Chapter 26
Sexually Transmitted Infections
Julia C. Phillippi and Gwen A. Latendresse
Throughout recorded history, infectious diseases have threatened humans. Even into the twentieth century, epidemics of diphtheria, typhoid, tuberculosis, cholera, and other catastrophic infections have decimated entire communities almost overnight (see Chapter 10). Despite medical advances, improved living standards, and better nutrition, epidemics still arise as major public health problems, and some pose lethal threats to individuals and communities. Some of these epidemics are caused by sexually transmitted infections (STIs). At this time, many people consider the number of individuals with acquired immunodeficiency syndrome (AIDS), human immunodeficiency virus (HIV), and human papillomavirus (HPV) infections to be at epidemic levels.
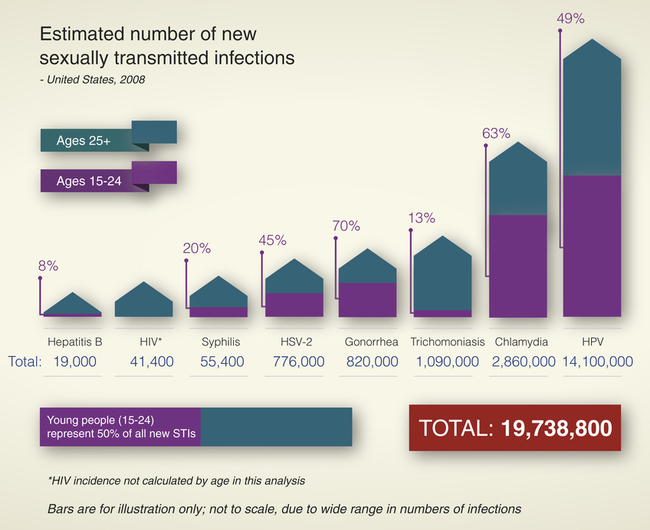
Sexually contracted infections affect more than 19 million Americans per year (see What’s New? Sexually Transmitted Infection [STI] Statistical Summary from 2010 CDC Report), and half of those are younger than 25 years1 and account for about one third of the reproductive mortality in the United States. They have been called the “hidden epidemic” by the Centers for Disease Control and Prevention (CDC)2 because of their stigma and overall lack of knowledge of the prevalence and consequences of sexually transmitted infections. Complications of STIs include pelvic inflammatory disease, infertility, ectopic pregnancy, chronic pelvic pain, neonatal morbidity and mortality, and genital cancers. Long-term sequelae of untreated or undertreated STIs can affect a person’s physical, emotional, and financial well-being.
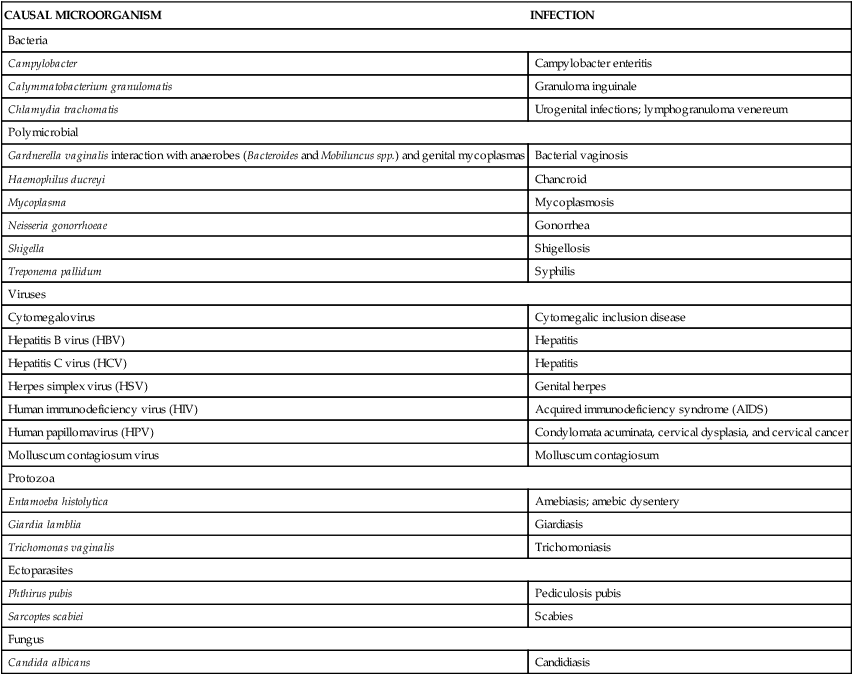
In the past an infection transmitted through sexual intercourse was called a venereal disease. Because of its limited scope, the term venereal disease has been replaced with sexually transmitted infection (STI). STIs are contracted by intimate, as well as sexual, contact and include systemic infections, such as tuberculosis and hepatitis, that can be spread to a sexual partner. The etiology of an STI may be bacterial, viral, protozoal, parasitic, or fungal (Table 26-1). Although the majority of STIs can be treated, viral STIs cannot be cured, only treated naturally or pharmacologically. Many infected individuals do not seek treatment because symptoms are absent, minor, or transient or because health services are inaccessible, unaffordable, or culturally insensitive. High-risk sexual behaviors (e.g., multiple partners) and poor health habits (e.g., failure to use a condom in nonmonogamous or new relationships, drug use) increase an individual’s risk of exposure or the severity of infection if exposed. Perhaps partly
TABLE 26-1
CURRENTLY RECOGNIZED SEXUALLY TRANSMITTED INFECTIONS
| CAUSAL MICROORGANISM | INFECTION |
| Bacteria | |
| Campylobacter | Campylobacter enteritis |
| Calymmatobacterium granulomatis | Granuloma inguinale |
| Chlamydia trachomatis | Urogenital infections; lymphogranuloma venereum |
| Polymicrobial | |
| Gardnerella vaginalis interaction with anaerobes (Bacteroides and Mobiluncus spp.) and genital mycoplasmas | Bacterial vaginosis |
| Haemophilus ducreyi | Chancroid |
| Mycoplasma | Mycoplasmosis |
| Neisseria gonorrhoeae | Gonorrhea |
| Shigella | Shigellosis |
| Treponema pallidum | Syphilis |
| Viruses | |
