Inflammation and the immune system are closely intertwined. Inflammation is composed of a complex web of responses to tissue injury and infection, characterized by the classic signs of rubor (redness), calor (heat), tumor (swelling), dolor (pain), and functio laesa (loss of function). The immune system includes the cells and soluble factors, such as antibodies and complement proteins, which mediate the inflammatory response; these cells and factors both eliminate the inciting inflammatory stimulus and initiate immunologic memory.
A normal inflammatory response is an acute process that resolves after removal of the inciting stimulus. Diseases of inflammation and immunity can occur due to inappropriate inflammation or when the normal inflammatory response progresses to chronic inflammation, either because of a long-term inappropriate response to a stimulus (e.g., allergies) or because the offending agent is not removed (e.g., chronic infection, transplantation, and autoimmunity).
Two pharmacologic strategies are used to target the pathophysiology of immune diseases. The first involves modification of the signaling mediators of the inflammatory process or suppression of components of the immune system. This is the rationale for drugs that modulate eicosanoid pathways (Chapter 43, Pharmacology of Eicosanoids), histamine (Chapter 44, Histamine Pharmacology), and cells of the immune system (Chapter 45, Pharmacology of Hematopoiesis and Immunomodulation, and Chapter 46, Pharmacology of Immunosuppression). This approach is still in its infancy, because it depends on understanding the molecular events in the relevant pathway, but it promises to yield multiple new drugs in the foreseeable future.
The second pharmacologic approach, used in diseases such as peptic ulcer disease (Chapter 47, Integrative Inflammation Pharmacology: Peptic Ulcer Disease), asthma (Chapter 48, Integrative Inflammation Pharmacology: Asthma), and gout (Chapter 49, Integrative Inflammation Pharmacology: Gout), involves modification of the underlying pathophysiologic stimulus, thus removing the impetus for inflammation. The difference between these two approaches is, at times, indistinct and will continue to overlap as the pathophysiology of chronic inflammatory disease is better understood at the molecular level.
This chapter provides sufficient background on the physiology of inflammation and the immune system to understand the subsequent chapters in this section of the textbook. The treatment is necessarily brief, with an emphasis on pharmacologically relevant targets of the inflammatory response. The chapter is organized in four parts. First, a general overview of the immune system is presented. Second, the molecular signals that mediate cellular communication and inflammation are introduced. Third, the immune and inflammatory cells and signaling molecules are discussed in the context of an integrated inflammatory response. Fourth, chronic inflammation, a pathologic state that is often associated with autoimmunity, is presented. For a more comprehensive presentation of this rapidly changing subject, see “Suggested Reading” at the end of this chapter.
 Mark is stressed—he has to take the US Medical Licensing Examination (USMLE) in 2 weeks, and he has barely begun to study. Throwing aside any pretense of a balanced lifestyle, Mark travels to the microbiology lab late one night to review techniques for performing a Gram stain. While applying the gentian violet component of the Gram stain, Mark cuts his thumb on the edge of the microscope slide. Thinking he lacks the time to clean his thumb properly, Mark continues to study furiously. Over the next 5 hours, Mark’s thumb becomes progressively swollen, warm, red, and tender. Mark retains focus and continues to study through the evening. By that night, however, he develops a fever and increased swelling in the thumb. By the third day, pus builds up at the site. By the fourth day, however, Mark’s body seems to have gotten the better of the offending agent. The swelling decreases, the site loses its distinctive angry red appearance, and his fever abruptly subsides. Relieved that he has not become a casualty of his own procrastination, Mark continues studying and performs well on his exam, not least because his wound has provided him with fundamental insights into immunology.
Mark is stressed—he has to take the US Medical Licensing Examination (USMLE) in 2 weeks, and he has barely begun to study. Throwing aside any pretense of a balanced lifestyle, Mark travels to the microbiology lab late one night to review techniques for performing a Gram stain. While applying the gentian violet component of the Gram stain, Mark cuts his thumb on the edge of the microscope slide. Thinking he lacks the time to clean his thumb properly, Mark continues to study furiously. Over the next 5 hours, Mark’s thumb becomes progressively swollen, warm, red, and tender. Mark retains focus and continues to study through the evening. By that night, however, he develops a fever and increased swelling in the thumb. By the third day, pus builds up at the site. By the fourth day, however, Mark’s body seems to have gotten the better of the offending agent. The swelling decreases, the site loses its distinctive angry red appearance, and his fever abruptly subsides. Relieved that he has not become a casualty of his own procrastination, Mark continues studying and performs well on his exam, not least because his wound has provided him with fundamental insights into immunology.
Questions
1. Which substance likely caused Mark’s immune system to activate in response to bacteria in his wound?
2. Which mediators accounted for Mark’s fever?
3. What changes in the vasculature accounted for the immediate swelling of Mark’s thumb?
4. Which chemical signals mediated the inflammatory response in Mark’s thumb?
The fundamental role of the immune system is to distinguish self from nonself. “Nonself” can be an infectious organism, a transplanted organ, or an endogenous tissue that is mistaken for something foreign. Because protection against infection is the classic role of the immune system, the terms infection and infectious agent are generally used to denote the inciting stimulus for an immune response. The immune system can be stimulated to react against any nonself agent.
Skin and other barrier tissues form the first line of defense against any infection. (In the introductory case, Mark’s infection occurred only after he cut his skin.) Once an offending agent penetrates these barriers, the immune system mounts a response. The immune response consists of innate and adaptive responses. Innate responses are stereotyped reactions to a stimulus (e.g., release of histamine, phagocytosis of a bacterium). In some cases, innate responses are sufficient to neutralize the offending agent. Cells of the innate immune system, especially antigen-presenting cells, can also process the offending agent into small fragments; this processing is necessary for activation of the adaptive immune system. Adaptive responses are neutralizing reactions that are specific to the offending agent (e.g., antibodies, cytotoxic T cells). In general, then, the innate immune system recognizes nonself and activates the response to an offending nonself agent; the adaptive immune system generates a response that specifically neutralizes or kills that agent.
Many different cell types are involved in the immune system, and these cell types interact in a complex web of signaling and communication to create the overall response. The cells of the immune system derive from two types of pluripotent cells in the bone marrow: myeloid stem cells and lymphoid stem cells. The lymphoid stem cell is sometimes called the common lymphoid stem cell because it gives rise to both B cells and T cells. In general, myeloid stem cells give rise to precursor cells of the innate immune system, whereas lymphoid stem cells generate precursor cells of the adaptive immune system; there are some exceptions. Figure 42-1 depicts the myeloid and lymphoid stem cells and the mature cell types into which the precursor cells differentiate. The derivation of these cell types is also discussed in Chapter 45. A helpful conceptual framework is to envision the innate immune system as the immunologic memory of a species, which is invariant over the lifetime of an individual and generally the same among individuals of the species. In contrast, the adaptive immune system establishes the immunologic memory of an individual over his or her lifetime, depending on that individual’s exposure to pathogens, vaccines, or other immunologic stimuli. Adaptive immunity, therefore, is relatively unique to each individual.
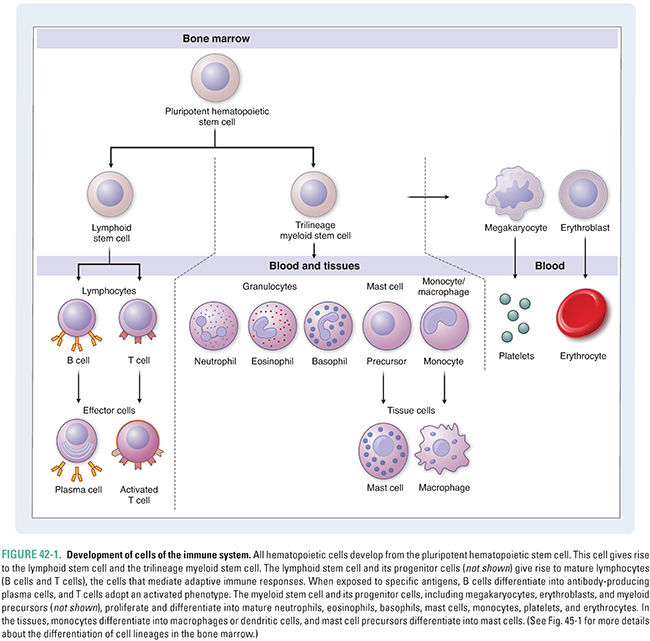
Cells of the innate immune system are the first responders to an offending agent that has penetrated the skin or another barrier (Table 42-1). Innate immune cells perform three important tasks. First, these cells defend against bacterial and parasitic infections, either by neutralizing the infectious agent with secreted cytotoxic proteins or by phagocytosis (engulfing) of the bacterium or parasite. Second, phagocytosis of the offending agent initiates proteolytic digestion of microbial macromolecules to fragments (antigens) that are then displayed, together with major histocompatibility complex (MHC) class II proteins, on the surface of antigen-presenting cells. In turn, these antigen-presenting cells, which include macrophages and dendritic cells, activate cells of the adaptive immune system. Third, innate immune cells secrete numerous cytokines (see below) that further amplify the immune response. The major cell types of the innate immune system include granulocytes (neutrophils, eosinophils, and basophils), mast cells, and antigen-presenting cells (macrophages and dendritic cells). Some immunologists consider natural killer (NK) cells, NK T cells, and γδ T cells to have innate immune roles; the biology of these cell types is beyond the scope of this text.
Granulocyte is a descriptive term based on the appearance of the cytoplasmic granules within these cells. Neutrophils, the most abundant cell type of the innate immune system and the “first responders” in inflammation, are phagocytic cells primarily responsible for defense against bacterial infection. These cells envelop invading bacteria in phagocytic vesicles and destroy the bacteria within these vesicles using enzymes such as myeloperoxidase. Eosinophils are circulating granulocytes primarily involved in defense against parasitic infections. Because parasites are often too large to engulf, eosinophils attach to a parasite’s exterior and secrete cytotoxic substances directly on the parasite. Both basophils (circulating) and mast cells (tissue-resident) bind IgE antibody, display this IgE on the cell surface, and maintain histamine-containing granules that are released when exogenous antigen binds to and cross-links the IgE. Basophils and mast cells are important in allergic responses. Eosinophils and basophils are so named because they exhibit eosinophilic and basophilic patterns, respectively, when stained with Wright-Giemsa stain.
Antigen-presenting cells (APCs) process the macromolecules (especially proteins) of an invading agent to display the processed fragments on the surface of the APC. In this form, the fragments serve as molecular fingerprints used by cells of the adaptive immune system to recognize the invading agent. APCs are important initiators of immune responses because, in addition to displaying nonself antigens to T cells (see below), they provide the costimulatory signals that are necessary for T-cell activation. The concept of costimulation, in which two separate signals are required to initiate an immune response to a stimulus, is discussed below.
Monocytes that exit the bloodstream and take up residence in the tissues can differentiate into macrophages. As “professional APCs,” macrophages process and present antigenic fragments of an invading pathogen for recognition by T cells. The ability of macrophages to envelop and destroy pathogens is enhanced by other components of the immune system, including antibodies and complement (which mediate opsonization) and cytokines (which enhance killing ability). In addition, macrophages produce cytokines such as TNF-α that modify immune responses. Dendritic cells are the most important APCs for the initiation of adaptive immune responses. In the nonlymphoid tissues, dendritic cells engulf and process foreign antigens. Dendritic cells then migrate to lymphoid tissues, where they present these cognate antigens to T cells via specific molecular interactions.
Activation of the Innate Immune Response
Innate immune cells respond to common determinants that are present on many invading agents (for example, lipopolysaccharide [LPS] in the outer membrane of Gram-negative bacteria). In this role, innate immune cells use pattern recognition to phagocytose a class of infectious agents rather than a specific infectious agent. In contrast, adaptive immune cells, as discussed below, mount a specific response to the three-dimensional conformation of a particular antigen, referred to as an epitope. From a teleological perspective, innate immunity provides a broad gating function, attempting to counteract harmful effects of foreign invaders in a rapid manner and to determine whether an infectious agent should be further attacked by adaptive immunity, while adaptive immunity provides a specialized response that is specific to the particular invading infectious agent. In an individual, innate immune cells respond in the same way and to the same extent to repeated infections with the same agent. In contrast, adaptive immune cells mount a faster and more intense response upon reexposure to the infectious agent.
The pattern recognition function of innate immune cells is mediated by multiple mechanisms. One important mechanism involves Toll-like receptors (TLRs). TLRs are transmembrane proteins that bind to shared microbial components such as LPS expressed by Gram-negative bacteria, mannans expressed by fungi, and double-stranded RNA expressed by viral pathogens. Ten TLRs are expressed in humans, and each has a characteristic immune cell distribution and set of pathogen-associated ligands. For example, TLR4 is expressed by antigen-presenting cells and it binds to LPS. Binding of TLRs to their ligands activates an intracellular signaling cascade that converges on the expression of proinflammatory cytokines, leading to further immune cell recruitment and activation of the inflammatory response. A fundamental role of innate immunity is to provide an immediate “alarm” that recruits elements of adaptive immunity. This alarm indicates that a molecular structure associated with a pathogen has been detected and serves as an early warning system that the adaptive immune system should initiate an immune response against the pathogen-associated antigens encountered in the context of a TLR agonist or another innate immune signal. Several pharmacologic agents are being investigated as modulators of TLR signaling. Imiquimod, discussed in Chapter 38, Pharmacology of Viral Infections, may function as a TLR agonist.
The main features of the adaptive immune system, specificity to foreign antigens and tolerance to self-antigens, rely on two principles. First, there must be a mechanism to generate a specific response to a foreign antigen. Second, adaptive immune cells must be able to distinguish native (self) cells and soluble factors from foreign (nonself) cells and soluble factors. The first property is provided by major histocompatibility complex (MHC) proteins and by somatic gene recombination in T cells and B cells, whereas the second property is provided by signals from the innate immune system, by regulated immune cell development, and by costimulation.
Major Histocompatibility Complex
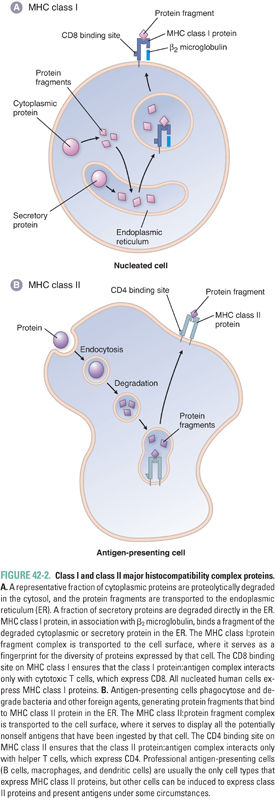
MHC proteins are transmembrane proteins that bind and display on their surface proteolytically degraded protein fragments and, in some cases, glycolipid antigens. There are two classes of MHC proteins: MHC class I and MHC class II. MHC class I proteins primarily display fragments of cytosolic proteins (Fig. 42-2). All nucleated cells express MHC class I proteins; the repertoire of protein fragments displayed by MHC class I proteins on a cell provides a fingerprint for all the proteins expressed within that cell. If a cell is expressing a recognizable pattern of proteins, then it will not be attacked by the immune system. However, if foreign (e.g., viral) proteins are being generated within the cell, then proteolytic fragments of those viral proteins will be displayed on MHC class I proteins at the surface of the cell, and the immune system will recognize that cell as virally infected. Antigens presented by MHC class I proteins are recognized by T cells bearing the cell surface protein CD8. (The designation “CD” stands for cluster of differentiation or cluster designation and is a system for naming an ever-growing list of cell-associated antigens. Each antigen must be defined by at least two different monoclonal antibodies in order to earn the CD designation. CD antigens now number in the hundreds and are present on leukocytes and other cell types.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



