INTRODUCTION
This chapter introduces the neurotransmitter serotonin (5-hydroxytryptamine; 5-HT), which is a target for many drugs used to treat psychiatric disorders related to depression and anxiety. Some of these medications also affect norepinephrine (NE) neurotransmission, and both neurotransmitter pathways are believed to be central to the modulation of mood. The various mechanisms by which drugs can alter serotonin and norepinephrine signaling are discussed. Although many such drugs function as antidepressants or anti-anxiety medications, interventions in this pharmacologic group are also effective treatments for migraine headache, irritable bowel syndrome, and other conditions. Lithium and some other drugs used to treat bipolar disorder are also discussed.
The major mood disorders are defined by the presence of depressive and/or manic or hypomanic episodes. Patients with recurrent depressive episodes and no history of mania or hypomania are said to have major depressive disorder (MDD); patients who have experienced at least one manic or hypomanic episode, with or without an additional history of depressive episodes, are said to have bipolar disorder (BD). The lifetime prevalence of MDD is approximately 17%, whereas that of BD is 1% to 2%. MDD can occur as an isolated illness or can be comorbid with other diseases such as stroke, dementia, diabetes, cancer, and coronary artery disease. Although twin studies suggest that up to 1/3 of the risk for MDD is heritable, environmental stress such as early traumatic experiences are also associated with risk. Aging and cerebral atherosclerosis are also associated with late-onset depression in the elderly. In addition to genetic and environmental triggers, many classes of drugs can precipitate or exacerbate depressive episodes (e.g., interferon, glucocorticoids, and chemotherapeutic agents). BD has a particularly strong heritable risk, even though environmental factors are often triggers for the mood episodes themselves. Although mania is a characteristic of BD, patients spend significant periods of their lives depressed, and depressive symptoms are strongly associated with the elevated risk for suicide among mood disorders. (Of note, in the majority of suicides, a physician [not necessarily a psychiatrist] will have seen the patient less than 1 month before the suicide.)
Both MDD and BD are major causes of morbidity worldwide, resulting in lost productivity and substantial use of medical resources. The World Health Organization (WHO) projects that a major depression will be the leading cause of disease burden by the year 2030, ahead of ischemic heart disease, road traffic incidents, and cerebrovascular disease.
 Mary R is a 27-year-old office worker who presents to her primary care physician, Dr. Lee, with an 8-lb. weight loss over the previous 2 months. Ms. R tearfully explains that she is plagued by near-constant feelings of sadness and by a sense of helplessness and inadequacy at work. She feels so terrible that she has not had a good night of sleep in more than a month. She no longer enjoys living and has recently become scared when new thoughts of suicide enter her mind. Ms. R tells Dr. Lee that she had felt like this once before, but it had passed after several months. Dr. Lee asks her about her sleep patterns, appetite levels, ability to concentrate, energy level, mood, interest level, and feelings of guilt. He asks her specific questions about thoughts of suicide, particularly whether she has formed a specific plan and whether she has ever attempted suicide. Dr. Lee explains to Ms. R that she has major depressive disorder, likely caused by specific abnormalities in the function of her brain circuitry, and he prescribes the antidepressant fluoxetine.
Mary R is a 27-year-old office worker who presents to her primary care physician, Dr. Lee, with an 8-lb. weight loss over the previous 2 months. Ms. R tearfully explains that she is plagued by near-constant feelings of sadness and by a sense of helplessness and inadequacy at work. She feels so terrible that she has not had a good night of sleep in more than a month. She no longer enjoys living and has recently become scared when new thoughts of suicide enter her mind. Ms. R tells Dr. Lee that she had felt like this once before, but it had passed after several months. Dr. Lee asks her about her sleep patterns, appetite levels, ability to concentrate, energy level, mood, interest level, and feelings of guilt. He asks her specific questions about thoughts of suicide, particularly whether she has formed a specific plan and whether she has ever attempted suicide. Dr. Lee explains to Ms. R that she has major depressive disorder, likely caused by specific abnormalities in the function of her brain circuitry, and he prescribes the antidepressant fluoxetine.
Two weeks later, Ms. R calls to indicate that the medicine is not working. Dr. Lee encourages her to continue taking the medicine, and after 2 more weeks, Ms. R begins to feel better. She no longer feels sad and demoralized; the feelings of helplessness and inadequacy that previously plagued her have diminished. In fact, when she returns to see Dr. Lee 6 weeks later, she reports feeling much better. She no longer needs much sleep and is always full of energy. She is now convinced that she is the most intelligent person in her company. She proudly tells Dr. Lee that she has recently purchased a new sports car and gone on a large shopping spree. After taking a more detailed history, Dr. Lee tells Ms. R that she may be having a manic episode and, in consultation with a psychiatrist, prescribes lithium and gradually tapers the fluoxetine. Ms. R is hesitant to take the new medication, arguing that she feels fine and that she is concerned about the adverse effects of lithium.
Questions
1. How is a depressive episode different from occasionally “feeling blue”?
2. What caused Ms. R’s mania? Why is it necessary to treat bipolar disorder if the patient “feels good”?
3. Why is there a delay in the onset of fluoxetine’s therapeutic effect?
4. What specific concerns might Ms. R have about the adverse effects of lithium?
 BIOCHEMISTRY AND PHYSIOLOGY OF SEROTONERGIC AND CENTRAL ADRENERGIC NEUROTRANSMISSION
BIOCHEMISTRY AND PHYSIOLOGY OF SEROTONERGIC AND CENTRAL ADRENERGIC NEUROTRANSMISSION
Serotonin (5-hydroxytryptamine; 5-HT) and norepinephrine (NE) have critical roles in modulating mood, the sleep–wake cycle, motivation and reward, cognitive processing, pain perception, neuroendocrine function, and other physiologic processes. Serotonergic projections to the spinal cord modulate pain perception, visceral regulation, and motor control, while projections to the forebrain are important in modulating mood, cognition, and neuroendocrine function. The noradrenergic system modulates vigilance, stress responses, neuroendocrine function, pain control, and sympathetic nervous system activity. The wide variety of behavioral and psychological processes regulated by these two neurotransmitters explains the similarly wide variety of disorders that can be treated by medications that alter the levels or postsynaptic signaling of 5-HT and/or NE.
5-HT and NE are primarily released from nonsynaptic neuronal varicosities. Unlike synapses, which form tight contacts with specific target neurons, varicosities release large amounts of neurotransmitter from vesicles into the extracellular space, establishing concentration gradients of neurotransmitter in the projection areas of the varicosities. 5-HT-containing cells within the raphe nuclei and NE-containing cells within the locus ceruleus project broadly throughout the cerebral cortex, while dopamine has a more focused pattern of projections. Each of these systems has prominent presynaptic autoreceptors that control local transmitter concentrations. This autoregulation results in coordinated firing, which causes spontaneous and synchronous waves of activity that can be measured as firing frequencies; for example, the cells within the raphe nuclei usually fire at rates between 0.3 and 7 spikes per second. Because the frequency of basal (tonic) firing does not change rapidly and the quanta of neurotransmitter released with each discharge are fairly well conserved, the neurotransmitter concentration in the vicinity of the varicosities is maintained within a narrow range.
The mean concentration establishes the baseline tone of activity in the target neurons that receive 5-HT and NE projections. In addition, specific stimuli can elicit rapid bursts of firing that are superimposed on the baseline tonic activity. Diffusely projecting systems can thus provide two types of information: a rapid and discrete neuronal firing akin to more traditional neurotransmission and a slower tonic firing that presumably allows for integration of information over a longer period of time.
Serotonin Synthesis and Regulation
Serotonin is synthesized from the amino acid tryptophan by the enzyme tryptophan hydroxylase (TPH), which converts tryptophan to 5-hydroxytryptophan. Aromatic L-amino acid decarboxylase then converts 5-hydroxytryptophan to serotonin (Fig. 15-1A). These enzymes are present throughout the cytoplasm of serotonergic neurons, both in the cell body and in cell processes. Serotonin is concentrated and stored within vesicles located in axons, cell bodies, and dendrites.

The biochemistry of norepinephrine synthesis and regulation is discussed in Chapter 11, Adrenergic Pharmacology. For review, the synthesis of norepinephrine is summarized in Figure 15-1B.
The serotonin metabolic cycle (Fig. 15-2) involves synthesis, uptake into synaptic vesicles, exocytosis, reuptake into the cytoplasm, and then either uptake into vesicles or degradation. The metabolic cycle of norepinephrine is summarized in Figure 15-3. Importantly, regulation of the levels of 5-HT and NE neurotransmission can occur at any of these steps.
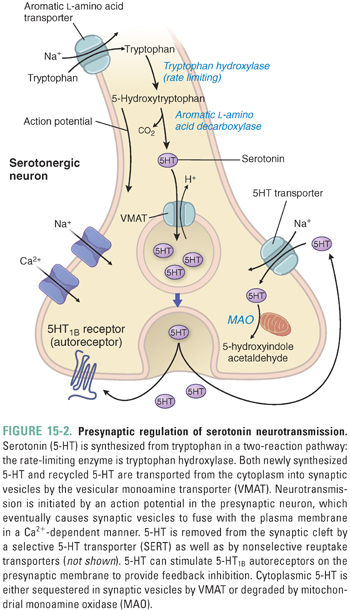

For all monoamines, the first synthetic step is rate-limiting. Thus, 5-HT synthesis is rate-limited by tryptophan hydroxylase (TPH), and DA and NE synthesis is rate-limited by tyrosine hydroxylase (TH). Both enzymes are tightly regulated by inhibitory feedback via autoreceptor-mediated signaling. 5-HT presynaptic autoreceptors respond to locally increased 5-HT concentrations by Gi protein signaling, which decreases TPH activity and serotonergic neuron firing. Although other explanations exist, this autoregulatory loop could be one explanation for the observed time course of clinical action of antidepressants, which is discussed below (see “The Monoamine Theory of Depression”).
5-HT is transported into vesicles by the vesicular monoamine transporter (VMAT). The transporter is a nonspecific monoamine transporter that is important for the vesicular packaging of dopamine (DA) and epinephrine (EPI) as well as 5-HT. Reserpine, an indole alkaloid historically used to treat hypertension and certain psychiatric symptoms, binds irreversibly to VMAT and thereby inhibits the packaging of DA, NE, EPI, and 5-HT into vesicles.
Selective serotonin reuptake transporters recycle 5-HT from the extracellular space back into the presynaptic neuron. Selective monoamine reuptake transporters are 12-transmembrane-spanning proteins that couple neurotransmitter transport to the transmembrane sodium gradient. Unlike VMAT, which is a nonspecific monoamine transporter, the individual monoamine reuptake transporters show selectivity, high affinity, and low capacity for each individual monoamine. The selective monoamine transporters, which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), are also capable of transporting the other monoamines, although less efficiently.
Once 5-HT is returned to the neuronal cytoplasm, the neurotransmitter is transported into vesicles via VMAT or degraded by the monoamine oxidase (MAO) system. MAOs are mitochondrial enzymes that regulate the levels of monoamines in neural tissues and inactivate circulating and dietary monoamines (such as tyramine) in the liver and gut. The two isoforms, MAO-A and MAO-B, differ according to substrate specificity: MAO-A oxidizes 5-HT, NE, and DA, and MAO-B preferentially oxidizes DA. Monoamine oxidases inactivate monoamines by oxidative deamination, using a covalently attached flavin adenine dinucleotide (FAD) cofactor as an electron acceptor. Catechol-O-methyltransferase (COMT) in the extracellular space is another important degradation enzyme for monoamines.
Fifteen 5-HT receptors have been characterized, and all but one are G protein-coupled (Table 15-1). In general, the 5-HT1 class of receptors inhibits cellular activity via the Gi pathway (thus decreasing adenylyl cyclase activity and opening K+ channels), the 5-HT2 class increases signaling through the Gq pathway to cause phosphatidylinositol turnover, and the 5-HT4, 5-HT6, and 5-HT7 classes signal through the Gs pathway to stimulate adenylyl cyclase. The only known ligand-gated ion channel is the 5-HT3 receptor. 5-HT1A receptors are expressed both on serotonergic cell bodies in the raphe nuclei (autoreceptors) and on postsynaptic neurons in the hippocampus and act to hyperpolarize neurons via the Gi pathway (as described above). Presynaptic 5-HT1B receptors are expressed on serotonergic nerve terminals, where they autoinhibit 5-HT neurotransmission. 5-HT2A and 5-HT2C signaling is excitatory and lowers the threshold for neuronal firing.

The various serotonin receptors are expressed differentially throughout the brain and are differentially innervated by raphe projections. For example, a subset of 5-HT projections to the cortex stimulates postsynaptic 5-HT2A receptors, while other projections to the limbic system stimulate postsynaptic 5-HT1A receptors. There is considerable overlap of receptor subtype expression, however, and the physiologic significance of this overlap is unclear.
The signaling mechanisms of norepinephrine (adrenergic) receptor subtypes are discussed in Chapter 11 and reviewed in Table 15-1.