| Cytomegalovirus | Cytomegalic inclusion disease |
| Hepatitis B virus (HBV) | Hepatitis |
| Hepatitis C virus (HCV) | Hepatitis |
| Herpes simplex virus (HSV) | Genital herpes |
| Human immunodeficiency virus (HIV) | Acquired immunodeficiency syndrome (AIDS) |
| Human papillomavirus (HPV) | Condylomata acuminata, cervical dysplasia, and cervical cancer |
| Molluscum contagiosum virus | Molluscum contagiosum |
| Protozoa | |
| Entamoeba histolytica | Amebiasis; amebic dysentery |
| Giardia lamblia | Giardiasis |
| Trichomonas vaginalis | Trichomoniasis |
| Ectoparasites | |
| Phthirus pubis | Pediculosis pubis |
| Sarcoptes scabiei | Scabies |
| Fungus | |
| Candida albicans | Candidiasis |
because of risk-taking behavior (unprotected intercourse or selection of high-risk partners), adolescents have the greatest risk for STI exposure and infection. In addition, adolescent women may have a physiologically increased susceptibility to infection because of cervical immaturity. Rates of gonorrhea, chlamydia, vaginitis, cervical condyloma, genital warts, and pelvic inflammatory disease (PID) are highest in adolescents and young women and decline exponentially with increasing age (Figure 26-1). The health consequences of STIs are both immediate and long-term. Women and infants bear the greatest health burden from STIs.
Sexually Transmitted Urogenital Infections
Bacterial Infections
Gonorrhea
Infection rates are highest in the southern region of the United States.2 Other demographic and lifestyle risk factors may include transient or urban residence, early onset of sexual activity, multiple serial or consecutive sex partners, drug use, prostitution, and previous gonorrheal or concurrent STI.2 The risk of developing gonorrhea from intercourse with an infected male partner is 50% to 80% for women, and with an infected female partner, it is 20% to 30% for men. The risk increases threefold to fourfold for men after four exposures to an infected partner. Men who have sex with infected men have a greater risk of contracting gonorrhea if they are the receptive partner in oral or anal intercourse.2
Treatment for gonorrhea is becoming more difficult because of rapidly developing resistance to available antibiotics. The CDC states that it is reasonable to expect that gonorrhea may become resistant to all known antibiotics in the near future.3
Pathophysiology
Humans are the only natural hosts for N. gonorrhoeae, which is an aerobic, non–spore-forming, oxidase-positive gram-negative coccal (round) microorganism that usually appears in pairs (diplococci), with the adjacent sides slightly flattened. Hairlike filaments, called pili, appear to help the microorganisms attach themselves to host cells: the epithelial cells of mucous membranes (Figure 26-2). Columnar, transitional, and stratified squamous epithelial cells are infected most often. First the microorganisms become attached to the plasma membranes (cell walls) of these cells, and then they invade the cells and begin to damage the mucosa. Generally a quick leukocytic (inflammatory) response and exudation at the site of infection occur.
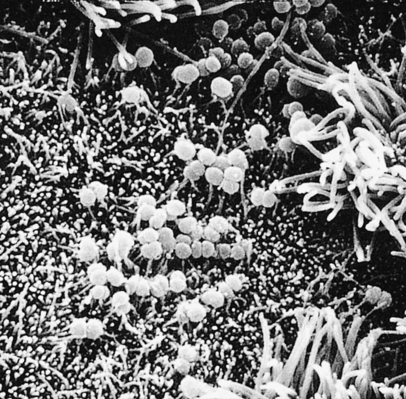
Scanning electron microscopy showing gonococci attaching to the nonciliated cells of human fallopian tube mucosa. (From Morse SA et al, editors: Atlas of sexually transmitted diseases and AIDS, ed 3, London, 2003, Mosby.)
