Drug-induced psoriasisiform eruption
Idiopathic psoriasis
Temporal association of drug initiation and onset of eruption
No association with initiation of drug(s)
Cessation of drug prevents disease progression
No recovery after cessation of drug(s)
Older age of onset
Young age
Possibly resistant to phototherapy
–
Recurrence of skin lesions on re-challenge with offending drug
–
Drugs Implicated and Pathogenesis
There are three categories of drugs implicated in psoriasiform drug eruptions: Strong association with psoriasis; Considerable but insufficient data to support association with psoriasis; and Occasionally reported to be associated with aggravation or induction of psoriasis (Table 14.2). They are discussed here in alphabetical order.
Table 14.2
Drugs and their relationship to psoriasis
Category of drugs/drug classes | Relationship to psoriasis |
|---|---|
Absolute causal relationship to psoriasis | β-Blockers, lithium, synthetic antimalarials, nonsteroidal anti-inflammatory drugs, tetracyclines |
Substantial but insufficient data supporting relationship with psoriasis | ACE inhibitors, interferons, terbinafine |
Drugs occasionally reported to be associated with induction or aggravation of psoriasis | Clonidine, digoxin, amiodarone, quinidine, dihydropyridine calcium antagonists, carbamazepine, valproic acid (sodium valproate), fluoxetine, acetazolamide, sulfonamides, penicillin, amoxicillin, ampicillin, morphine, procaine, cimetidine, ranitidine, gold, mercury, oxandrolone, progesterone, gemfibrozil, potassium iodide, granulocyte-macrophage colony-stimulating factors |
Abatacept
A fully human CTLA4-IgG, abatacept is used in the treatment of refractory rheumatoid arthritis. On multiple accounts, it has been reported to cause psoriasiform dermatitis, confirmed on withdrawal and rechallenge. Latent periods ranging from 2 months to 14 months have been reported. Reported morphologies include a widespread psoriasiform eruption, scalp involvement, palmoplantar involvement, and nail dystrophy with onycholysis and yellowing of the nail plate.
Angiotensin-Converting Enzyme Inhibitors (ACEI)
Angiotensin-converting enzyme (ACE) is a zinc-metallopeptidase that converts angiotensin I to the potent vasoconstrictor angiotensin II. It is expressed in a variety of tissues including vascular endothelium, skin, and cells of the immune system. ACE inhibitors (ACEI) are prescribed for the treatment of hypertension and function by blocking the conversion of angiotensin I to angiotensin II by competing with kininase II. Frequently prescribed ACEI include captopril, enalapril, lisinopril, perindopril and ramipril.
ACEI can trigger both induction and exacerbation of psoriasis with an intermediate latency period between 4 and 12 weeks. Case reports in the literature describe various clinical manifestations including guttate, plaque-type, palmoplantar, pustular, and erythrodermic forms of psoriasis associated with the use of ACEI. Biopsies show typical histopathology of psoriasiform dermatitis with hyperkeratosis, parakeratosis, epidermal acanthosis, and variable neutrophilic exocytosis. Resistance to standard treatment modalities is typical for ACEI-induced psoriasis. However, discontinuation of the drug leads to improvement in the psoriatic lesions usually within a few days.
Pathogenesis
Three mechanisms have been postulated in the development of ACEI-induced psoriasis: (1) an allergic, immune-mediated reaction, supported by captopril-induced psoriasis with a positive mast cell degranulation (MCD) test; (2) a pharmacologic dose-dependent response resulting from augmentation of kinin levels in the skin; and (3) increased levels of substance P. Studies suggest that patients with a family history of psoriasis and a specific ACE genotype exhibiting low ACE activity may be more susceptible to developing psoriasis with ACEI therapy.
Angiotensin Receptor Blockers (ARBs)
Also known as angiotensin II receptor antagonists and AT1-receptor antagonists or sartans, angiotensin receptor blockers (ARBs) are a group of pharmaceuticals that modulate the renin-angiotensin-aldosterone system. Their main uses are in the treatment of hypertension, diabetic nephropathy, and congestive heart failure. Examples of ARBs include candesartan, cilexetil, losartan, irbesartan, and valsartan. A relationship between ARB treatment and psoriasis has been suggested in several reports. This includes development of psoriasis de novo as well as exacerbation of disease in patients with a history of psoriasis. In the largest report of nine patients, psoriasis developed within six weeks and nine months of initiation of ARB therapy. Interruption of treatment led to regression of the cutaneous lesions over weeks to months. One patient experienced induction of psoriasis by two different ARB agents. The clinical attributes of the ARB-induced psoriasis differed from classic psoriasis, with lesions predominating in sun-exposed areas of hands and forearms, and with severe ungual involvement noted in some patients.
Pathogenesis
ARBs increase angiotensin II levels by inhibiting retroactive control of angiotensin II on renin secretion. Angiotensin II stimulates keratinocyte proliferation and this has been postulated as a mechanism for induction or exacerbation of psoriasis.
Antiepileptics
The aromatic anticonvulsants, phenytoin, carbamazepine, phenobarbital, and lamotrigine are the most common causes of cutaneous drug reactions in this class. These medications have been associated with Stevens-Johnson syndrome and toxic epidermal necrolysis. Other anticonvulsants such as valproate, levetiracetam, and topiramate have been less commonly associated with cutaneous drug eruptions. Both carbamazepine and sodium valproate have been associated with psoriasiform eruptions. Carbamazepine has been reported to cause a generalized psoriasiform eruption, as well as palmoplantar psoriasis with a non-scarring alopecia.
Pathogenesis
The mechanism of action for these eruptions possibly includes formation of a superantigen, delayed hypersensitivity, altered lymphocyte activation, and alterations in epidermal cAMP levels.
Antimalarials
Synthetic antimalarials have long been reported to flare existing psoriasis. Reports of antimalarial-induced psoriasiform dermatitis in patients with no prior history do not exist to date. It has been reported that as many as 18 % of psoriatics flare when treated with synthetic antimalarials. These medications are commonly encountered in treatment of arthritis, connective tissue diseases, and malaria prophylaxis. Reports of progression from plaque-type psoriasis to pustular flares and erythroderma exist. Chloroquine is a more frequent offender than hydroxychloroquine. The pharmacologically related anti-arrhythmic quinidine has also been reported to cause a psoriasiform eruption. The time between initiation of an antimalarial therapy and onset of psoriasis flare ranges from four to twelve weeks.
Pathogenesis
The chemical structure of the antimalarial drugs is very similar to that of dansyl-putrescine, a strong transglutaminase inhibitor. The mechanism of anti-malarial-induced psoriatic flares is thought to be via inhibition of cutaneous transglutaminase enzymes by the antimalarials. This leads to cellular proliferation, thus flaring psoriasis.
β-Blockers
β-adrenergic receptors are present in many cells of the human body, including the immune system and the epithelial skin cells. Two classes are recognized, non-selective and selective, referring to their selectivity of β1- and β2-adrenergic receptors. β-blockers have been reported to cause, aggravate, or induce psoriasiform skin eruptions and/or pre-existing psoriasis (Fig. 14.1), typically 1 month to 2 years after drug initiation. This variation in onset is likely contributed to racial and genetic traits. Many of these agents demonstrate significant cross-reactivity (notably propranolol, oxprenolol, and atenolol).
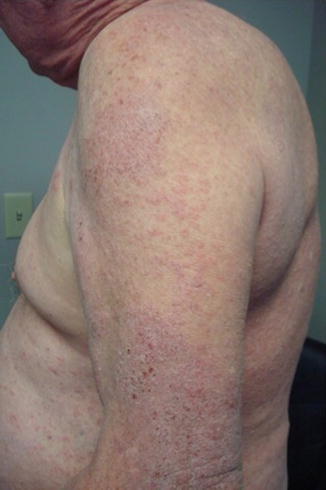

Fig. 14.1
Psoriasiform dermatitis due to propanolol that slowly improved after discontinuation of the drug. Notice thick adherent scale with suggestion of silvery-white scale near the elbow
Researchers are unable to determine if β–blocker-induced cutaneous reactions are true psoriasis, as the psoriasiform eruption occurs more commonly in those with a negative family history of psoriasis. Drug-aggravated psoriasis improves with cessation of the drug, but may not clear completely, suggesting there are other factors at play.
Propranolol (Inderal), the first non-selective β-blocker, has been reported to cause hyperkeratosis and parakeratosis, consistent with epidermal psoriasiform alteration in guinea pigs after topical application. This suggested that propranolol works immediately on cutaneous β2 receptors. Several cases of oxyprenolol (Trasicor)-induced and exacerbated psoriasiform eruptions have been reported in the literature. Cumberbatch reported that 2–3 weeks after oxyprenolol initiation, an “intense, firey, annular erythema and underlying oedema” developed, consistent with exacerbation of underlying psoriasis, present for 10 years prior. A similar case was reported shortly after, both switching from oxypranolol to propranolol with subsequent disappearance of symptoms. Skin reactions to practolol, a selective β-blocker, are a known side effect, specifically psoriasiform eruptions and exacerbation of pre-existing psoriasis. Three patients have been reported who developed psoriasiform skin eruptions following oral practolol therapy. Many other reports worldwide of psoriasiform drug eruptions following β-blocker administration have been noted: atenolol (Tenormin), cetamolol (Betacor), metoprolol (Lopressor, Seloken), and nadolol (Corgard). Topical application of timolol (Timoptol), in the treatment of chronic open angle glaucoma, was reported to induce psoriasis and to transform psoriasis vulgaris into psoriatic erythroderma, probably through the passage of the β-blocker into the systemic circulation via the conjunctiva, nasal mucosa, or uveal circulation. Tsankov et al. reported the conversion of plaque-type psoriasis in a 50-year-old woman to pustular psoriasis after initiation of pindolol (Visken).
Pathogenesis
The exact mechanism of action of β-blockers on psoriasis still remains unknown. Epidermal cell division is slowed by β-adrenergic stimulation via an increase in cyclic-AMP (cAMP). Therefore, the use of β-blockers presumably interferes with cAMP production via epidermal β-receptors. This, in turn, decreases cAMP concentration in the epidermis, increases epidermal proliferation, and increases epidermal glycogen levels. This is the picture of a psoriatic dermatitis. Like lithium, β-blockers have been shown to increase phosphorylation in psoriatic T-cells, which may affect intracellular calcium levels as well. Other hypotheses include immunologic mechanisms and delayed-type hypersensitivity.
Botulinum Toxin A (Botox A)
Botox A is a neurotoxin produced by the bacterium Clostridium botulinum. It is used therapeutically for the treatment of upper motor neuron syndrome, hyperhidrosis, cervical dystonia, chronic migraine, blepharospasm, strabismus, and glabellar furrows. A case of a psoriasiform eruption temporally related to the injection of botulinum toxin A into the medial rectus muscle to treat an ocular motility disorder was reported. Conversely, other studies have shown efficacy of botulinum toxin A injection for the treatment of inverse psoriasis.
Calcium Channel Blockers (CCBs)
Also referred to as calcium channel antagonists or calcium antagonists, calcium channel blockers (CCBs) are medications that disrupt the movement of calcium through calcium channels. CCBs are used to treat hypertension, angina, and arrhythmia. Examples of CCBs include diltiazem, nifedipine, nicardipine, and verapamil. In an early report from Japan, there were a notable number of psoriasiform eruptions associated in patients treated with CCBs, which resolved or were easily controlled after discontinuation of the drug. The possible role of CCBs as precipitating or exacerbating factors in patients with psoriasis was also supported in a case control study of 150 patients. The median latent period between the beginning of intake of CCBs and psoriasiform eruption is 28 months.
Pathogenesis
It is postulated that CCBs can trigger psoriasis by interfering with calcium influx, which is necessary for normal keratinocyte proliferation and differentiation.
Chlorthalidone
As a diuretic, chlorthalidone is used to treat hypertension and edema. It acts similarly to the thiazide diuretics but does not contain the benzothiadiazine molecular structure. There is a report of two patients who experienced a psoriasiform eruption while taking chlorthalidone.
Cimetidine
Cimetidine is a histamine H2-receptor antagonist that is largely used to treat heartburn and peptic ulcers. There is a rare report of exacerbation of psoriasis during treatment with cimetidine. Other literature supports use of cimetidine in the treatment of psoriasis, especially in HIV-positive individuals.
Digoxin
As a purified cardiac glycoside, digoxin is used for the treatment of many cardiac conditions, including atrial fibrillation and flutter as well as heart failure. A psoriasiform eruption induced by digoxin was reported and confirmed upon re-exposure. Given a positive migration inhibition factor in this case, it is theorized that the patient had a hypersensitivity reaction to digoxin that caused Koebner phenomenon.
Erlotinib
Erlotinib is a reversible tyrosine kinase inhibitor that acts on the epidermal growth factor receptor (EGFR). It is used to treat non-small cell lung cancer, pancreatic cancer, and several other forms of cancer. Although there are reports of improvement of psoriasis in patients treated with erlotinib for cancer, there is a report of a psoriasiform eruption triggered by erlotinib. It occurred simultaneously with the more common acneiform form erlotinib-induced rash that is thought to portend a good prognosis.
Fluoxetine
Fluoxetine is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. Fluoxetine-induced psoriasis has been reported in several patients, with or without a personal history of psoriasis.
Pathogenesis
SSRI drugs modulate serotonergic function, a factor that may contribute to the pathophysiology of psoriasis. A serotonergic influence in the pathogenesis of psoriasis may be possible together with a pharmacogenetic difference in the drug metabolism of these patients.
Gemfibrozil
Gemfibrozil is an oral medication used to lower lipids levels. It reduces triglycerides and increases cholesterol carried in high density lipoprotein (HDL) in the blood. It may cause exacerbation of psoriasis, but the mechanism is unknown.
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
Colony-stimulating factors (CSFs) are commonly used for the treatment of pancytopenia following chemotherapy. Granulocyte-macrophage colony-stimulating factor (GM-CSF) has effects on neutrophil function and chemotaxis. There are several reports implicating CSF in the induction or exacerbation of psoriasis.
Pathogenesis
Due to its role in enhancing the function of neutrophils and macrophages, it is suggested that therapeutically administered GM-CSF may amplify and modulate the inflammatory reactions and activated T-cells in psoriasis.
Imiquimod
Imiquimod is an immune response modifier that is approved for treatment of superficial basal cell carcinomas, actinic keratoses, and genital warts. There are several published reports describing cases of psoriasis triggered by imiquimod cream. Most reports are of exacerbation of pre-existing psoriasis with a rare case of induction of psoriasis de novo.
Pathogenesis
Imiquimod activates immune cells through the toll-like receptor 7 (TLR7), commonly involved in pathogen recognition. Cells activated by imiquimod via TLR-7 secrete cytokines (primarily interferon-α (INF-α), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)). These cytokines, particularly INF-α, are capable of inducing psoriasis.
Interferon (IFN)
The immune effects of interferons have been exploited to treat various diseases. Recombinant IFN-α is used for systemic therapy of hematologic malignancies, malignant melanoma, hepatitis B and C, and carcinoid syndrome. There are a number of reports of induction or exacerbation of psoriasis during treatment with IFN-α. The lesions generally resolved within 2 weeks to 6 months after cessation of IFN-α. Psoriatic lesions have also been induced at injection sites of INF-γ.
Pathogenesis
Interferons have various actions that could be related to the pathogenesis of psoriasis, including activation of macrophages, intensification of phagocytosis, and induction of interleukin-1 production and release by keratinocytes.
Lithium
Lithium, used as lithium carbonate, lithium citrate, or lithium benzoate, has a variety of known cutaneous adverse effects, with a documented prevalence of 3.4–45 %. A relationship between psoriasis and lithium was first suspected and then described in 1972 and 1976, respectively. Lithium salts are found in mineral water, and although the mechanism of action remains unclear, is extensively used in the treatment of many psychiatric disorders and as a uricolytic. The therapeutic range for lithium is 0.6–0.12 mEq/L, and plasma levels exceeding 1.5 mEq/L can lead to severe systemic reactions involving the skin, central nervous system, kidneys, thyroid, and gastrointestinal system.
Lithium-associated psoriasis is the most common cutaneous reaction that may or may not be dosage-related. Lithium-associated psoriasis includes the following: exacerbation of established psoriasis, new onset psoriasis, pustular psoriasis, psoriatic arthropathy, psoriatic erythroderma, and nail alterations. Of these, exacerbation of pre-existing psoriasis is the most common. In some, treatment-resistant scalp psoriasis is the first manifestation. A rare case of fingernail psoriasis has been reported as the sole manifestation. Many patients report feelings of stress during the recovery and treatment period, which alone can exacerbate psoriasis. Interestingly, patients with psoriasis who had not been treated with lithium or lithium-containing compounds have been reported to have increased lithium plasma concentrations in their blood. Of note, when lithium is used to treat urological issues, cutaneous side effects are not reported, probably owing to the short duration of therapy.
Lithium–triggered psoriasis: The latency period between starting lithium and the exacerbation of pre-existing psoriasis is relatively long, averaging 20 weeks, and may occur after mental status has improved. Generalized pustular psoriasis has been reported after lithium therapy in a patient with pre-existing psoriasis vulgaris.
Lithium–induced psoriasis: The latency period is often longer, averaging 48 weeks, and may also occur after mental status has improved. The true relationship between lithium and de novo psoriasis is questionable, although there has been a reported association with de novo palmoplantar pustular psoriasis. Some have reported that onset of new disease is no higher than in a control group not taking lithium. Lithium-induced psoriasis is often resistant to standard treatments, and some may require dose modification or discontinuation of lithium.
Pathogenesis
The precise mechanism(s) by which lithium compounds exert their effects are still being elucidated. It is clear, however, that lithium directly inhibits cell differentiation and potentiates an increase in the concentration of polymorphonuclear leukocytes in psoriatic lesions, presumably via a deficiency of cyclic-AMP (cAMP). Initial hypotheses regarding lithium’s mechanism of action suggested that the induction/aggravation of psoriasis was secondary to a reduction in intraepidermal cAMP levels via a decrease in adenyl cyclase. Chronic lithium therapy, however, has been shown to increase cAMP concentration in the epidermis, presumably via a compensatory mechanism, and it is in chronic lithium therapy that the psoriasis-associated reactions most often occur. More recent studies have not revealed reduced levels of cAMP in psoriatic T-cells. A more recent and promising hypothesis involves recycling of inositol in the epidermis, which is essential for intracellular calcium release. Lithium inhibits the monophosphatase enzyme, which is required for inositol recycling. Thus, calcium release is inhibited, calcium levels drop, and increased proliferation and lack of keratinocyte differentiation result, ultimately triggering psoriasis. Oral inositol supplementation reverses these side effects.
There is contradicting literature on cytokine production in lithium-associated psoriasis. Some researchers report IL-2, IL-6, IL-8, tumor necrosis factor-α, and interferon-γ are elevated in lithium-associated psoriasis, and others say they are closer to normal. These cytokines presumably interfere with the communication of psoriatic keratinocytes. Lithium has been shown to increase the release of inflammatory mediators, via lithium-stimulated neutrophils. Another finding is increased tyrosine phosphorylation in psoriatic T-cells compared to normal cells. This might be relevant to the development of psoriasis.
Interestingly, the antigens most commonly associated with psoriasis have been documented to be sparsely represented in lithium-induced psoriasis, specifically HLA B13, B17, and/or Bw37. Further epidemiologic studies are necessary to determine details regarding the causal relationship between lithium and psoriasis.
Metformin
Metformin is an oral antihyperglycemic agent in the biguanide class that is used in the management of non-insulin-dependent diabetes mellitus (type 2). Metformin works by suppressing hepatic glucose production, decreasing intestinal absorption of glucose, and improving insulin sensitivity by increasing peripheral glucose uptake and utilization. It lowers both basal and postprandial plasma glucose, and reduces insulin resistance, in type 2 diabetes mellitus. A psoriasiform eruption was described in patient 1 week after initiating therapy with metformin hydrochloride at 850 mg daily. The eruption resolved with cessation of the medication and recurred with rechallenge.
Mitomycin-C
Mitomycin-C is a chemotherapeutic agent rarely associated with psoriasiform dermatitis. A report of new plaque-type psoriasis in a patient undergoing chemotherapy for breast carcinoma exists. Intravesicular mitomycin-C in urothelial carcinoma has been reported to cause widespread psoriasiform dermatitis.
NSAIDs
Exacerbation of psoriasis and induction of generalized pustular psoriasis have been associated with nonsteroidal anti-inflammatory drugs (NSAIDs). A case of indomethacin-induced psoriasis has been reported in a 51-year-old woman. Psoriatic flares have been seen with local and perioral indomethacin treatment. A clinical exacerbation of psoriasis occurred following systemic and local therapy in 14 of 20 patients with pre-existing psoriasis subsequently treated with indomethacin. Generalized pustular psoriasis has been reported in a patient treated with phenylbutazone.
Although there is the risk of worsening pre-existing psoriasis or rarely inducing de novo psoriasis, NSAIDs are still implicated in treatment of psoriasis for several reasons. Corticosteroids successfully treat/manage psoriasis, as they decrease levels of arachidonic acid (AA), which is the building block for prostaglandin synthesis. Additionally, in patients with psoriatic arthritis, NSAIDs have been shown to restrict leukocyte chemotaxis into the epidermis. The lipoxygenase inhibiting-NSAIDs are also promising treatments for psoriasis. When the COX pathway is inhibited, the bioavailability of AA is increased, which subsequently activates the lipoxygenase pathway. These metabolites are integral in psoriatic inflammation.
Pathogenesis
NSAIDs influence the metabolism of arachidonic acid by inhibiting either the cyclo-oxygenase or the lipoxygenase pathway, thus preventing the formation of inflammatory mediators such as prostaglandins, prostacyclins, and leukotrienes. Elevated levels of these inflammatory mediators in psoriatic skin have been found or indirectly confirmed in a several studies.
Potassium Iodide
Potassium iodide has therapeutic applications, either in tablet form or saturated solution, for treatment of hyperthyroidism, radiation poisoning, sporotrichosis, and erythema nodosum Potassium iodide, 500 mg administered orally, was reported to induce generalized pustular psoriasis in two patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


