Antibiotics
Antiarrythmics-antihypertensives
Vaccines
Actinomycin
Amoxicillin
Ampicillin
Cephalexin
Ciprofloxacin
Chloroquine
Dactinomycin
Levofloxacin
Penicillin
Rifampin
Ca± channel blockers
Amlodipine
Nifedipine
ACE inhibitors
Captopril
Enalapril
Lisinopril
β-blockers
Nadolol
Practolol
Angiotensin II antagonists
Losartan
Influenza
Swine flu
Tetanus toxoid
HZV
Hexavalent combined vaccines
NSAID
Salicylates
Other
Azapropazone
Diclofenac (topical)
Ibuprofen
Mefenamic acid
Phenacetin
Aspirin
Sulphasalazine
Salicylazosulfapyridine
Arsenic
Clonidine
Erlotinib
Fluoxetine
Flupenthixol
Gabapentin
Galantamine hydrobromide
Gold thiosulfate
Interleukin-2
Levetiracetam
Methyldopa
Terbinafine
Omeprazole
Psoralens with UVA
Placental extracts
Potassium iodide
Risperidone
Sulfonamide
Diuretics
Antidiabetics
Furosemide
Spironolactone
Sitagliptin
Tolbutamide
Vildagliptin
Anti TNF-α
Antirheumatics
Adalimumab
Efalizumab
Etanercept
D-penicillamine
Tiobutarit
Clinical Presentation
Like idiopathic bullous pemphigoid, the clinical picture of DIBP is heterogeneous. The varying presentations can make diagnosis difficult. Morphology ranges from classic tense bullae arising from erythematous urticarial basis that inconsistently involves the oral mucosa, to few bullous lesions with no erythematous bases, to target lesions, to scarring plaques and nodules with bullae or excoriations (papular or nodular pemphigoid). It can even mimic other diseases such as bullous erythema multiforme and pemphigus. DIBP is typically present in a younger population than idiopathic bullous pemphigoid. Other common clinical features of DIBP include positive Nikolsky sign, appearance of lesions on normal-appearing skin, target lesions on the palms and soles, involvement of the lower legs, and mucosal involvement. Nikolsky’s sign involves the application of pressure to a blister, resulting in exfoliation of the superficial layers of the skin. Table 18.2 presents a summary of suggested differences between DIBP and classic bullous pemphigoid.
Table 18.2
Suggested differences between drug-induced bullous pemphigoid and classic bullous pemphigoid
Drug-induced bullous pemphigoid | Classic bullous pemphigoid | |
|---|---|---|
History: | Receives multiple therapeutic regimens | May or may not receive multiple therapeutic regimens |
Patient was treated with a new drug recently | Patient did not receive a new drug recently | |
Clinical picture: | Younger age of onset | Older age of onset |
Possible positive Nikolsky sign | Nikolsky sign is negative | |
Appearance of lesions on normally appearing skin | Frequent appearance of lesions on an erythematous and urticarial base | |
Mucosal involvement may be present | Mucosal involvement is rare | |
Histology findings: | Marked eosinophilic infiltrate | Eosinophilic infiltrate present |
Intraepidermal vesicles may be present | Intraepidermal vesicles are not present | |
Necrotic keratinocytes may be present | Necrotic keratinocytes are rarely seen | |
Thrombus formation may be seen | Thrombus formation is very rarely seen | |
Laboratory findings: | Marked eosinophilia in serum | Eosinophilia present |
Clinical course: | Responds rapidly to treatment with oral corticosteroids | May exhibit prolonged course despite oral corticosteroid treatment |
Improves after discontinuation of inciting drug | No inciting drug is identified | |
– | Rarely relapses | Relapses often |
Offending Drugs
Because many associated drugs are commonly prescribed yet only a few patients develop DIBP, drugs likely act as triggers in patients with underlying genetic susceptibility. A number of mechanisms have been proposed regarding the pathogenesis of DIBP. These include:
1.
Drugs may act as haptens, binding to proteins in the lamina lucida and changing their antigenic properties, resulting in anti-BMZ antibody production. This theory may explain the association of thiols with DIBP. This class of drugs is the most commonly associated with DIBP. Thiols contain or release sulfhydryl groups either in their initial form or as a metabolite. The thiol group may allow the molecule to combine with proteins in the lamina lucida and act as a hapten. Thiols associated with DIBP include penicillamine, captopril, penicillin and penicillin derivatives, furosemide, and some cephalosporins.
2.
Drugs may stimulate an autoimmune response by causing structural modifications in molecules that result in exposure of otherwise hidden epitopes, which are the part of an antigen molecule to which an antibody attaches itself. The immune system subsequently recognizes and targets these epitopes resulting in disease.
3.
Sulfur-containing drugs may cause a biochemical, dermo-epidermal split with no immune mediation.
4.
T-regulatory cells may be modulated such that they have decreased suppressor activity, resulting in increased production of autoantibodies against bullous pemphigoid antigens. Penicillamine may contribute to DIBP by this mechanism.
Vaccinations have rarely been reported to trigger bullous pemphigoid. In less than 20 cases in recent years, anti-influenza vaccine, tetanus toxoid booster, and tetracoq vaccine have been associated with the onset of bullous pemphigoid. It has been postulated that the underlying mechanism is due to inflammation in the skin at the vaccination site with disruption of the basement membrane architecture and subsequent generation of anti-basement membrane-specific antibodies. Alternatively, vaccinations may trigger an enhanced autoimmune response in patients with a predisposition to or subclinical bullous pemphigoid. In addition, cases of DIBP have also been linked to topical treatments, radiation therapy, and UV therapy.