In women the endocervical canal (inner portion of the cervix) is the usual site of initial gonococcal infection, although urethral colonization and infection of glands (paraurethral [Skene glands] and greater vestibular [Bartholin glands]) near the urethra and vagina also are common. Several factors can facilitate ascent of gonococci into the uterus and the fallopian tubes, where they cause PID. Among these factors are: (1) disintegration of the cervical mucous plug and an increase in vaginal pH to greater than 4.5 during menstruation, (2) uterine contraction that may cause retrograde menstruation into the fallopian/uterine tubes, and (3) various microbes that possess virulent potentiating factors for chlamydia or gonococcal PID. Bacteria (N. gonorrhoeae, Chlamydia trachomatis) also may adhere to sperm and be transported to the fallopian/uterine tubes. In the fallopian/uterine tubes, progressive mucosal and submucosal invasion and sloughing of normal, ciliated tubal epithelium are accompanied by a marked inflammatory response, causing the tubes to fill with exudate (see Chapter 25 for more on PID). In men the gonococci typically infect the urethra or rectum. Untreated urethral infection can cause epididymitis in men, and, if untreated, urethral stricture, fistula, and sterility.4 Concurrent oropharyngeal and anorectal infection can be found in infected men and women.2 Virulence is determined by variations in the bacterial properties and host response.
Clinical Manifestations
The clinical manifestations of gonorrhea can be categorized as local or systemic and uncomplicated or complicated. Uncomplicated local infections are seen as urethral infections in men and urogenital infections in women. The majority of infected men will have sudden onset of painful urination or purulent penile discharge, or both, within a week of infection.4 These severe symptoms encourage most men in developed countries to seek treatment.2,4 However, some men have little discharge or urethral itching (i.e., pruritus) only, and 5% to 10% never have signs or symptoms. Most cases of untreated gonococcal urethritis resolve spontaneously after several weeks, and more than 95% of individuals are asymptomatic by 6 months after infection (although they may still be infectious). Some men develop urethritis even after being appropriately treated. If untreated, gonorrhea can cause penile abscesses, fistulae, and strictures.4
In women, the incubation period varies, but those who develop symptoms usually manifest within 10 days of exposure or within 1 to 2 days after the next menstrual period. More than half of gonorrhea infections in women are initially asymptomatic. Symptoms often do not appear until the infection has spread to the upper reproductive tract (uterus, fallopian/uterine tubes, and ovaries). Symptoms can include dysuria, increased vaginal discharge, abnormal menses (increased flow or dysmenorrhea), dyspareunia, lower abdominal/pelvic pain, and fever. Physical examination may disclose cervical friability and erythema (redness) and mucopurulent discharge from the cervical os (Figure 26-3). There may be a discharge from the paraurethral (Skene) or greater vestibular (Bartholin) glands if these sites are involved.
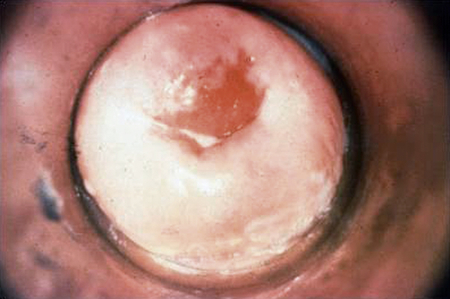
The cervix is involved in 85% to 90% of cases in women, but the resultant discharge is profuse enough to be recognized in only 10%. (From Centers for Disease Control and Prevention: STD clinical slides (website). Available at www.cdc.gov/std/training/clinicalslides/slides-dl.htm. Accessed March 5, 2013.)
Anal and rectal gonococcal infection is found in 30% to 50% of women diagnosed with urogenital gonorrhea. In women, anorectal infection is usually asymptomatic and not necessarily related to anal intercourse. Symptomatic anorectal gonorrhea occurs more commonly in men who have sex with men.2 Symptoms of anorectal gonorrhea range from mild anal pruritus (itching), mucopurulent rectal discharge, and slight rectal bleeding to severe rectal pain, tenesmus (painful and ineffectual straining at stool), and constipation. Physical examination findings include anal erythema and discharge and evidence of mucosal damage to the anus and rectum, such as friability, edema, and purulent exudate.
Complicated gonococcal infections include prostatitis, epididymitis, lymphangitis, and urethral stricture in men and salpingitis, PID, and bartholinitis in women. Chronic salpingitis or perididymitis can cause scarring and adhesions that lead to sterility in men and women. Approximately 10%2 of women with untreated cervical gonorrhea develop salpingitis or PID. Importantly, salpingitis has the potential for long-term sequelae, especially infertility and ectopic pregnancy.
