Fig 37.1
Acute retroviral syndrome includes a diffuse maculopapular eruption in 70–80 % patients (Image content provider: CDC Public Health Image Library, donated by Brian Hill, New Zealand.)
Despite the current (2015) availability of 26 antiretroviral drugs (with an additional 10 fixed-dose combination [FDC] ARV pills), and currently preferred/recommended HAART consisting of low complexity (most taken once daily) and low daily pill burden (1–3 pills per day) regimens comprised from less toxic, more tolerable medications than were available in the past, managing HIV infection/AIDS remains extremely challenging.
Only 25–28 % of HIV-infected patient in the United States are consistently meeting the treatment goal of fully suppressing HIV replication which allows for immune system recovery (or stability if treatment is begun prior to clinically significant immune system depression) and a near normal life expectancy. Reasons for this are multifaceted, but include: an estimated 18 % of patients who are HIV-infected are yet to be diagnosed and are unaware of their condition. Of those who are diagnosed, a significant number fail to engage in care and/or remain engaged in care due to denial, depression, shame, stigma, problems with access to care, and issues competing for the individual’s attention (especially mental illness and substance abuse). Not all who are engaged in care are prescribed and/or start a HAART regimen, and of those prescribed ARVs, not all are able to adequately tolerate and/or adhere well enough to their HAART regimen to achieve full viral suppression and continued regimen viability.
Maintaining perfect or near-perfect adherence day-in-day-out, year after year is clearly a major challenge for many of our patients, as it is for all patients. The factors that influence medication-taking behavior and the ways we can effectively encourage and foster the near-perfect adherence level necessary for success are beginning to be better understood.
For those who are able to successfully engage in care and navigate the above-mentioned barriers to receiving and being fully adherent to a potentially suppressive ARV regimen, three of the largest remaining roadblocks to successful long-term viral suppression include:
1.
Adverse drug reactions (ADRs) which are primarily immune-related (Fig. 37.2) and involve the skin (exclusively cutaneous or cutaneous combined with systemic symptoms and multi-organ system manifestations (Figs. 37.3 and 37.4).
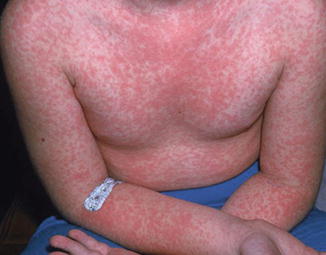
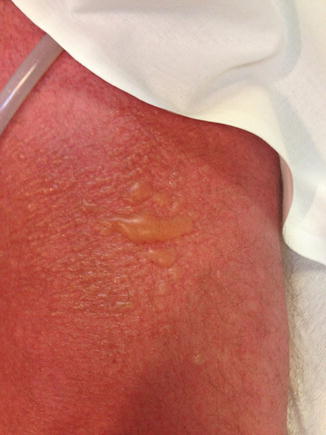
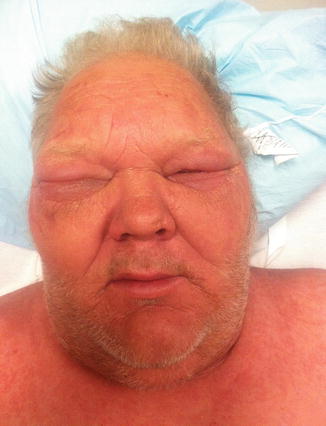

Fig 37.2
Typical morbilliform (maculopapular or exanthematous) rash seen with TMP/SMX, NNRTIs, and other medications utilized in the treatment of HIV-1-infected patients (Photo courtesy of HIV Web Study at the University of Washington)

Fig. 37.3
Morbilliform eruption evolving to a diffuse erythroderma with edematous, infiltrated, blistering lesions in a patient with DiHS/DRESS

Fig. 37.4
Facial edema including periorbital swelling in a patient with DiHS/DRESS
2.
Lipodystrophy and other appearance-related AEs/side effects which many patients worry will “out” them (i.e., bring out the fact that they are living with HIV/AIDS). These appearance-related AEs or fear of them developing may therefore lead to non-engagement in care in the first place and/or depression, despondency, HAART discontinuation, non-adherence with HAART, and/or the patient “falling out of care”.
3.
Immune response inflammatory syndrome (IRIS), a paradoxical worsening in the patient’s condition during suppressive HAART where the awakening, increasingly competent cell-mediated (T-cell) immune system recognizes and appropriately and vigorously responds to the antigenic stimuli present on cells from previously disseminated (and occasionally localized) foreign microbial (mostly opportunistic) pathogens.
These will be all discussed in greater detail in this chapter.
Epidemiology/Risk Factors
HIV predisposes patients to drug hypersensitivity reactions, with an estimated 100-fold increase in the risk of drug rashes compared with the general population.
ADRs are the 4th to 6th leading causes of death in the developed world. In a meta-analysis of inpatient ADR prospective studies, 15.1 % of patients sustained ADRs during their hospitalizations, 6.7 % experienced serious ADRs, and 0.32 % fatal ADRs. ADRs result in death in 0.1 % of medical and 0.01 % surgical inpatients, adversely affect surviving patients’ quality-of-life (QOL), and cause patients to lose confidence in their providers. They also often mimic other diseases, resulting in unnecessary investigations and delays in treatment.
Cutaneous adverse drug reactions (cADRs) are the most frequent ADRs comprising 10–30 % of all ADRs. cADRs account for 1 % of outpatient antibiotic prescription and 1–3 % of inpatient admissions. These range from mildly discomforting to life-threatening. Prior to the recognition of HIV infection and AIDS in the early 1980s and continuing into the HIV era cADRs were/are most commonly associated with anti-infective and anticonvulsant drugs, especially the aromatic anticonvulsants (phenytoin, phenobarbital, carbemazepam) but in the AIDS era have been joined by several ARV agents (Tables 37.1 and 37.2).
Table 37.1
Rash and hypersensitivity with HIV drugs I: older drugs
Drug | Rash (%) | Severe rash (%) | Rx D/C (%) | Reportsa (#1/#2/#3) | Reaction |
|---|---|---|---|---|---|
TMP/SMP | – | 4 | – | 125/16/61 | Exanthema, SJS, TEN, DiHS |
PCP Rx | 27–64 | 10–28 | 15–25 | – | – |
PCP Prophy | 3–34 | – | – | – | – |
Sulfadiazine | 10–40 | 0.6 | 3 | 6/5/4 | Exanthema, SJS, TEN, DiHS, |
Dapsone | – | – | – | 43/43/10 | Exanthema, Sulfone Rxn |
PCP Rx | 17–53 | – | – | – | – |
PCP Prophy | 5–10 | – | – | – | – |
Abacavir | 5–8 | Rare | 5 | 15/44/3 | Exanthema, DiHS, anaphylaxis |
Zidovudine | 17 | Rare | – | 16/1/5 | Exanthema |
Delavirdine | 14–18 | 4 | 4 | 13/1/3 | Exanthema |
Nevirapine | 9–16 | 6–8;hepatic rxn -5; SJS/TEN 0.3 | 7 | 23/13/29 | Exanthema, SJS, TEN, DiHS |
Amprenavir | 20–27 | 3 | 3 | 6/0/1 | Rash, DiHS, TEN |
Fosamprenavir | 2–16 | <1 | <1 | 2/2/1 | Rash, DiHS |
Lopinavir/ritonavir | <5 | NR | 5/2/0 | Rash | |
Tripanavir | 2–14 | rare | 0.5 | -/-/- | Rash, dyslipidemia |
Table 37.2
Rash and hypersensitivity with HIV drugs II: newer preferred drugs
Drug | Rash (%) | Severe rash (%) | Rx D/C (%) | Reportsb (#1/#2/#3) | Reaction |
|---|---|---|---|---|---|
Efavirenz | 10 | 0.1–0.7 | 2 | 13/3/1 | Exanthema, DIHS, SJS, TEN |
Etravirine | 12 | 3 cases | 2 | 7/1/1 | Rash, SJS, TEN |
Rilpivirine | 3–8 | <0.1 | 0.1 | 0/0/0 | – |
Tenofovir | 1–6 | – | – | – | – |
Atazanavir | 1–6 | – | 0.4 | 9/0/3 | Rash |
Darunavir | 7 | <1 | 0.3 | 4/0/2 | Rash, DiHS |
Raltegravir | 0 | – | – | – | – |
Elvitegravir | – | – | – | NR | – |
Dolutegravir | 1 | – | <1 | NR | – |
Enfuvirtide | 98a | <1 | – | – | ISRa, DIHS |
Maraviroc | 5/100 pt-years | – | – | – | Exanthem |
Adverse drug reactions can be classified by mechanism of the reactions (or by their clinical manifestations (Tables 37.3 and 37.4).
Table 37.3
Prototypical drug reactions involving the skin/skin structures in patients with HIV infection
Drug manifestation | Drug/drug class |
|---|---|
Maculopapular (morbilliform) | Sulfamethoxazole Nevirapine/(NNRTIs) Amprenavir, fosamprenavir |
SJS/TEN | Nevirapine Sulfamethoxazole |
DIHS/DRESS | Abacavir Nevirapine Sulfamethoxazole Fosamprenavir Darunavir Enfuvirtide |
Retinoid effects (ingrown nails/alopecia/xerosis/cheilosis) | Indinavir |
Injection site reactions | Enfuvirtide |
Table 37.4
Severe cutaneous adverse reactions (SCAR) in patients with HIV infection
Type of Rxn | Drug/drug classa | Incidence (%) | Mortality (%) |
|---|---|---|---|
DIHS/DRESSb | TMP/SMX Neviripine Efavirenz Enfuvirtide | 4 6–8 Rare (1 case) | 10 |
SJS/TENb | Neviripine TMP/SMX Etravirine | 0.3 | 10 (SJS) 30–50 (TEN) |
Acute generalized, exanthematous pustulosis (AGEP) | Clindamycin Lopinavir/r | Rare Rarec | 5 |
The most common cADRs are mild to moderate maculopapular (morbilliform) eruptions without systemic/other organ system involvement. These can frequently be managed without drug discontinuation or with later drug reintroduction (graded challenge/test dosing/direct rechallenge) while tolerance induction procedures such as dose escalation and true desensitization are sometimes indicated and performed. Patients with more severe, potentially life-threatening, systemic drug hypersensitivity reactions (with multi-system/multi-organ involvement) as in DIHS/DRESS and those with SJS/TEN should never undergo graded challenge or direct rechallenge and rarely, if ever, should tolerance induction procedures be attempted.
Risk factors for cADRs include being on multiple medications (polypharmacy), HIV infection, other viral infections (Epstein Barr virus [EBV]/cytomegalovirus [CMV] mono, human herpesvirus 6 [HHV-6], parvovirus B19, viral hepatitis viruses [in some but not all studies]), female sex, and age (especially age <3 years), immune status, and the presence of various genetic polymorphisms.
Pathogenesis, Pharmacogenetics/Genomics and Resulting Heterogeneity in Cutaneous Drug Eruptions
Most cADRs occurring in those living with HIV are Type B (idiosyncratic) reactions and of these, the vast majority are immunologically mediated Type IV (delayed, T-cell-mediated) hypersensitivity (allergic) reactions, although basically all categories of the immunologic reactions within the Gell and Coombs classification have been described. Clinically, reactions involving T-lymphocytes are characterized by prominent skin findings because the skin is a repository for an enormous number of T-cells.
It is unclear why HIV patients are so prone to adverse drug reactions, but the underlying reasons are likely multifactorial. Mechanisms include altered metabolism affecting the rate of inactivation and behavior of toxic metabolites, enhanced sensitivity of HIV-infected cells to cytotoxicity, immune system dysregulation, and activation including activation of T-cells via increased antigen presentation by major histocompatibility complex molecules or direct activation by parent drugs or metabolites. The “danger signal” hypothesis endorses the idea that HIV infection itself induces cytokine release and inflammatory signals, alerting the immune system to danger and promoting hyperactivity.
Heterogeneity in the reactions seen with individual ARV and OI agents and between agents can be explained by preferential recruitment and activation of various effector cells, distinct and varied T-cell recruitment and cytokine profiles, as well as host genetic predisposition influences.
Further research into the mechanism of hypersensitivity to commonly used medications in HIV infected patients is vital to improving tolerability and adherence to these therapies. Further identification and characterization of specific genetic risk profiles and additional specific risk factors/scores for more common as well as the rare severe cADRs should help to mitigate these burdensome and potentially dangerous reactions.
Spectrum of Reactions Seen in Patients with HIV
Adverse cutaneous drug reactions in HIV-infected patients include severe cutaneous adverse drug (SCAR) reactions including DiHS/ DRESS, SJS/TEN, angioedema/anaphylaxis, and acute generalized exanthematous pustulosis (AGEP) (Fig. 37.5) which are potentially life-threatening, and bullous fixed drug eruption [bFDE] which is not; non-SCAR reactions including the very common exanthematous (morbilliform, maculopapular) eruptions (which can frequently be managed without drug discontinuation or with later drug reintroduction), other less common reactions and manifestations, and non-immune ADRs such as lipodystrophy (Fig. 37.6). Prototypical drug reactions involving the skin and/or skin structures in patients with HIV infection are discussed further below.
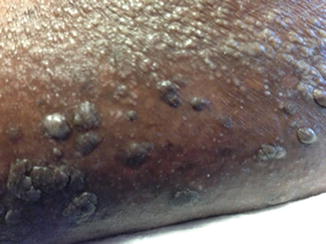
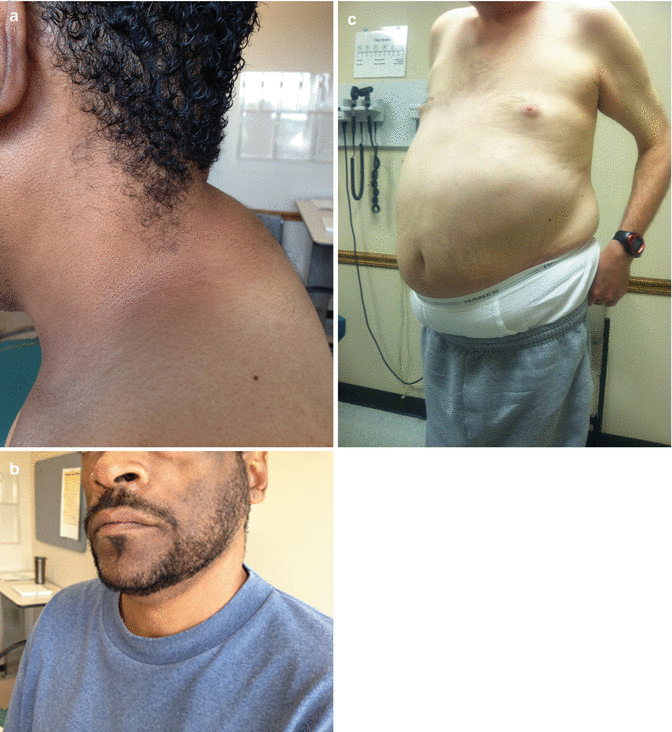

Fig. 37.5
Acute generalized exanthematous pustulosis in a patient treated with clindamycin

Fig. 37.6
(a–c)—Lipodystrophy associated with antiretroviral therapy (a) dorsocervical lipohypertrophy; (b) facial lipoatrophy; (c) abdominal lipohypertrophy (HIV-associated adipose Redistribution Syndrome [HAARS] with visceral adiposity confirmed by abdominal computed tomography [CT] scan)
HIV-Related Issues Which Make Diagnosis of Cutaneous Drug Eruptions Particularly Challenging
The diagnosis of cADRs is mostly clinical. The medical history regarding the reaction is extremely important as it helps determine if the patient has had or is having a cADR versus another clinical condition in the differential diagnosis (see Table 37.5), guide choice of diagnostic tests, determine which medicine is the likely culprit drug and determine whether it might be safe to continue or reintroduce the medication. If possible, the original medical record that describes the reaction should be reviewed. Expertise is essential to optimizing the outcomes for our patients with cADRs. Consultation should be sought early and urgently in order to safely and effectively manage both the reaction and the patient’s ARV and other HIV-specific needs.
Table 37.5
Differential diagnosis of cutaneous exanthems
Reaction type | Differential diagnosisa |
|---|---|
Exanthematous (maculopapular, morbilliform) | Viral (EBV, CMV, HIV [ARS rash], HHV6, parvovirus B19, rubella, rubeola, enteroviruses [Echo, Coxsackie], arbovirus, hepatitis viruses), bacterial (secondary syphilis, scarlet fever, typhoid fever, rheumatic fever, rat-bite fever, leptospirosis, erysipeloid, toxic shock syndrome, Lyme disease, ehrlichiosis), RMSF (early), murine (endemic) typhus, scrub typhus, trichinosis, toxoplasmosis, pityriasis rosea, acute graft-vs-host reaction, Kawasaki disease, Adult Onset Still’s Disease, SLE |
Acute Generalized Exanthematous Pustulosis (AGEP) | Generalized acute pustular psoriasis (von Zumbusch type); SJS/TEN; DRESS; bullous impetigo; subcorneal pustular dermatosis (Sneddon-Wilkinson disease) |
DRESS/DiHS | Acute viral infections (incl. HHV6), idiopathic hypereosinophilic syndrome, , angioimmunoblastic T cell lymphoma, Sezary syndrome, acute cutaneous lupus erythematosus, AGEP |
SJS/TEN | Exfoliative dermatitis, staphylococcal scalded skin syndrome, AGEP, paraneoplastic pemphigus |
Components of a drug allergy history are contained within the “RASHES ITCH” mnemonic device:
R xn description
A ction taken
S ystems involved
H x – when given (date of the reaction)
E xposed before or since the reaction (tolerated re-exposure/continuation)
S imilar symtoms while not taking medication?
I ndication – for the medication
T iming – when the reaction occurred relative to the initial dose
C oncomittant meds
H x – does the patient have predisposing underlying conditions?
Additional history details are described below:
R eaction description: onset, timing, severity, evolution, manifestations; IgE – mediated phenomena, hypotension?
A ctions taken: what treatment was needed? Was the drug continued or discontinued? Was intensive care unit care, vasopressors, or intubation needed? Were other treatment needed?
S ystems involved? Rash only versus rash plus fever versus rash plus systemic manifestations (other organ systems involved—hepatic, renal, pulmonary, cardiovascular, hematologic?)
H istory: How long ago did the reaction occur? What date?
E xposed before or since the reaction? (Was the patient previously on this medication and if so when? Has the medication or a similar medication been given since the reaction and if so how was it tolerated?)
S imilar symptoms occurring when not on the medication? If this is the case perhaps this was not a drug eruption. If the reaction was urticarial does the patient have idiopathic chronic recurrent urticaria or hereditary angioedema, or a polyserositis syndrome (systemic lupus erythematosis, Familial Mediteranean fever)?
I ndication: Why was the patient on the medication ? Is it still needed or are there reasonable alternatives?
T iming – When in relation to the first dose (in hours if possible) did the reaction occur? If patient was rechallenged or had been on the medication previously how soon after re-initiation did the reaction occur?
C oncomitant meds – What other medications was the patient receiving at the time of or within a few days of the reaction?
H x – Does the patient have underlying diseases or conditions (systemic lupus erythematosus, HIV, other) which may predispose them to hypersensitivity reactions?
HIV-Related Issues Which Make Management and Prevention of Cutaneous Drug Eruptions Particularly Challenging
With the need to simultaneously suppress HIV as well as prevent and/or treat opportunistic infections, and the multiple comorbidities often encountered in these patients, it is important to anticipate and manage drug–drug interactions and avoid, if possible, initiating at the same time more than one medication with higher chances of a cutaneous drug eruption. For example, if you anticipate the need for Pneumocystis jiroveci (formerly P. carinii) pneumonia (PCP) and/or toxoplasmosis prophylaxis and a neviripine-containing or an abacavir-containing HAART regimen (in an HLA-B*5701 non-screening setting), consider either avoiding TMP/SMX DS (i.e. use either atovaquone or dapsone for PJP prophylaxis with concomitant pyrimethamine if Toxoplasma gondii prophylaxis is also needed [i.e., Toxoplasma IgG antibody positive and CD4 <100]) or staggering these by starting the TMP/SMX DS first and then starting HAART 1–3 months later. Use of both abacavir (in an HLA-B*5701 non-screening setting) and neviripine in the same HAART regimen is not recommended and neither drug is recommended for PEP, sPEP, or PrEP.
The use of fixed-dose combination (FDC) agents, while decreasing pill burden, improving patient quality of life (QOL) and adherence to ARV therapy, can prove to be challenging and problematic in the setting an adverse drug reaction. First the culprit medication is not always clear. HIV suppression requires combination, multi-drug regimens. HIV replication is incredibly dynamic and this, along with differential intracellular half-lives of component drugs, the risk of resistance mutations occurring during “management” of drug reactions is quite real.
Discontinuing a fixed-dose combination ARV drug when the component medications within the FDC-ARV have vastly different half-lives entails a large concern for resistance development to the agent with the longer half-life due to “virtual monotherapy,” which occurs as the shorter half-life component drugs dissipate and are cleared. This is made even more difficult when in a severe reaction stopping the culprit medication early on can be critically important. Stopping one drug at a time or alternatively discontinuing all potential medication causes of a hypersensitivity reaction and reintroduction of one agent at a time is often not tenable, due to resistance concerns even when the current or proposed new regimen is comprised of agents which all have similar half-lives.
Drug-Related Lipodystrophy
Both lipodystrophy and immune reconstitution inflammatory syndrome (IRIS) have cutaneous manifestations and affect the patient’s appearance, which is often of great concern as these appearance-related adverse effects (AEs) can be quite stigma-inducing, and therefore hold the potential to lead to drug discontinuation and/or poor adherence both of which causes loss of viral control, potential for HIV drug resistance, and concomitant clinical and immune system deterioration.
Even prior to the HAART era, nucleoside analog-induced lipodystophy (also called HIV- associated adipose redistribution syndrome [HAARS] and HIV-associated metabolic and morphological abnormality syndrome [HAMMAS]) consisting of increasing visceral adiposity with concomitant facial and extremity fat wasting/lipoatrophy with or without trunchal fat accumulation/lipohypertrophy were seen and described.
Lipoatrophy is considered a consequence of mitochondrial toxicity seen especially with earlier nucleoside analogue reverse transcriptase inhibitors, didanosine (ddI), zalcitabine (ddc), and stavudine (D4T), collectively referred to as “D drugs” and somewhat with zidovudine (ZDV, also initially know as “AZT” for azidothymidine).
Lipohypertrophy and cardiometabolic syndrome/insulin resistance subdivisions of lipodystrophy are more associated with protease inhibitor-based HAART. Carr et al. identified lipodystrophy in 83 % of 113 HIV-infected patients treated with protease inhibitors (PIs) compared with 4 % of controls (HIV-infected, not using PIs). The lipodystrophy occurred after a mean of 21 months of therapy and failed to resolve following cessation of treatment.
Treatment strategies for lipodystrophy are limited and until now have had only a modest impact on those already affected. The incidence of HIV lipodystrophy appears to be declining as a result of the use of newer antiretroviral drugs. Recent studies confirm that stavudine and zidovudine are the nucleoside analogues responsible for most lipoatrophy, but also suggest that different protease inhibitors have opposing effects on lipoatrophy. Antiretroviral regimens excluding stavudine and zidovudine offer substantial protection against lipoatrophy. Established lipoatrophy improves gradually with the cessation of these drugs.
Thiazolidinediones, uridine, and pravastatin may also improve lipoatrophy, although their effects have not been shown to be sustained. Metformin and growth hormone and its analogues are effective in reducing abdominal fat accumulation, although they aggravate lipoatrophy and generally have only transient effects. Tesamorelin, a synthetic growth hormone releasing hormone, which is FDA approved for lipodystrophy was shown after 6 months of treatment to decrease substantially both visceral adipose tissue and hepatic lipid measurements. No medical intervention has been shown to have a sustained and substantial benefit on either lipoatrophy or visceral fat accumulation, so results with this agent at longer follow up are eagerly awaited. Cosmetic surgery can modestly improve facial lipoatrophy.
Cutaneous Manifestations of Immune Response Inflammatory Syndrome (IRIS)
IRIS reveals itself in paradoxical deterioration in clinical status/worsening of a preexisting infectious process occurring after initiation of effective HAART. It is caused by inflammation (localized or systemic) related to a reconstituting cell-mediated immune response to a preexisting infection which may have been previously diagnosed and treated or may be subclinical and unmasked by the host’s regained capacity to mount an inflammatory response.
The immune reconstitution inflammatory syndrome (IRIS) has also been variably referred to as immune recovery disease, immune reconstitution disease, immune reconstitution syndrome, immune restoration disease, immune rebound illness, steroid withdrawal disease, immunorestitution disease, and immune response reaction.
Up to 25 % of patients started on HAART will experience some manifestations of IRIS, most of which include cutaneous findings. Ratnum and colleagues undertook a retrospective study of all patients starting HAART during a 2-year period. Of the 199 patients studied, 50 % were male, 59 % were black African, 29 % were white, and 10.5 % were black Caribbean. Fourty-four (22.7 %) of these patients experienced an IRIS event at a median of 12 weeks after HAART initiation; 22 events (50 %) involved genital herpes, 10 (23 %) involved genital warts, 4 (9 %) mollusscum contagiosum, and 4 (9 %) involved varicella zoster virus infection. Five patients had mycobacterial infections, four had hepatitis, one had Pneumocystis jiroveci infection, and one had Kaposi sarcoma. The strongest independent predictors of IRIS were younger age at HAART initiation (P = .003), baseline CD4 cell percentage of <10 % (OR, 2.97) and CD4+ %/CD8+ % of <0.15 (OR, 3.45 95 % CI, 1.27–9.1).
IRIS is usually self-limited, however it may cause significant morbidity and is rarely fatal. IRIS is important as it may lead to HAART interruption/failure, tests/procedures, and require medical or surgical management. Early examples of immune reconstitution inflammatory syndrome targeting disseminated extrapulmonary tuberculosis with angry, hot, swollen, tender, and pointing anterior cervical lymph nodes with dual NRTI ARV clinical therapy and “hepatic IRIS” versus hepatitis B and C from blinded zidovudine plus neviripine versus zidovudine plus placebo clinical trial protocol-based therapy (leading to a temporary international halting of the trial) were seen in the early 1990s by this author.
IRIS is not unique to AIDS and HAART. It is well described as “paradoxical rxns” during tuberculosis treatment in patients without HIV/AIDS. Localized and disseminated infections with mycobacteria other than TB (MOTT) worsening during steroid withdrawal is another example.
IRIS is characterized by clinical features which are not particularly typical of naturally occurring AIDS-related OIs yet the similarities are close enough to sometimes cause confusion especially if IRIS is not initially suspected or considered. IRIS must be differentiated from drug reaction/toxicity, especially DIHS (Table 37.6)—e.g., neviripine HSR, efavirenz HSR, abacavir HSR in HLA-B*5701 negative patients or non-screening settings—OI progression due to drug resistant pathogen, non-adherence with OI prophylactic treatment with a lack of immune system restoration due to nonadherence with HAART or HIV resistance, and other non-immune/non-inflammatory processes.
Table 37.6
Delayed cutaneous hypersensitivity RXNs: warning signs and management
Red Swollen (edematous or infiltrated) lesions | |
Sign | Action |
Edema of central face | Stop drug and treat as required |
Diffuse erythematous swelling | Stop drug and treat as required |
Involvement of extended body surface | Stop drug and treat as required |
Erythroderma | Stop drug and treat as required |
Atypical targets | Stop drug and treat as required |
Infiltrated plaques | Stop drug and treat as required |
Hemorrhagic “Bloody” Lesions | |
Sign | Action |
Necrotic lesions | Stop and treat as required |
Hemorrhagic lesions | Stop and treat as required |
Palpable purpura | Stop as early as possible, treat as required |
Vesiculobullous lesions | |
Sign | Action |
Painful “Skin” (early initial symptom) | Immediately stop drug and treat |
Positive Nikolsky’s sign | Immediately stop drug and treat |
Epidermolysis | Immediately stop drug and treat |
Vesicles, bullae | Stop drug and treat as required |
Mucosal erosions | Stop drug and treat as required |
It is important to understand that IRIS will resolve in most individuals with continued HAART, continued OI coverage, with or without the institution of anti-inflammatory medications (with fairly rapid resolution). However, IRIS causing significant morbidity including that due to perforation, obstructive phenomena, and mass effect, has been described.
The most common IRIS entities described include: mycobacterium avium complex lymphadenitis, pulmonary infiltrates and nodules, paradoxical exacerbation of pulmonary and central nervous system CNS) M. tuberculosis disease (with potential for serious CNS sequelae), IRIS-related dermatomal zoster (varicella zoster infection), Pneumocystis jiroveci (formerly P.carinii) pneumonia/respiratory failure, paradoxical worsening of viral hepatitis (HBV, HCV), progressive multifocal leukoencephalopathy (PML) worsening (including contrast enhancing PML and fatal PML), paradoxical exacerbation of cryptococcal disease (meningitis; pulmonary disease, necrotizing mediastinal lymphadenopathy), cytomegalovirus (CMV) uveitis, optic nerve neovascularization, cutananeous ulcers, and pneumonitis.
The antigenic target in IRIS, when known, is often but not always a component of an opportunistic microorganism or a more common cutaneous viral condition such as genital herpes and warts (human papilloma virus) and includes abscess formation with or without fluctuance and sinus tract formation, nodules, pustules, vesicles, exanthema, plaques, ulcers, verucous lesions, inflamed tender elevation of tattoos, and alopecia universalis.
Other less common variants include: IRIS-induced pre-eclampsia, IRIS-related sarcoidosis (new-onset or recurrent/relapsing), relapsing Guillian Barre syndrome, Reiter’s syndrome, tuberculoid leprosy with “reversal reactions” and autoimmune thyroid disease (AITD).
When IRIS is considered and recognized it is important to continue HAART attempting to “treat through” the IRIS. When recognized one can often defer/avoid diagnostic evaluation procedures and their attendant risks. Exceptions to this strategy include serious, life-threatening manifestions or non-IRIS conditions which are not easily differentiated from IRIS which may be occurring and/or prove difficult to manage and the results of the diagnostic procedure would likely result in a major change in therapy.
Usual IRIS management measures include reassurance, NSAIDs, corticosteroids, and, less commonly, IVIG, thalidomide, and TNF alpha drugs. Surgery is occasionally needed for MAC/Mtb lymphadenopathy that is threatening spontaneous suppuration and drainage/sinus formation, and other situations where there is uncontrolled inflammation in a closed space causing mass effect or other serious sequelae. Continued or renewed treatment directed at the pathogen is indicated when a live antigenic stimulus for IRIS is suspected while specific pathogen directed treatment would not be indicated or helpful (and in fact might be detrimental) when the IRIS is directed against non-viable, dead microbial antigenic products. It is unknown whether treatment which leads to lysis of the organism compared with non-lytic therapies (as in cell wall active bactericidal bacterial meningitis treatment and Jarish-Herxheimer reactions with syphilis treatment) are of importance in OI treatment or prevention in the setting of IRIS.
Another strategy which has been employed successfully in some cases of IRIS is that of staggered therapy, for example 1–4 months of directed or empiric pathogen-specific therapy to decrease “antigen load” of the implicated pathogen, providing the antigenic stimulus against which the IRIS is directed, then after this interval treatment period adding HAART with the hope and expectation that the intensity of IRIS signs and symptoms would be less severe and more easily tolerated.
Reactions Seen with Drugs Used to Treat and Prevent Opportunistic Infections
Trimethoprim – Sulfamethoxazole
One drawback in the use of TMP/SMX is the high rate of ADRs (mostly cADRs) in HIV-infected patients. Besides penicillins, sulfonamide antibiotics are the most common cause of drug-induced allergic reactions, most commonly delayed maculopapular/morbilliform eruptions, and are the most common cause of SJS/TEN. The incidence of SCARs is estimated to be between 1:1000 and 1:100,000. IgE-mediated reactions (urticaria/angioedema, anaphylaxis) are infrequent.
Sulfonamide antibiotic ADRs are greatly increased in HIV-infected patients occurring in 40–80 % in HIV patients given TMP/SMX versus 3–5 % of healthy subjects given the drug. In patients receiving high (treatment)-dose TMP-SMX for PCP, the rate of cADRs in HIV-infected patients is reported to be 5.4 times higher than in those immunocompromising conditions other than HIV/AIDS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