Diagnosis
Diagnosis is confirmed by histopathological staining and immunofluorescence studies. Typical histopathological findings in DIBP include perivascular infiltrate of lymphocytes with eosinophils and neutrophils, subepidermal blisters, intraepidermal vesicles, foci of necrotic keratinocytes, and thrombi in dermal vessels. Blister cavities may contain numerous eosinophils, neutrophils, and fibrin. Of these histologic features, necrotic keratinocytes, intraepidermal vesicles, and thrombi are commonly seen in DIBP but are not associated with classic bullous pemphigoid. Notably, samples taken from normal skin do not show diagnostic findings.
Direct immunofluorescence and indirect immunofluorescence are consistent with idiopathic bullous pemphigoid. Direct immunofluorescence in bullous pemphigoid demonstrates IgG antibodies and C3 linear along the basement membrane zone in 90 % of cases. Circulating IgG antibodies are detected in 75 % of cases with indirect immunofluorescence.
Labs may show marked eosinophilia in serum and increased soluble IL-2 receptor. Other immunological markers such as macrophage migration inhibitory factor may be present. Mast cell degranulation toward the offending drug may also be present.
Treatment and Course
The course of the disease varies based on the type of DIBP. Two main types have been defined, with the most common being the pure drug eruption form of bullous pemphigoid. In this case, the disease is acute and self-limited; relapses are uncommon. It resolves with cessation of the culprit drug with or without steroid therapy (Figs. 18.1 and 18.2).
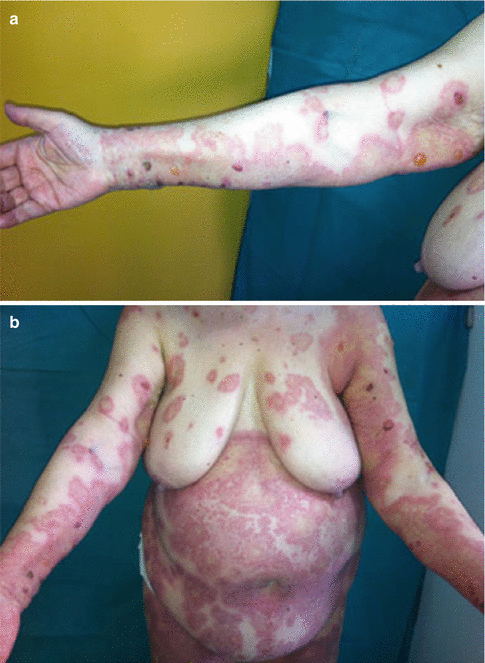
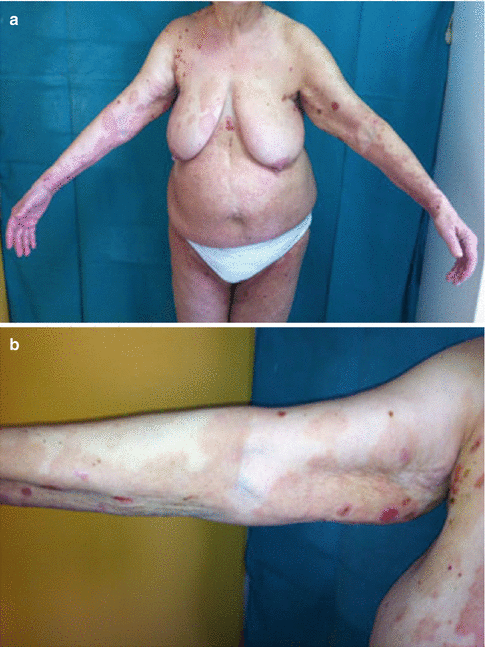

Fig. 18.1
(a) Tense bullae appearing on an erythematous base. The patient reported receiving antibiotic treatment (quinolone) 2 weeks prior to the appearance of the eruption. (b) Tense bullae on an erythematous base, accompanied by an erythema multiforme type eruption (Reproduced with permission from John Wiley and Sons, from Stavropoulos et al. (2014). Copyright © 2014)

Fig. 18.2
(a) Marked improvement of bullous pemphigoid lesions after discontinuation of the possible inciting drug and 3 weeks of treatment with oral prednisolone. (b) Cessation of the appearance of new lesions and marked improvement of inflammation was observed within 3 weeks from initiation of oral corticosteroid treatment and after discontinuation of the inciting drug. Two years later the patient is still in remission and is not receiving any treatment (Reproduced with permission from John Wiley and Sons, from Stavropoulos et al. (2014). Copyright © 2014)
The second type is better described as drug-triggered bullous pemphigoid. Drug administration seems to precipitate the onset of a chronic form of bullous pemphigoid that evolves to have all features of the classic form of the disease. Additional therapy aside from drug withdrawal is often necessary in these cases, which can be managed similarly to idiopathic bullous pemphigoid.
Drug-Induced Pemphigus
Pemphigus is a group of intraepidermal blistering disorders caused by a disruption of desmosomes leading to acantholysis, or a loss of keratinocyte adhesion. There are five main variants of pemphigus classified by the level of intraepidermal acantholysis: pemphigus vulgaris (PV), pemphigus foliaceus (PF), pemphigus erythematosus, drug-induced pemphigus, and paraneoplastic pemphigus. Drug-induced pemphigus is an increasingly common variant of pemphigus, accounting for approximately 10 % of all reported cases. In drug-induced pemphigus, PF is most often reported; however, PV can be seen as well. Most cases have been described in patients ranging from 30 to 90 years of age.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