 PATHOPHYSIOLOGY OF AFFECTIVE DISORDERS
PATHOPHYSIOLOGY OF AFFECTIVE DISORDERS
Major depressive disorder (MDD) and bipolar disorder (BD) are characterized by mood dysregulation. MDD is typified by single or recurrent depressive episodes, whereas BD is defined by the presence of mania or hypomania as well as periods of depression.
The monoamine hypothesis proposes that decreased serotonin and/or norepinephrine levels cause mood disorders, based largely on the molecular mechanism of action of known antidepressants as well as animal models suggested to correspond to depression or mania. More current research suggests that these disorders reflect complex disturbances in neural circuit activity rather than a simple chemical imbalance. However, because the underlying etiologies of these disorders are still not well understood at a physiologic or molecular level, diagnostic criteria rely solely on clinical evaluation. To date, despite intriguing findings from neuroimaging and transcriptomic studies, no reliable biomarkers for these disorders have been identified. The American Psychiatric Association diagnostic criteria for MDD and BD are summarized in Boxes 15-1 and 15-2.
BOX 15-1 Criteria for Major Depressive Disorder (MDD), abbreviated from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) |
A. Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous functioning; at least one of the symptoms is either (1) depressed mood or (2) loss of interest or pleasure. 1. Depressed mood most of the day, nearly every day, as indicated by either subjective report (e.g., feels sad, empty, hopeless) or observation made by others (e.g., appears tearful). 2. Markedly diminished interest or pleasure in all, or almost all, activities most of the day, nearly every day (as indicated by either subjective account or observation). 3. Significant weight loss when not dieting or weight gain (e.g., a change of more than 5% of body weight in a month), or a decrease or increase in appetite nearly every day. 4. Insomnia or hypersomnia nearly every day. 5. Psychomotor agitation or retardation nearly every day (observable by others, not merely subjective feelings of restlessness or being slowed down). 6. Fatigue or loss of energy nearly every day. 7. Feelings of worthlessness or excessive or inappropriate guilt (which may be delusional) nearly every day (not merely self-reproach or guilt about being sick). 8. Diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others). 9. Recurrent thoughts of death (not just fear of dying), recurrent suicidal ideation without a specific plan, or a suicide attempt or a specific plan for committing suicide. B. The symptoms cause clinically significant distress or impairment in social, occupational, or other important area of functioning. C. The episode is not attributable to the physiological effects of a substance or to another medical condition. D. The occurrence of the major depressive episode is not better explained by schizoaffective disorder, schizophrenia, schizophreniform disorder, delusional disorder, or other specified and unspecified schizophrenia spectrum and other psychotic disorders. E. There has never been a manic episode or a hypomanic episode. Reprinted with permission from the American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. |
BOX 15-2 Criteria for Bipolar Disorder (BD), abbreviated from the Diagnostic andStatistical Manual of Mental Disorders, Fifth Edition (DSM-5) |
BIPOLAR I DISORDER For a diagnosis of bipolar I disorder, it is necessary to meet the following criteria for a manic episode. The manic episode may have been preceded by and may be followed by hypomania or major depressive episodes. Manic Episode: A. A distinct period of abnormally and persistently elevated, expansive, or irritable mood and abnormality and persistently increased goal-directed activity or energy, lasting at least 1 week and present most of the day, nearly every day (or any duration of hospitalization is necessary). B. During the period of mood disturbance and increased energy or activity, three (or more) of the following symptoms (four if the mood is only irritable) are present to a significant degree and represent a noticeable change from usual behavior: 1. Inflated self-esteem or grandiosity. 2. Decreased need for sleep (e.g., feels rested after only 3 hours of sleep). 3. More talkative than usual or pressure to keep talking. 4. Flight of ideas or subjective experience that thoughts are racing. 5. Distractibility (i.e., attention too easily drawn to unimportant or irrelevant external stimuli), as reported or observed. 6. Increase in goal-directed activity (either socially, at work or school, or sexually) or psychomotor agitation (i.e., purposeless non-goal-directed activity). 7. Excessive involvement in activities that have a high potential for painful consequences (e.g., engaging in unrestrained buying sprees, sexual indiscretion, or foolish business investments). C. The mood disturbance is sufficiently severe to cause marked impairment in social or occupational functioning or to necessitate hospitalization to prevent harm to self or others, or there are psychotic features. D. The episode is not attributable to the psychological effects of a substance (e.g., a drug of abuse, a medication, other treatment) or to another medical condition. BIPOLAR II DISORDER For a diagnosis of bipolar II disorder, it is necessary to meet the following criteria for a current or past hypomanic episode and the following criteria for a current or past depressive episode. Hypomanic Episode: A. A distinct period of abnormally and persistently elevated, expansive, or irritable mood and abnormality and persistently increased goal-directed activity or energy, lasting at least 4 consecutive days and present most of the day, nearly every day. B. During the period of mood disturbance and increased energy or activity, three (or more) of the following symptoms (four if the mood is only irritable) represent a noticeable change from usual behavior and have been present to a significant degree: 1. Inflated self-esteem or grandiosity. 2. Decreased need for sleep (e.g., feels rested after only 3 hours of sleep). 3. More talkative than usual or pressure to keep talking. 4. Flight of ideas or subjective experience that thoughts are racing. 5. Distractibility (i.e., attention too easily drawn to unimportant or irrelevant external stimuli), as reported or observed. 6. Increase in goal-directed activity (either socially, at work or school, or sexually) or psychomotor agitation. 7. Excessive involvement in activities that have a high potential for painful consequences (e.g., engaging in unrestrained buying sprees, sexual indiscretion, or foolish business investments). C. The episode is associated with an unequivocal change in functioning that is uncharacteristic of the individual when not symptomatic. D. The disturbance in mood and the change in functioning are observable by others. E. The episode is not severe enough to cause marked impairment in social or occupational functioning or to necessitate hospitalization. If there are psychotic features, the episode is, by definition, manic. F. The episode is not attributable to the psychological effects of a substance (e.g., a drug of abuse, a medication, other treatment). Reprinted with permission from the American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. |
Clinical Characteristics of Affective Disorders
Major depressive disorder (MDD) is characterized by single or recurrent episodes of depressed mood, social isolation (including apathy, decreased ability to experience pleasure, and feelings of worthlessness), and characteristic somatic symptoms (decreased energy, changes in appetite and sleep, muscle pain, and slowing of movement with speech latency). Episodes are sometimes precipitated by major life events or stresses, although they may also occur spontaneously. A single depressive episode must last 2 weeks or longer and must interfere significantly with the patient’s daily functions, such as work and personal relationships. An episode is not considered to be MDD if it is due to a general medical condition such as hypothyroidism or Cushing’s disease.
In all depressed patients, it is crucial to determine whether there is any suicidality and whether there is evidence of psychosis. Although psychosis is more typical of BD, severely depressed patients may become psychotic, and either suicidality or psychosis is an indication for prompt psychiatric evaluation in a secure setting.
Psychotic depression is among the most severe and disabling forms of MDD. SSRIs and antipsychotics are considered first-line agents for this subtype of depression, but patients may require electroconvulsant therapy if the symptoms are refractory to first-line agents.
A manic episode is associated with irritable, elevated, or euphoric mood, as well as increased overall activity. Associated symptoms often include an inflated sense of self-worth (termed grandiosity) and distractibility. Rather than speech latency and soft speech, as seen in depression, there is increased, rapid, and loud speech that is often difficult to interrupt. Rather than the sense of fatigue and need for sleep seen in depression, there is often decreased need for sleep. At the extreme, patients may not sleep at all, and rather than feeling tired, they feel energized. Manic episodes are also characterized by disorganized, racing thoughts, often to point where patients cannot stay on topic for more than a few seconds. While not core features of mania, these episodes may be associated with psychosis (delusions or hallucinations). Mania is associated with high risk for adverse outcomes (e.g., traffic accident, arrest, or psychiatric hospitalization), particularly in the absence of treatment. When some symptoms of a manic episode and a depressive episode are present simultaneously, the depressive or manic episode is said to have “mixed features.”
If a patient has manic symptoms for at least 4 days without such an adverse outcome, and without causing significant distress to the patient, it is then by definition a hypomanic episode (literally, a “little mania”). In the introductory case, there is insufficient detail to determine whether Ms. R has experienced significant adverse consequences yet. If Dr. Lee had not intervened, her symptoms might be expected to worsen and her risk for such consequences would increase.
Although BD is characterized by manic symptoms (either mania or hypomania), the disorder is also notable for depression, which may be prolonged and debilitating. The depressive episodes may occur before any mania is experienced, and these patients are often mistakenly diagnosed with MDD. Patients with BD sometimes experience rapid “switches” into mania when taking antidepressants (as in the case of Ms. R), or more frequent mood episodes referred to as rapid cycling. The drug classes used to treat BD are discussed at the end of the pharmacology section and in the past were referred to as mood stabilizers. More recently, such drugs may be described in terms of their relative antidepressant or antimanic properties, or their ability to prevent such episodes. In many patients with BD, combinations of medications are required to achieve adequate control of mood symptoms and recurrences.
The Monoamine Theory of Depression
The biological basis for depression began to be understood in the 1940s and 1950s, when keen observers noticed that imipramine, iproniazid, and reserpine had unexpected effects on mood.
In the late 1940s, the tricyclic drug imipramine was developed for use in the treatment of psychotic patients, but it was subsequently noted to have strong antidepressant effects. Imipramine preferentially blocks the 5-HT transporter (SERT), and its active metabolite desipramine preferentially blocks the NE transporter (NET). By these mechanisms, imipramine allows 5-HT and NE to persist in the extracellular space at higher concentrations and for longer durations, yielding increased activation of 5-HT and NE receptors.
In 1951, the antituberculosis drug iproniazid was shown to have antidepressant effects. Iproniazid inhibits monoamine oxidase (MAO) and thereby prevents the degradation of 5-HT, NE, and DA. The resulting increase in cytosolic neurotransmitter leads to increased neurotransmitter uptake into vesicles and, consequently, to greater release of neurotransmitter after exocytosis.
In the 1950s, the antihypertensive agent reserpine was noted to induce depression in 10–15% of patients. Researchers then found that reserpine could induce depressive symptoms in animal models as well as in humans. Reserpine depletes 5-HT, NE, and DA in presynaptic neurons by inhibiting the transport of these neurotransmitters into synaptic vesicles. The drug binds irreversibly to VMAT and ultimately destroys the vesicles. The 5-HT, NE, and DA that accumulate in the cytoplasm are degraded by mitochondrial MAO. The resulting decrease in monoamine neurotransmission is thought to be responsible for inducing a depressed mood.
The findings described above strongly suggested that the central monoaminergic serotonin and norepinephrine systems are involved in the pathogenesis of depression. The monoamine theory of depression holds that depression results from pathologically decreased serotonin and/or norepinephrine neurotransmission. Based on this hypothesis, it follows that increasing serotonin and/or norepinephrine neurotransmission could ameliorate or reverse depression. As a biological disease related to long-term pathologic alterations in monoamine activity, MDD should thus be treatable by medications.
Limitations of the Monoamine Theory
Although nearly all of the antidepressants are pharmacologically active at their molecular and cellular sites of action almost immediately, their full antidepressant effects are generally not seen until the drugs have been administered for 6 or more weeks of continuous treatment. Similarly, although reserpine rapidly depletes neurotransmitter in monoaminergic systems, it takes several weeks of continuous treatment with reserpine to induce depression. The unexplained delay in the onset of full effect of these drugs remains a central conundrum and strong challenge to the monoamine theory.
In some patients, drugs that selectively increase 5-HT neurotransmission decrease depressive symptoms, while drugs that selectively increase NE neurotransmission have little or no effect. In other patients, drugs affecting the NE system are more beneficial than those affecting the 5-HT system. Overall, each individual drug is effective in about 70% of patients with depression, and drugs that have markedly different efficacies in blocking the reuptake of NE and/or 5-HT may have similar clinical effectiveness when tested in large populations. These clinical observations are not easily explained by the monoamine theory.
The time lag in the clinical effectiveness of antidepressants may reflect autoregulatory mechanisms in presynaptic monoaminergic neurons and/or in postsynaptic neural circuitry. Acute treatment with antidepressants actually produces a decrease in neuronal firing in the locus ceruleus and/or raphe nucleus (depending on the drug), due to acute feedback inhibition via 5-HT1A and α2 autoreceptors on 5-HT- and NE-containing neurons, respectively. This causes a concomitant, acute decrease in the synthesis and release of 5-HT and NE.
In contrast, chronic use of antidepressants causes the inhibitory autoreceptors themselves to be down-regulated, leading to enhancement of neurotransmission. The change in autoreceptor sensitivity takes several weeks to occur, consistent with the time-course of the therapeutic response in patients. This could explain the lag in full therapeutic response; only after chronic antidepressant therapy does the gradual desensitization of autoreceptors allow increased neurotransmission (Fig. 15-4). Although speculative, this hypothesis regarding changes in monoamine receptor sensitivity offers an explanation for the delay in onset of the therapeutic action of fluoxetine experienced by Ms. R.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