The onset of symptoms for women may be rapid and usually occurs during menses. Women may experience chills, fever, nausea, vomiting, and lower abdominal/pelvic pain that worsen with movement, such as walking, coughing, sneezing, or intercourse. Abdominal palpation often discloses bilateral lower quadrant tenderness and rebound tenderness resulting from peritoneal irritation caused by tubal exudate. Marked tenderness of the internal genitalia is often noted during pelvic examination. Enlargement or masses may be palpable in the upper genital tract. Tubal infertility is found in 12% of women after one episode of PID. If a woman has three or more episodes of PID, she has a 50% risk of tubal infertility.5 Disseminated gonococcal infection (DGI) is a rare systemic complication caused by the spread of infection through the bloodstream. Symptoms of this life-threatening condition include a generalized rash and severe joint pain. Proliferation of N. gonorrhoeae to the liver causes a condition known as perihepatitis or Fitz-Hugh–Curtis syndrome. C. trachomatis also has been identified as a causative agent of this condition. Inflammation of the capsule of the liver produces sudden and intense right upper quadrant pain.6 This complication usually develops after acute salpingitis in women and is very rare in men.
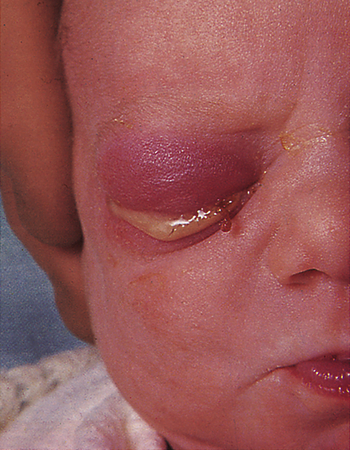
If a mother has untreated gonorrhea at the time of birth, she can transmit the infection to her child, regardless of whether the baby is delivered vaginally or by cesarean section. Most states require all infants to receive prophylactic ophthalmic antibiotics to prevent gonococcal eye infection (ophthalmia neonatorum) (Figure 26-4) and risk for associated blindness. Newborns born to infected mothers also may develop gonorrheal rhinitis, anorectal infection, or an abscess at the site of electrode placement for fetal monitoring. Onset of symptoms generally occurs 1 to 12 days after birth, with a mean of 4 to 6 days. Affected newborns usually are born to mothers who have suffered from prolonged ruptured membranes. In these cases, immediate treatment with a topical antibiotic is not effective because the infection is already established. Established infection causes bilateral corneal ulceration, with a profuse yellow or gray purulent exudate, and is followed by necrosis, scarring, and permanent blindness.
Evaluation and Treatment
Clinical signs and symptoms are not sufficient for the differential diagnosis of gonococcal infections. Microscopic evaluation of Gram-stained slides of clinical specimens is deemed positive for N. gonorrhoeae if gram-negative diplococci with typical “kidney bean” morphology are seen inside polymorphonuclear leukocytes. Such a finding is considered adequate for the diagnosis of gonococcal urethritis in a symptomatic man. For women, the Gram-stain technique is less accurate and reliable and is replaced with a single sample of endocervical secretions. Most clinic settings now use nucleic acid hybridization tests because these samples do not require an anaerobic incubation, are highly sensitive, and can be easily transported to a laboratory for testing. Because of the large percentage of infected women without symptoms, routine screening for at-risk women (i.e., those younger than age 25 or with a new sexual partner) is recommended. Samples can be obtained from the urine or the vagina, endocervix, urethra, rectum, or oropharynx. Although nucleic acid hybridization is acceptable for screening, the CDC prefers direct culture for testing because this will provide information about antibiotic susceptibility and help provide a cure.3
N. gonorrhoeae has developed resistance to many antibiotics and has the potential to become resistant to all current treatment drugs.3 Several types of drug-resistant strains have been identified, including penicillinase-producing N. gonorrhoeae (PPNG), which is resistant to penicillin; tetracycline-resistant N. gonorrhoeae (TRNG), which is resistant to tetracycline; chromosomal control of mechanisms of resistance of N. gonorrhoeae (CMRNG), which is resistant to penicillin and tetracycline; and increasingly a fluoroquinolone-resistant N. gonorrhoeae (QRNG).7,8 Until 2007 fluoroquinolones were the first-line treatment recommended by the CDC. In 2010 more than 27% of CDC-monitored samples were resistant to one or more antibiotics.2 The only class of drugs still recommended for treatment of gonorrhea are the cephalosporins; however, there is increasing resistance to this type of medication in the United States, Canada, and around the world. There is currently only one CDC recommended drug, ceftriaxone, to treat gonorrhea although less-effective alternatives are available.3 To prevent further drug resistance, the CDC recommends a multidrug treatment for gonorrhea that also is effective against chlamydia.3
The CDC is closely observing the drug resistance of gonorrhea through monitoring of samples obtained at sexually transmitted disease (STD) clinics around the country.3 Treatment for gonorrhea is influenced by three factors: (1) the spread of infection caused by drug-resistant strains, (2) the high frequency of chlamydia infection accompanying gonorrhea, and (3) the recognition of the serious complications of chlamydia and gonorrhea infections. CDC treatment guidelines are updated regularly, and the most recent edition should be used. Current CDC treatment guidelines for uncomplicated gonorrheal infections are listed in Box 26-1; complicated infections require intravenous antibiotic therapy and possibly hospitalization.
Syphilis
Syphilis, a disease with local and systemic manifestations, has been well-known throughout history. Many famous figures from the ancient world and from the royal families of Europe were thought or known to have had syphilis.9 In the early half of the 1900s, an estimated 1 in 4 to 1 in 20 Americans were infected.9 With the advent of antibiotics and intensive public health efforts during and after World War II, the prevalence of syphilis declined sharply. Rates of syphilis declined in the 1990s and reached a record low in 2000 (2.2 cases per 100,000). However, between 2000 and 2009, the syphilis rate in the United States increased. In 2010, the rate of syphilis infection was 7.9 cases per 100,000 population for men and 1.1 cases per 100,000 for women.2 The rate of primary and secondary syphilis has risen dramatically among men. Data suggest this increase is driven by increased transmission of syphilis among men who have sex with men (MSM), and accounts for 67% of these cases. The rate of primary and secondary syphilis is now nearly six times greater in men than women, whereas rates were almost equivalent a decade ago. Syphilis remains a problem in certain geographic regions, particularly in the South. Syphilis facilitates the transmission of human immunodeficiency virus (HIV) infection and seems to contribute to HIV transmission in those parts of the United States where rates of both infections are high. From 2003 to 2009, the rates have increased for all groups; in 2010 the reported rate among non-Hispanic whites was 2.1 per 100,000, among Hispanics the rate was 4.6 per 100,000, among Asian/Pacific Islanders the rate was 1.3 per 100,000, among Native American/Alaskan Natives the rate was 2.5 per 100,000, and among blacks the rate was 16.8 per 100,000.
While rates in men have increased dramatically, rates in women have decreased slightly from a recent high in 2008.2 There also has been a slight decline in the rate of congenital syphilis, currently reported as 8.7 cases of congenital syphilis per 100,000 live births.2 During pregnancy, untreated early syphilis results in perinatal death in as many as 40% of cases and may lead to fetal infection in more than 70% of cases because the spirochete that causes syphilis can cross the placental membrane to infect the fetus.10
Race, ethnicity, and gender alone do not alter STI risk but rather act as risk markers that correlate with other more fundamental determinants of health status, such as poverty, access to quality care, and health-seeking behavior. Higher infection rates have been associated with urban areas, with the exchange of sex for drugs, and with prison populations.2,11 A growing concern is the incidence of co-infection with HIV among MSM.2 Since a syphilitic chancre is an open wound, it greatly increases the risk of acquiring other STIs such as HIV.
Pathophysiology
Treponema pallidum, the cause of syphilis, is an anaerobic bacterium that cannot be cultured in vitro. The treponema (individual microorganism) resembles a corkscrew, with regular, tight spirals and a rotary motion; it can infect any organ or tissue. Because the bacterium is present in exudate from moist mucosal or cutaneous lesions, the spirochete is usually transmitted to others during the first few years of infection. Transmission generally occurs through minor abrasions during sexual intercourse but can occur extragenitally as well. Approximately 30% to 50% of partners who have sexual intercourse with an individual in early stage syphilis develop the disease.2 Although condoms can decrease the likelihood of infection, syphilis can be easily transmitted by contact with areas not covered by the condom.
Syphilis becomes a systemic disease shortly after infection and can be transmitted from a pregnant woman to her fetus as early as the ninth week of gestation. The risk of transmission to the fetus gradually declines with each subsequent pregnancy; therefore, a mother who has given birth to several children with severe congenital syphilis may later have a healthy child.12 The course of untreated syphilis consists of four stages: primary, secondary, latent, and tertiary (Box 26-2).
Primary syphilis begins at the site of bacterial invasion (Figure 26-5), where T. pallidum multiplies in the epithelium and produces a granulomatous tissue reaction called a chancre. Some microorganisms drain with lymph into adjacent lymph nodes. Within the nodes and at the site of the chancre, the cell-mediated and humoral immune responses are stimulated.
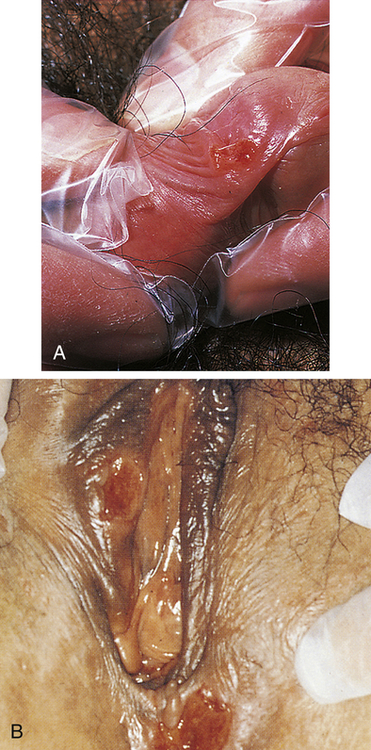
A, Penile chancre. B, Vulval chancres; the labia and perineum show induration and edema of chancres. (A from McMillan A, Scott GR: Sexually transmitted infections, ed 2, London, 2000, Churchill Livingstone; B courtesy Barbara Romanowski, MD, from Morse SA et al, editors: Atlas of sexually transmitted diseases and AIDS, ed 4, London, 2013, Saunders.)
Latent syphilis may be subdivided into early and late stages; however, no specific criteria delineate one from the other.11 Medical history and serologic studies show that syphilis is present, but the individual has no clinical manifestations. Transmission is possible during the late and early latent stages.
Tertiary syphilis is the most severe stage, involving significant morbidity and mortality. The pathogenesis of syphilitic manifestations at this stage remains unclear. The destructive skin, bone, and soft tissue lesions (called gummas) of tertiary syphilis probably are caused by a severe hypersensitivity reaction to the microorganism. Within the cardiovascular system, infection with T. pallidum may cause aneurysms, heart valve insufficiencies, and heart failure. Within the central nervous system (CNS), the presence of T. pallidum in cerebrospinal fluid may cause the manifestations of neurosyphilis, which can occur within any stage of syphilis infection.11
Congenital syphilis is estimated to cause a half million fetal and neonatal deaths every year worldwide.12 The risk of acquiring congenital syphilis (CS) is estimated at up to 80% in primary syphilis and declines with advancing stages of the disease. Intrauterine infection causes fetal or perinatal death in 40% of affected infants and many live-born infants have permanent lifelong morbidity.12
Clinical Manifestations
Primary Stage
In adults, the incubation period of syphilis ranges from 12 days to 12 weeks after exposure and averages 3 weeks. A sore, or hard chancre, develops at the site of treponemal entry. Typically the chancre is an eroded, painless, firm, and indurated (hard) ulcer that may be a few millimeters to 2 cm in diameter. Firm, enlarged, and nontender regional lymph nodes accompany chancres. Figure 26-5 shows typical chancres of the penis and vulva. Syphilitic chancres are not always typical, however, and syphilis should be considered in the presence of any open lesion. Secondary infection can cause chancres to become necrotic and painful, and lesions on the fingers may be dry, scaly, and papular or moist and vegetative. If left untreated, the chancre of primary syphilis heals in 2 to 8 weeks and then spontaneously disappears, usually without leaving a scar.
Secondary Stage
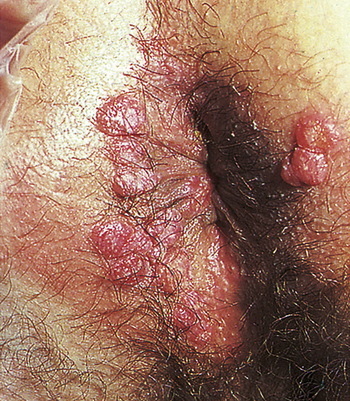
Clinical manifestations of secondary syphilis usually develop 6 weeks after the first appearance of the chancre but may overlap with those of the primary stage. Typically this stage presents with variable systemic symptoms, including low-grade fever, malaise, sore throat, hoarseness, anorexia, generalized adenopathy, headache, joint pain, and skin or mucous membrane lesions or rashes. Cutaneous (skin) rashes are generally papulosquamous (raised and scaly), but any variation or combination of macular (flat), papular (raised), and pustular (pus-filled) lesions may be seen. Often lesions are widespread and bilateral and appear on the palms and soles (Figure 26-6). Some lesions become hypertrophied, flat, moist, and wartlike or vegetative (e.g., cauliflower-like). These lesions, called condylomata lata, are highly contagious and develop on the perineum, vulva, and groin of women (Figure 26-7) and around the inner thigh and the anal area in men and women. Besides skin sores, oral mucous membrane lesions (known as mucous patches), lymphadenopathy, pruritus, and alopecia are common. Some individuals develop anemia, leukocytosis, increased sedimentation rate, hepatitis, transitory proteinuria, arthritis, electrocardiographic abnormalities, and central nervous system (CNS) symptoms. Regardless of whether treatment is given, the cutaneous lesions generally heal in 2 to 10 weeks, but relapses may occur for several years.11
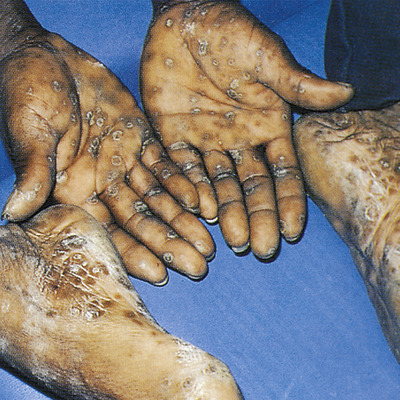
Secondary syphilis to the palms and plantar surfaces. (From Morse S et al, editors: Atlas of sexually transmitted diseases and AIDS, ed 4, London, 2013, Saunders.)
Congenital Syphilis
Congenital syphilis (CS) is characterized by vasculitis, necrosis, fibrosis, and distribution of T. pallidum throughout the tissues; it is divided into early and late stages. Signs and symptoms of early CS manifest in the first 2 years of life, and clinical manifestations of the late stage often occur near puberty. Affected newborns often are premature and have growth abnormalities, hepatosplenomegaly, bone marrow depression, destructive bone and skin lesions (see Figure 26-6), retinal inflammation, glaucoma, blood dyscrasia, nephrotic syndrome, and varying degrees of CNS involvement.9 Late manifestations of classic congenital syphilis are rare and are similar to those of tertiary syphilis in the adult.
Evaluation and Treatment
Because T. pallidum cannot be cultured in vitro, early definitive diagnosis of primary or secondary syphilis depends on darkfield microscopy of a specimen taken from an infected site. If the initial result is negative, the darkfield examination is repeated on 2 successive days. In addition to darkfield microscopy, serologic testing is useful in diagnosis. An algorithmic approach to the diagnosis of genital ulcers is presented in Figure 26-8.
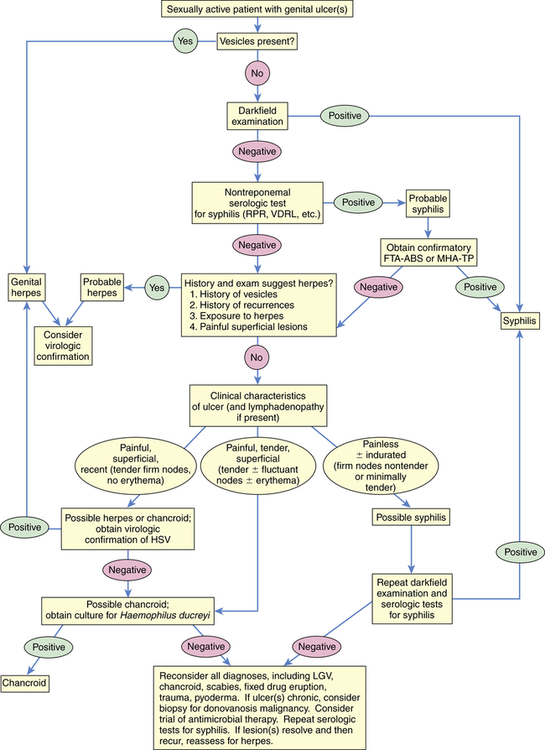
Algorithm outlining an approach to the diagnosis of an individual who presents with a genital ulceration. FTA-ABS, Fluorescent treponemal antibody absorption; HSV, herpes simplex virus; LGV, lymphogranuloma venereum; MHA-TP, microhemagglutination assay–Treponema pallidum; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory. (Redrawn from Pitot P, Plummer FA. In Holmes KK et al, editors: Sexually transmitted diseases, ed 2, New York, 1990, McGraw-Hill.)
Two categories of serologic testing exist: nontreponemal antigen tests and treponemal antibody tests.9 Nontreponemal antigen tests, which demonstrate the presence of reagin (a group of antibodies present in syphilis) in serum, provide indirect evidence of infection. Examples of nontreponemal analysis are the Venereal Disease Research Laboratory (VDRL) antigen and the rapid plasma reagin (RPR) tests (Box 26-3). These tests yield a positive result (presence of reagin) in more than 50% of individuals with primary syphilis and in 100% of individuals in the secondary phase of disease. Because the VDRL and RPR tests have high rates of false positives, a treponemal test is performed if the screening test is positive. Treponemal tests are serologic-specific tests that are used to assess antibody response to T. pallidum and include the fluorescent treponemal antibody absorption (FTA-ABS) test and the passive particle agglutination (TP-PA) assay.9
Preferred treatment for all stages of syphilis is parenteral injection of benzathine penicillin G. If the individual has manifested signs of the disease for less than 1 year, a single intramuscular dose is appropriate. If signs have been present for more than 1 year, the treatment is three weekly injections. This therapy is also appropriate for pregnant women.9 There is no evidence to date that T. pallidum has developed resistance to penicillin. In fact, it is highly sensitive; but because of the slow replication time, serum levels must be maintained for 7 to 14 days. Nonpregnant women who are allergic to penicillin may receive oral doxycycline, 100 mg twice daily for 14 days. Pregnant women with a penicillin allergy should be desensitized and then treated with benzathine penicillin G as recommended by the CDC because tetracycline will cause permanent, lifelong discoloration of the forming teeth of the fetus.9 Repeated assessment of VDRL or RPR titers is used to determine effectiveness of treatment. Titers should decrease fourfold if treatment has been successful. Sexual partners also are examined and treated, and the use of condoms is recommended until effective treatment is verified.
Newborns of mothers with documented syphilis need careful evaluation after birth. Definitive diagnosis of congenital syphilis is made by microscopic identification of T. pallidum in material from skin lesions, nasal discharge, or placental tissue. Nontreponemal tests are performed on the infant’s blood and compared with maternal levels.9 In all cases of maternal syphilis, the goal is to treat the mother to prevent congenital syphilis through early maternal treatment with penicillin. If the infant requires treatment, penicillin is the drug of choice. Such infants are then given serologic tests for syphilis every 2 to 3 months until the test becomes nonreactive or the titer has decreased fourfold.9
Chancroid
Chancroid, or soft chancre, is an acute infectious disease that was first differentiated from syphilis in 1852. It is caused by Haemophilus ducreyi, a gram-negative bacillus. The incidence of chancroid is decreasing worldwide. The incidence in the United States is low; 24 cases were reported to the CDC in 2010.2 Sporadic outbreaks occur throughout the world and tend to be associated with conditions such as poverty, prostitution, and illicit drug use, in which individuals continue to engage in intercourse in spite of the presence of a painful lesion.2
Pathophysiology
H. ducreyi is a gram-negative bacillus with rounded ends. Under a microscope it is commonly observed in small chains or clusters along mucous strands. Transmission can occur through sexual contact and autoinoculation. There is no evidence of maternal-fetal transfer before or after delivery. Chancroid lesions usually are found throughout the genital region, most commonly on the internal surface of the foreskin or at its point of attachment to the penis (the frenulum) in men and on the labia, clitoris, or fourchette in women. Initially the papule enlarges; it then erodes into a soft, circumscribed ulcer containing a superficial exudate. Beneath the ulcer is a lesion characterized by edema, endothelial proliferation, and a base of granulation tissue that is full of lymphocytes and plasma cells. Adjacent lymph nodes are acutely inflamed and full of polymorphonuclear leukocytes and necrotic cells.13
Clinical Manifestations
Chancroid has an incubation period of 3 to 10 days.13 Generally, women are asymptomatic, but depending on the site of infection, can present with less obvious symptoms (dysuria, dyspareunia, vaginal discharge, pain on defecation, or rectal bleeding). Constitutional symptoms are unusual. At the site of inoculation, an initial vesicopustule lesion forms and erodes into a soft ulcer with a necrotic base, surrounding erythema, and a ragged, serpiginous (spreading) border (Figure 26-9). Unilateral, painful, local lymphadenopathy presents in about half of infected individuals—primarily men. (Women tend to have multiple lesions.) Inguinal buboes (unilocular abscess of the inguinal lymph nodes) develop 7 to 10 days after the initial chancre and fill with exudate. In 25% to 60% of cases, the buboes spontaneously rupture out onto the skin, spreading the infection through autoinoculation.
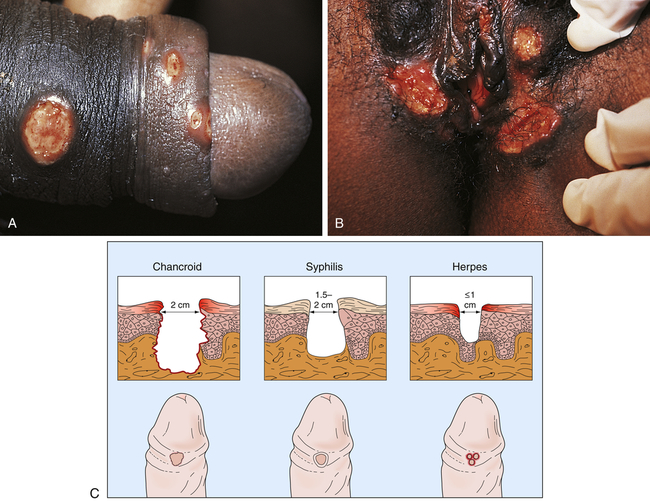
A, Ulcers on the penile shaft. B, Multiple vulvar lesions. C, Differences in clinical appearance among chancroid, syphilis, and genital herpes. (From Morse SA et al, editors: Atlas of sexually transmitted diseases and AIDS, ed 4, London, 2013, Saunders.)
Evaluation and Treatment
Chancroid is easily confused with other types of genital ulcers, particularly those of syphilis, genital herpes, and granuloma inguinale (see Figure 26-9). Unlike the syphilitic ulcer, chancroidal ulcer is painful, tender, and nonindurated. Microscopic analysis of a Gram-stained smear from the chancroid helps to identify the microorganism. Definitive diagnosis depends on recovery of H. ducreyi from cultured specimens; however, the culture medium is not widely available.9 Because 10% of infected individuals are coinfected with syphilis or herpes simplex virus (HSV), testing includes serologic examination for syphilis and viral culture for HSV. In addition, HIV testing is recommended: chancroid is a cofactor for transmission of HIV.
Resistance to recommended antibiotics has emerged in isolated instances worldwide. Recent treatment recommendations include a single intramuscular injection of ceftriaxone (250 mg) or a single dose of oral azithromycin (1 g). Effective oral multiple-dose regimens include ciprofloxacin, 500 mg orally twice daily for 3 days; or erythromycin, 500 mg three times daily for 7 days. Persons infected with HIV have higher rates of treatment failure with single-dose therapy and may require a longer treatment regimen. As a palliative measure, buboes can be aspirated through adjacent, healthy skin. In approximately 5% of cases, relapses at the site of original ulcer have occurred.9 Simultaneous treatment of sexual partners and use of condoms are recommended to prevent reinfection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree