pituitary and regulate the surge of LH at midcycle.21 Rising estrogen and progesterone levels during the luteal phase may inhibit the anterior pituitary, and thus reduce LH and FSH secretion. Just before the onset of menstruation, FSH and LH levels begin to increase slightly, probably because of declining estrogen and progesterone levels (see Figure 23-9).
A variety of growth factors and autocrine/paracrine peptides influence hormonal control and follicular response.1 During the early follicular stage, FSH stimulates FSH and LH receptors, insulin-like growth factor 1, and production of inhibin and activin in the ovary. Activin from granulosa cells stimulates the secretion of FSH and increases the pituitary response to GnRH, and increases FSH-binding in the granulosa cells in the dominant follicle. FSH stimulates inhibin secretion from granulosa cells and it in turn suppresses FSH synthesis. Inhibin B is primarily secreted in the follicular phase of the cycle but sharply spikes when ovulation occurs. Inhibin A is secreted in the luteal phase and further suppresses FSH. Inhibin also restrains prolactin and growth hormone release, interferes with GnRH receptors, and promotes breakdown of intracellular gonadotropins. In summary, the balance between activin and inhibin regulates FSH secretion, and follistatin inhibits activin and boosts inhibin activity. Inhibin and activin also regulate LH stimulation of androgen synthesis in theca cells.22,23 Figure 23-9 depicts fluctuating estrogen, progesterone, gonadotropin, and inhibin levels. Research continues to advance understanding of the function and structural complexity of these polypeptides and their interaction with GnRH, gonadotropins, and sex hormones.24
Ovarian Cycle
By stimulating follicles, gonadotropins initiate their growth and maturation. The most important hormonal event is a rise in FSH. The decline in the late luteal phase of estrogen, progesterone, and inhibin secretion allows FSH to rise; concurrently there is a slight increase in LH levels (see Figure 23-9). FSH stimulates granulosa cell growth and initiates estrogen production in these cells in the next cycle. At this time a group of ovarian follicles is recruited and begins to mature; the exact number depends on the remaining pool of inactive follicles. As the follicles mature, granulosa cells multiply, increasing estradiol secretion. Within a few days of the cycle, one follicle becomes dominant and the others atrophy. The mechanism for follicular recruitment or dominance is unknown.25 The dominant follicle begins to secrete progressively larger amounts of estrogen (estradiol), which exerts an increase in GnRH receptor concentration and an increase in pituitary sensitivity to GnRH, creating a positive-feedback effect causing an FSH and LH surge. Ovulation generally occurs 1 to 2 hours before the final progesterone surge, or about 12 to 36 hours after the onset of the FSH and LH surge. Progesterone, proteolytic enzymes, and prostaglandins (E and F series) trigger mechanisms controlling follicular rupture and release of the ovum.1 Possible mechanisms include thinning, stretching, degradation, and digestion of the follicular wall and contraction of smooth muscle cells of the follicle. The role of prostaglandins is essential to ovulation, and infertility patients should be advised to avoid the use of drugs that inhibit prostaglandin synthesis.26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree