Most potent phenol and catechol derivatives
Non-phenolic/catecholic agents
Hydroquinone
Mercury
Monobenzyl ether of hydroquinone
Arsenic
p-tert-Butylchatechol
Cinnamic aldehyde
p-tert-Butylphenol
p-phenylenediamine (PPD)
p-tert-Amylphenol
Benzyl alcohol
–
Azelaic acid
–
Corticosteroids
–
Physostigmine
–
Chloroquine
–
Fluphenazine
While contact leukoderma is classically associated with industrial materials, there are a number of commonly encountered products that can cause leukoderma. Phenols and catechols have been identified in commonly encountered products, such as deodorants, detergents, latex gloves, adhesives, insecticides, disinfectants, perfumes, varnish resins, photographic chemicals, rubber sandals, and paints. PPD, found commonly in hair dyes, is the most common cause of contact leukoderma from cosmetics. While it classically affects the scalp/face of patients exposed to hair dyes, it can also be an occupational exposure in hairstylists. More recently, synthetic black-henna tattoos (which are formulated with PPD) have been associated with contact leukoderma. PPD found in black socks and shoes can cause leukoderma of the feet. Azo dyes in eyeliners, lipliners, and lipsticks have also been implicated in contact leukoderma.
Unintentional Leukoderma from Topical Prescription Medications
Imiquimod is commonly prescribed for dermatologic conditions such as warts and non-melanoma skin cancers. There are reports in the dermatology literature of imiquimod inducing vitiligo-like skin hypopigmentation in patients treated for superficial basal cell carcinoma as well as from treatment of genital warts. It is unclear if the pigmentary changes are due to benzyl alcohol within the vehicle (previously reported cause of leukoderma) or T-cell mediated cytotoxicity toward melanocytes. The effect seems to be persistent in follow-up as long as 18 months after discontinuation of imiquimod.
Topical corticosteroids are a mainstay in the treatment of dermatologic conditions. The clinician should remain vigilant of the multiple cutaneous side effects of topical corticosteroids, including epidermal atrophy and skin hypopigmentation. There have been reports of streaks of linear hypopigmentation after unintentional administration of intralesional corticosteroids into veins during cutaneous injections.
Intentional Leukoderma from Prescription Medications
Cultural fascination with skin lightening dates back to ancient times. Arsenic and mercury were used in creams for bleaching skin, and arsenic was used in face powders by European aristocrats in 1400 AD. While the toxicity of these heavy metals has prompted elimination from skin bleaching creams, other agents have been formulated for their depigmenting properties.
Hydroquinone (1,4-dihydroxybenzene) is found in both over-the-counter and prescription skin-lightening agents. It causes oxidation of melanin, tyrosinase, and phenol oxidases into reactive species (semiquinones and quinones) that prevent melanogenesis by inhibition of tyrosinase. Over-the-counter skin bleaching creams usually contain 2 % hydroquinone, while prescription strength can range from 3 to 4 %. A number of over-the-counter topicals that are not necessarily marketed as skin-bleaching creams may contain hydroquinone, and can thus cause unwanted leukoderma. Chronic use of hydroquinone (usually 6–8 % formulations) can cause paradoxical hyperpigmentation in the form of exogenous ochronosis (discussed in a later section). Monobenzylether of hydroquinone (monobenzone, 4-(benzyloxy)phenol) is used for permanent depigmentation in patients with extensive vitiligo of greater than 50 % body surface area.
Systemic Medications That Can Cause Leukoderma
Several medications have been implicated in drug-induced leukoderma. Photo-exposed sites tend to be preferentially affected.
Tyrosine kinase inhibitors have been developed for the treatment of many malignancies, including chronic myelogenous leukemia and gastrointestinal stromal tumors (imatinib); metastatic renal cell carcinoma (sunitinib); and non-small cell lung cancers (gefitinib, a single tyrosine kinase inhibitor). Depigmentation results from inhibition of c-kit, a tyrosine protein kinase involved in melanocyte development. Imatinib can cause localized or widespread hypo- or depigmentation in darker-skinned patients. Much less commonly, it can cause hyperpigmentation of the skin, hair, nails, and oral mucosa. Sunitinib has been reported to cause early facial depigmentation in a patient with no personal or family history of vitiligo and has also been associated with intermittent leukotrichia. Gefitinib has recently been reported to induce leukoderma in a patient undergoing treatment for metastatic squamous cell carcinoma of the parotid gland.
Methylphenidate applied as a topical patch for the treatment of attention-deficit-hyperactivity disorder has been associated with application-related contact dermatitis, urticaria, and hair loss. It has recently been implicated in contact leukoderma (confirmed by Wood’s lamp examination and biopsy) at sites of application of the methylphenidate patch. It is unclear whether the true culprit was the laminate film (which contains polyester/ethylene vinyl acetate), adhesive (acrylic or silicone), or the active agent methylphenidate itself. It was not clear in this study whether there was any spontaneous repigmentation after discontinuation of the methylphenidate patch.
Other medications associated with cutaneous hypopigmentation include clonidine, chloroquine, minoxidil, botulinum toxin, and thiotepa.
Poliosis or Leukotrichia Caused By Medications
Poliosis circumscripta (patch of white hair among a group of otherwise normal follicles) is generally associated with genetic syndromes, such as piebaldism, Waardenburg syndrome, and tuberous sclerosis. Though very rare, it bears mention that several topical and systemic medications have been reported to cause poliosis.
Topical Agents Associated with Poliosis
Chloramphenicol (topical antibiotic) has been associated with whitening of eyelashes and periorbital cutaneous hypopigmentation following allergic contact dermatitis to the agent in a patient who had undergone surgery for eyelid ptosis. The proposed mechanism of the pigmentary changes was attributed to T-cell mediated hypersensitivity that caused selective loss of either melanin or melanocytes. In this case, the poliosis and hypopigmentation persisted at 9-month follow-up.
Imiquimod can induce leukoderma (see above) as well as poliosis. While unclear, purported mechanisms include the possibility of benzyl alcohol (fragrance preservative), induced chemical leukoderma, or activation of cytotoxic T-cells that destroy melanocytes.
Prostaglandin f2α analogs, such as latanoprost, travaprost, and bimatoprost, are used topically in the treatment of glaucoma. They have been reported to cause whitening of a few eyelashes scattered among normal-colored lashes. They are thought to limit melanogenesis via tyrosinase inhibition. It is unclear how long the effect persists, as one report demonstrated near complete repigmentation within 10 months after the medication was discontinued, and another report saw persistent poliosis at 2 months follow-up of 7 patients. This paradoxical poliosis is of particular interest to the dermatologist, since bimatoprost is prescribed to promote hypertrichosis and darkening of lashes and has been noted to cause darkening of the iris.
Systemic Medications Associated with Poliosis
Acitretin is FDA-approved for the treatment of psoriasis, but off-label uses include treatment of various disorders of keratinization, such as Darier’s disease, pityriasis rubra pilaris, keratodermas, and diseases on the ichthyosis spectrum. It is well known to induce alopecia but was recently reported to cause diffuse poliosis concurrently with alopecia of the scalp and body. The patient’s hair regained normal pigmentation after cessation of the medication.
Cetuximab, an epidermal growth factor receptor inhibitor used in the treatment of metastatic colorectal cancer and head/neck squamous cell carcinomas, has been implicated in numerous cutaneous adverse effects. Recently, it was associated with poliosis of the eyelashes in conjunction with trichomegaly in a patient with metastatic colorectal cancer. When the medication was discontinued due to disease progression, the patient’s poliosis resolved within a month. The mechanism of poliosis is not understood.
Chloroquine is classically associated with blue-gray hyperpigmentation of the skin. However, there are reports associating this medication with hypopigmentation of hair in blondes and red-heads and even hypopigmentation of freckles. Sunitinib has been associated with intermittent leukotrichia following rounds of treatment (with repigmentation in between treatments). It also causes leukoderma (see above).
Yellow, Orange, and Red Dyspigmentation
Systemic Medications That Cause Yellow, Orange, or Red Dyspigmentation
Quinacrine (mepacrine) is an antimalarial agent for the treatment of lupus erythematosus and is generally added onto either hydroxychloroquine or chloroquine to avoid the theoretical additive rentinal toxicity of the latter two medications and to improve efficacy. Chronic ingestion can cause yellow to yellow-brown discoloration of the skin, sclera, and nails that mimics jaundice. Histologically, yellow-brown pigment within histiocytes can be seen throughout the dermis. The dyspigmentation is short-lived, often resolving within a few months after discontinuation of the medication. This dyspigmentation will not be apparent in darker-skinned individuals.
Multikinase inhibitors sorafenib and sunitinib have been reported to cause deep yellow dyspigmentation diffusely in patients as early as the first few weeks of treatment. Notably, sclerae and mucous membranes are spared, and the discoloration resolves upon discontinuation of the medications. Sorafenib is approved for the treatment of metastatic renal cell carcinoma, unresectable hepatocellular carcinoma, and radioactive iodine-resistant thyroid cancer. Sunitinib is approved for GI stromal tumors, metastatic renal cell carcinoma, and pancreatic neuroendocrine tumors.
Clofazimine is FDA-approved for the treatment of leprosy, though its anti-inflammatory properties are harnessed in off-label treatment of several inflammatory and granulomatous skin diseases. When this deep red-to-orange colored lipophilic riminophenazine dye localizes to the fat, it can cause orange-red dyspigmentation of the skin, conjunctivae, and body fluids. This early discoloration can start within a couple of weeks of medication initiation and resolves within months after discontinuation of treatment. With longer duration of treatment, patients can develop violet-brown to blue-gray discoloration of lesional skin.
Sunless-Tanning Agents That Can Cause Orange Discoloration
Canthaxanthin is a synthetic carotenoid that, when combined with beta-carotene, produces a color that resembles a natural tan (golden-orange color) upon deposition within the subcutaneous fat. In addition, this pill causes stool to become a deep red, imparts an orange hue to plasma, and causes deposits within the retina. It has been associated with both liver and retinal damage.
Dihydroxyacetone is the active ingredient in sunless tanning products. It reacts with free amino acids in sweat and keratin to produce a brown color called melanoidin, which resembles a suntan in people of fair skin types. Dermatologists should remind their patients to apply sunscreens after using self-tanning creams and lotions, as they do not confer any degree of photoprotection.
Melanocyte-stimulating hormones (MSH) are a class of peptide hormones that stimulate skin and hair melanogenesis via stimulation of melanocortin receptors. Synthetic analogs of α-MSH have been developed for the purpose of photoprotection. These include afamelanotide (melanotan) and melanotan II. The former seems to decrease photosensitivity in patients with solar urticaria and erythropoietic porphyria, and afamelanotide is already prescribed in Italy and Switzerland for patients with erythropoietic protoporphyria. It has not yet gained FDA approval in the United States. Afamelanotide with narrow-band UVB phototherapy seems to be a promising new treatment approach for vitiligo.
Dietary Intake That Can Cause Orange Discoloration
Carotenemia presents with a yellow-orange dyspigmentation of the skin due to excessive consumption of carotene-containing foods (carrots, squash, pumpkin, yellow turnips, sweet potatoes, peaches, apricots, papayas, mangoes, and egg yolk). Carotene is excreted through the liver and epidermis. During times of heavy excretion, the stratum corneum can reabsorb carotene, thus explaining the prominent pigmentation on the palms and soles, which bear thicker stratum corneum. Other sites include areas of pressure overlying joints (elbows, knees, knuckles, and ankles), and face (nasolabial grooves, upper eyelids). Patients with hypothyroidism and eating disorders can also present with carotenemia. Patients of darker skin types may only present with pigmentation of the palms and soles. Importantly, patients are not pruritic, have no involvement of the sclerae and mucous membranes, and have normal-colored urine and stool, thus differentiating carotenemia from jaundice. Carotenemia can also lead to xanthochromia of cerebrospinal fluid. Lycopenemia presents as orange-yellow discoloration that occurs from over-consumption of lycopene, a red carotenoid found in fruits and vegetables, such as tomatoes, beats, chili beans, and berries. Once patients reduce their dietary intake of lycopene-containing foods, their discoloration gradually resolves over the ensuing weeks.
Green Dyspigmentation
Copper is well-known to cause blue-green dyspigmentation of skin, hair, and nails. There has been a report of a green dyschromia of multiple seborrheic keratoses in a patient who swam regularly in a pool that had copper concentration double the levels approved by the U.S. Environmental Protection Agency. This dyspigmentation quickly resolved after the patient stopped swimming in the pool. Extended exposure to chlorine can cause green discoloration of light-colored hair.
Bronze Dyspigmentation
Arsenic is a ubiquitous heavy metal used in the production of numerous commercial products, such as pesticides, herbicides, insecticides, feed additives, and wood preservatives. Globally, the most common source of arsenic exposure is through contaminated well- and groundwater. Cutaneous manifestations of arsenic exposure occur after long-term exposure, when skin levels are greater than 200 mcg/L. The lipid-soluble trivalent form absorbs through the skin and can deposit within the epidermis and dermis as well as stimulate epidermal melanin synthesis. Patients present with non-photo-distributed bronze hyperpigmentation, particularly on the trunk, with accentuation of the folds. Some patients can develop diffuse hyperpigmentation or melanotic macules. Arsenic can also deposit within the nail bed and appear as transverse white bands (Mee’s lines, which can also be seen with thallium deposition).
Iron salts can cause permanent hyperpigmentation at sites of intravenous, intramuscular, or topical administration (the latter with Monsel’s solution, ferric subsulfate, which is used to attain superficial wound hemostasis). The genetic iron-overload condition hemochromatosis can cause generalized bronze hyperpigmentation. Histology will reveal pigment bound to collagen fibers and deposition within dermal macrophages. This results in a reddish-brown, bronze, or blue-gray hyperpigmentation (Fig. 9.1). While the hyperpigmentation is generally thought to be permanent, there is a recent report of Monsel solution-induced facial hyperpigmentation that improved over 20 months with adapalene 0.1 % cream.
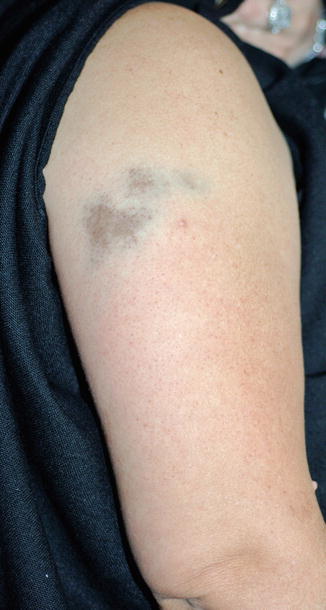

Fig. 9.1
Gray-blue/tan dyspigmentation on the right upper arm following administration of intramuscular iron replacement in an anemic 47-year-old woman. Histology revealed iron deposition throughout the dermis (Courtesy of Dr. Richard A. Johnson, MD (Boston, MA))
Brown Dyspigmentation
Table 9.2 summarizes drug-induced brown dyspigmentation. Oral contraceptives can cause hyperpigmentation of the nipples, stimulate generation of new melanocytic nevi, and cause melasma. Histologically there are greater numbers of melanocytes and increase in melanin synthesis. They are also associated with fixed drug eruptions (see below).
Table 9.2
Drug-induced brown dyspigmentation
Medication | Indication | Sites | Comments |
|---|---|---|---|
Hydroquinone | Skin bleaching | Sites of application | Can cause irritant dermatitis, exogenous ochronosis (blue-black hyperpigmentation) |
Imatinib | Chronic myelogenous leukemia | Oral mucosa, nails, hair | Usually leukoderma; can see repigmentation of gray hairs, melanonychia |
Oral contraceptives | Face, nipples | Melasma, stimulate production of new melanocytic nevi | |
Psoralens | PUVA (psoriasis, vitiligo, mycosis fungoides, etc.) Furocoumarin exposure through fruit/vegetables/plants | Photo-exposed | Also phytophotodermatitis |
Zidovudine | HIV | Skin, nails, mucosa; possible accentuation in photo-exposed sites | Longitudinal melanonychia; hyperpigmentation more prominent in darker skin types |
Chemotherapy | |||
Bleomycin | Hodgkin’s disease, testicular carcinoma, pancreatic cancer, head/neck squamous cell carcinoma | Sites of scratching, joints, pressure | Flagellate hyperpigmentation |
Busulfan | Chronic myelogenous leukemia; conditioning regimen for SCT | Face, chest, forearms, abdomen | Negative iron stains |
Carmustine | Brain tumors, multiple myeloma, lymphoma, off-label mycosis fungoides | – | Applied topically for MF |
Cyclophosphamide | Off-label JIA, lupus neprhritis | Skin and mucosa (nails, palmoplantar, teeth) | – |
Dactinomycin | Wilms’ tumor, rhabdomyosarcoma, Ewing’s sarcoma, gestational trophoblastic neoplasia, malignant hydatidiform mole | Face | – |
Daunorubicin | Leukemia, lymphoma, breast, uterine, ovarian, lung cancers | Photo-exposed | – |
Doxorubicin | Leukemia, lymphoma, breast, uterine, ovarian, lung cancers | Palmoplantar, small joints hands | – |
Fluorouracil | Solid tumors | Photo-exposed Overlying veins | Photosensitive eruption; supravenous serpentine hyperpigmentation |
Hydroxyurea | Hematologic malignancies, sickle cell anemia, off-label for derm | Back, sites of pressure | Also lichenoid drug eruption |
Methotrexate | Oncology Rheumatology Dermatology | Photo-exposed | UV recall dermatitis |
Nitrogen mustard (meclorethamine) | Mycosis fungoides (topical) | Lesional skin | – |
Zidovudine (AZT), a nucleoside reverse transcriptase inhibitor for HIV treatment, is classically associated with longitudinal melanonychia. However, it can also cause diffuse mucocutaneous hyperpigmentation, particularly in patients of darker skin types. There can be accentuation in photo-exposed areas. Histologically, there is increased melanin in macrophages within the epidermis and dermis. This temporary discoloration will fade gradually after discontinuation of AZT.
Hydroquinone, which is used as a topical bleaching agent, can cause an irritant dermatitis that leads to post-inflammatory hyperpigmentation. Prolonged use of a 6–8 % formulation can cause paradoxical hyperpigmentation in the form of exogenous ochronosis. This is characterized by blue-black hyperpigmentation, and histologically shows yellow-brown irregularly shaped fragments within the dermis.
Imatinib can rarely cause hyperpigmentation, though hypo- or depigmentation are far more common pigmentary changes. Repigmentation of gray hair can also be seen, as can melanonychia and oral mucosal dyspigmentation.
Psoralens can cause generalized brown dyschromia after UVA exposure (oral psoralens, PUVA) or linear/circumscribed hyperpigmentation after topical psoralen application (or in the form of phytophotodermatitis). On skin biopsy, there is increased number of follicular melanocytes and increased melanin. Similar reactions may be seen with other plants and herbal supplements that contain furocoumarins, such as limes, celery, fennel, and parsnip.
Unintentional skin hyperpigmentation can result from the use of cosmetics. A classic example is Riehl melanosis, a photoallergic contact dermatitis to cosmetic agents that contain fragrances and essential oils.
Chemotherapeutic Agents That Cause Brown Hyperpigmentation
Nitrogen mustard (mechlorethamine) applied topically for the treatment of mycosis fungoide can cause a diffuse hyperpigmentation, with accentuation of lesional skin. Pathology will show keratinocytes with disaggregated melanosomes and increased melanocytes.
Methotrexate is used for treatment applications in oncology, rheumatology, and dermatology. It can cause not only hyperpigmentation in photo-exposed areas, but also a photosensitive eruption that resolves with temporary post-inflammatory hyperpigmentation from a photosensitivity eruption. Methotrexate is also implicated in photo-recall type reactions. This can be a delayed response if high-dose methotrexate is given after exposure.
Bleomycin is a glycopeptide antibiotic that inhibits DNA, RNA, and protein synthesis. It is used in the treatment of a number of cancers, including Hodgkin’s disease, testicular carcinoma, pancreatic cancer, and squamous cell carcinoma of the head and neck. Bleomycin causes a brown hyperpigmentation with unique flagellate appearance (thought to be the result of minor trauma, such as scratching). There can also be hyperpigmentation at sites of pressure and over joints. Histologically, a normal number of melanocytes but increased epidermal melanin is seen. While docetaxel, shiitake mushrooms, dermatomyositis, and adult-onset Still’s disease are also associated with flagellate erythema, hyperpigmentation was considered unique to bleomycin until a recent report of bendamustine-induced flagellate hyperpigmentation in a patient undergoing treatment for chronic lymphocytic leukemia.
Busulfan is an alkylating agent used for the treatment of chronic myelogenous leukemia and as part of conditioning regimens for stem cell transplantation. It can cause widespread hyperpigmentation (particularly on the face, chest, forearms, and abdomen) that mimics Addison’s disease but with notable sparing of palmar creases. The dyschromia resolves within months after stopping the medication and will recur with re-exposure. Histology reveals increased melanin within basal keratinocytes and melanin within dermal macrophages. Of note, there are normal numbers of melanocytes, and iron stains are negative, thus indicating melanin rather than hemosiderin deposition. While the exact mechanism of hyperpigmentation is unknown, it is thought that this alkylating agent inactivates sulfhydryl groups within the skin, thereby releasing inhibition of tyrosinase, thereby allowing for melanin production and subsequent hyperpigmentation.
Carmustine (BCNU) is an alkylating agent for the treatment of brain tumors (glioblastoma multiforme, and other malignant gliomas), multiple myeloma, Hodgkin’s disease, and non-Hodgkin’s lymphoma. It is prescribed in dermatology for the off-label treatment of mycosis fungoides. When applied topically, carmustine causes brown hyperpigmentation of the skin. Skin biopsy shows basal melanocyte hyperplasia and pigment deposition within keratinocytes.
Cyclophosphamide is another alkylating agent used for treatment of internal malignancies and various rheumatologic conditions. It can cause generalized hyperpigmentation of both skin and mucosal surfaces. The pigment has a tendency to deposit in nails, palmoplantar surfaces, and teeth. After the medication is discontinued, the pigmentation will slowly fade, usually over 6–12 months.
Several antibiotics used as chemotherapies have also been associated with skin and nail hyperpigmentation. Dactinomycin is used for treatment of a number of solid tumors and also causes generalized hyperpigmentation, particularly of the face. The dyschromia tends to fade after discontinuation of the medication. The anthracycline antibiotics intercalate into DNA and are used in the treatment of leukemias, lymphomas, breast, uterine, ovarian, and lung cancers. Daunorubicin produces hyperpigmentation that tends to occur in photo-exposed regions but can also involve the nails. Doxorubicin hyperpigmentation localizes to the palmoplantar surfaces and over the small joints of the hands. Histologically, there is both increased melanocyte proliferation and epidermal melanin content.
5-Fluorouracil is a pyrimidine analog used in the treatment of solid tumors. It can cause a photosensitive eruption that results in post-inflammatory hyperpigmentation. It can also cause cutaneous hyperpigmentation overlying veins into which the chemotherapy was infused (supravenous serpentine hyperpigmentation) as well as target other sites, such as the dorsal hands, palmoplantar surfaces, and radiation ports.
Hydroxyurea is used in the treatment of hematologic malignancies and sickle cell anemia, and has off-label dermatologic applications. It can cause prominent hyperpigmentation on the back and over sites of pressure. This hyperpigmentation is reversible, as is the post-inflammatory hyperpigmentation that can occur after a hydroxyurea-induced lichenoid eruption.
Fixed Drug Eruptions
Fixed drug eruptions are characterized by the appearance of well-demarcated round or oval patches or plaques of erythema (or even bullous variants), preferentially on acral (hands and feet) and mucosal (oral and genital) sites. Initial exposure can produce the eruption 1–2 weeks after starting an offending medication; subsequently, the eruption recurs at the same site(s) within a day after re-exposure. It is possible that with repeated exposures, the number of sites involved can increase. Symptoms associated with fixed drug eruptions are minimal, and patients are otherwise well. Fixed drug eruptions heal with crust and eventually develop a dusky brown color that is more prominent in darker skinned individuals.
Though fixed drug eruptions are thought to be allergic reactions, the pathophysiology is not well understood. Skin biopsy is characterized by interface changes and mixed dermal infiltrate consisting of lymphocytes, neutrophils, histiocytes, and eosinophils. Histology of older plaques will show prominent pigment incontinence. Sites of recurrent fixed drug eruptions will reveal deeper melanophages within the dermis.
While many medications have been associated with fixed drug eruptions, the most common culprits include sulfonamides (with trimethoprim-sulfamethoxazole or co-trimoxazole as the most common cause of fixed drug eruption), tetracyclines, acetaminophen, barbiturates, and NSAIDs. The frequency of phenolphthalein-induced fixed drug eruptions has decreased since it is no longer used in the formulation of laxatives. Piroxicam, pseudoephedrine, and sorafenib have been reported to cause non-pigmenting variants that do not leave residual hyperpigmentation. Table 9.3 provides a list of medications associated with fixed drug eruptions (not exhaustive).
Table 9.3
Medications associated with fixed drug eruptions
Antimicrobials | Anticonvulsants | Cardiac | Miscellaneous |
TMP-SMXa | Phenytoin | Beta-blockers | Allopurinol |
Acyclovir | Barbiturates | Clopidogrel | Amide local anesthetics |
Amoxicillinb | Carbamazepine | Flecainide | Bismuth |
Ceftriaxone | Chlordiazepoxide | Heparin | Colchicine |
Clarithromycin | Lamotrigine | Hydrochlorothiazide | Cyclophosphamide |
Erythromycin | Anthistamines | Nifedipine | Dapsone |
Fluconazole
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|