41
Pancreatic Hormones & Antidiabetic Drugs
CASE STUDY
A 56-year-old Hispanic woman presents to her medical practitioner with symptoms of fatigue, increased thirst, frequent urination, and exercise intolerance with shortness of breath of many months’ duration. She does not get regular medical care and is unaware of any medical problems. Her family history is significant for obesity, diabetes, high blood pressure, and coronary artery disease in both parents and several siblings. She is not taking any medications. Five of her six children had a birthweight of over 9 pounds. Physical examination reveals a BMI (body mass index) of 34, blood pressure of 150/90 mm Hg, and evidence of mild peripheral neuropathy. Laboratory tests reveal a random blood sugar of 261 mg/dL; this is confirmed with a fasting plasma glucose of 192 mg/dL. A fasting lipid panel reveals total cholesterol 264 mg/dL, triglycerides 255 mg/dL, high-density lipoproteins 43 mg/dL, and low-density lipoproteins 170 mg/dL. What type of diabetes does this woman have? What further evaluations should be obtained? How would you treat her diabetes?
 THE ENDOCRINE PANCREAS
THE ENDOCRINE PANCREAS
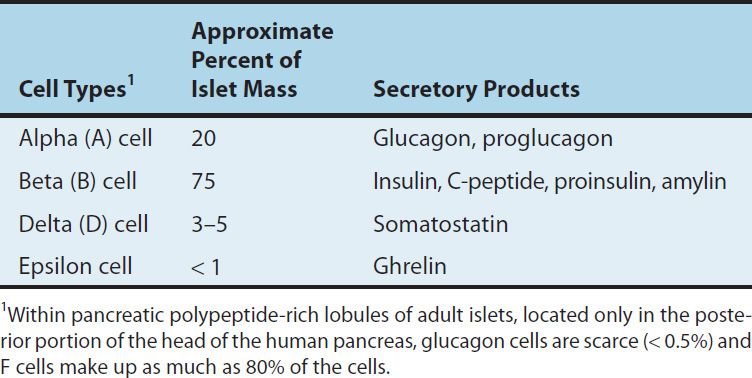
The endocrine pancreas in the adult human consists of approximately 1 million islets of Langerhans interspersed throughout the pancreatic gland. Within the islets, at least five hormone-producing cells are present (Table 41–1). Their hormone products include insulin, the storage and anabolic hormone of the body; islet amyloid polypeptide (IAPP, or amylin), which modulates appetite, gastric emptying, and glucagon and insulin secretion; glucagon, the hyperglycemic factor that mobilizes glycogen stores; somatostatin, a universal inhibitor of secretory cells; pancreatic peptide, a small protein that facilitates digestive processes by a mechanism not yet clarified; and ghrelin, a peptide known to increase pituitary growth hormone release.
TABLE 41–1 Pancreatic islet cells and their secretory products.
Diabetes mellitus is defined as an elevated blood glucose associated with absent or inadequate pancreatic insulin secretion, with or without concurrent impairment of insulin action. The disease states underlying the diagnosis of diabetes mellitus are now classified into four categories: type 1, type 2, other, and gestational diabetes mellitus.
Type 1 Diabetes Mellitus
The hallmark of type 1 diabetes is selective beta cell (B cell) destruction and severe or absolute insulin deficiency. Type 1 diabetes is further subdivided into immune-mediated (type 1a) and idiopathic causes (type 1b). The immune form is the most common form of type 1 diabetes. Although most patients are younger than 30 years of age at the time of diagnosis, the onset can occur at any age. Type 1 diabetes is found in all ethnic groups, but the highest incidence is in people from northern Europe and from Sardinia. Susceptibility appears to involve a multifactorial genetic linkage, but only 10–15% of patients have a positive family history. Most patients with type 1 diabetes have one or more circulating antibodies to glutamic acid decarboxylase 65 (GAD 65), insulin autoantibody, tyrosine phosphatase IA2 (ICA 512), and zinc transporter 8 (ZnT8) at the time of diagnosis. These antibodies facilitate the diagnosis of type 1a diabetes and can also be used to screen family members at risk for developing the disease.
For persons with type 1 diabetes, insulin replacement therapy is necessary to sustain life. Pharmacologic insulin is administered by injection into the subcutaneous tissue using a manual injection device or an insulin pump that continuously infuses insulin under the skin. Interruption of the insulin replacement therapy can be life-threatening and can result in diabetic ketoacidosis or death. Diabetic ketoacidosis is caused by insufficient or absent insulin and results from excess release of fatty acids and subsequent formation of toxic levels of ketoacids.
Some patients with type 1 diabetes have a more indolent autoimmune process and initially retain enough beta cell function to avoid ketosis. They can be treated at first with oral hypoglycemic agents but then need insulin as their beta cell function declines. Antibody studies in northern Europeans indicate that up to 10–15% of “type 2” patients may actually have this milder form of type 1 diabetes (latent autoimmune diabetes of adulthood; LADA).
Type 2 Diabetes Mellitus
Type 2 diabetes is characterized by tissue resistance to the action of insulin combined with a relative deficiency in insulin secretion. A given individual may have more resistance or more beta-cell deficiency, and the abnormalities may be mild or severe. Although insulin is produced by the beta cells in these patients, it is inadequate to overcome the resistance, and the blood glucose rises. The impaired insulin action also affects fat metabolism, resulting in increased free fatty acid flux and triglyceride levels and reciprocally low levels of high-density lipoprotein (HDL).
Individuals with type 2 diabetes may not require insulin to survive, but 30% or more will benefit from insulin therapy to control blood glucose. Although persons with type 2 diabetes ordinarily do not develop ketosis, ketoacidosis may occur as the result of stress such as infection or the use of medication that enhances resistance, eg, corticosteroids. Dehydration in individuals with untreated or poorly controlled type 2 diabetes can lead to a life-threatening condition called nonketotic hyperosmolar coma. In this condition, the blood glucose may rise to 6–20 times the normal range and an altered mental state develops or the person loses consciousness. Urgent medical care and rehydration are required.
Other Specific Types of Diabetes Mellitus
The “other” designation refers to multiple other specific causes of an elevated blood glucose: pancreatectomy, pancreatitis, nonpan-creatic diseases, drug therapy, etc. For a detailed list the reader is referred to the reference Expert Committee, 2003.
Gestational Diabetes Mellitus
Gestational diabetes (GDM) is defined as any abnormality in glucose levels noted for the first time during pregnancy. Gestational diabetes is diagnosed in approximately 7% of all pregnancies in the USA. During pregnancy, the placenta and placental hormones create an insulin resistance that is most pronounced in the last trimester. Risk assessment for diabetes is suggested starting at the first prenatal visit. High-risk women should be screened immediately. Screening may be deferred in lower-risk women until the 24th to 28th week of gestation.
 INSULIN
INSULIN
Chemistry
Insulin is a small protein with a molecular weight in humans of 5808. It contains 51 amino acids arranged in two chains (A and B) linked by disulfide bridges; there are species differences in the amino acids of both chains. Proinsulin, a long single-chain protein molecule, is processed within the Golgi apparatus of beta cells and packaged into granules, where it is hydrolyzed into insulin and a residual connecting segment called C-peptide by removal of four amino acids (Figure 41–1).
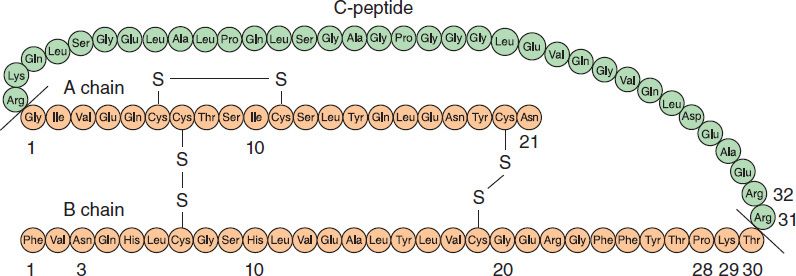
FIGURE 41–1 Structure of human proinsulin (C-peptide plus A and B chains) and insulin. Insulin is shown as the shaded (orange color) peptide chains, A and B. Differences in the A and B chains and amino acid modifications for the rapid-acting insulin analogs (aspart, lispro, and glulisine) and long-acting insulin analogs (glargine and detemir) are discussed in the text. (Adapted, with permission, from Gardner DG, Shoback D [editors]: Greenspan’s Basic & Clinical Endocrinology, 9th ed. McGraw-Hill, 2011. Copyright © The McGraw-Hill Companies, Inc.)
Insulin and C-peptide are secreted in equimolar amounts in response to all insulin secretagogues; a small quantity of unprocessed or partially hydrolyzed proinsulin is released as well. Although proinsulin may have some mild hypoglycemic action, C-peptide has no known physiologic function. Granules within the beta cells store the insulin in the form of crystals consisting of two atoms of zinc and six molecules of insulin. The entire human pancreas contains up to 8 mg of insulin, representing approximately 200 biologic units. Originally, the unit was defined on the basis of the hypoglycemic activity of insulin in rabbits. With improved purification techniques, the unit is presently defined on the basis of weight, and present insulin standards used for assay purposes contain 28 units per milligram.
Insulin Secretion
Insulin is released from pancreatic beta cells at a low basal rate and at a much higher stimulated rate in response to a variety of stimuli, especially glucose. Other stimulants such as other sugars (eg, mannose), amino acids (especially gluconeogenic amino acids, eg, leucine, arginine), hormones such as glucagon-like polypeptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), glucagon, cholecystokinin, high concentrations of fatty acids, and β-adrenergic sympathetic activity are recognized. Stimulatory drugs are sulfonylureas, meglitinide and nateglinide, isoproterenol, and acetylcholine. Inhibitory signals are hormones including insulin itself, somatostatin, and leptin; α-adrenergic sympathetic activity; chronically elevated glucose; and low concentrations of fatty acids. Inhibitory drugs include diazoxide, phenytoin, vinblastine, and colchicine.
One mechanism of stimulated insulin release is diagrammed in Figure 41–2. As shown in the figure, hyperglycemia results in increased intracellular ATP levels, which close the ATP-dependent potassium channels. Decreased outward potassium efflux results in depolarization of the beta cell and opening of voltage-gated calcium channels. The resulting increased intracellular calcium triggers secretion of the hormone. The insulin secretagogue drug group (sulfonylureas, meglitinides, and D-phenylalanine) exploits parts of this mechanism.
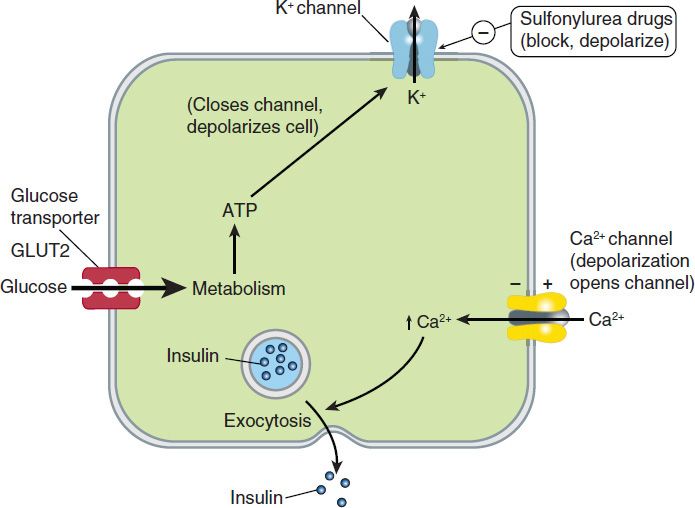
FIGURE 41–2 One model of control of insulin release from the pancreatic beta cell by glucose and by sulfonylurea drugs. In the resting cell with normal (low) ATP levels, potassium diffuses down its concentration gradient through ATP-gated potassium channels, maintaining the intracellular potential at a fully polarized, negative level. Insulin release is minimal. If glucose concentration rises, ATP production increases, potassium channels close, and depolarization of the cell results. As in muscle and nerve, voltage-gated calcium channels open in response to depolarization, allowing more calcium to enter the cell. Increased intracellular calcium results in increased insulin secretion. Insulin secretagogues close the ATP-dependent potassium channel, thereby depolarizing the membrane and causing increased insulin release by the same mechanism.
Insulin Degradation
The liver and kidney are the two main organs that remove insulin from the circulation. The liver normally clears the blood of approximately 60% of the insulin released from the pancreas by virtue of its location as the terminal site of portal vein blood flow, with the kidney removing 35–40% of the endogenous hormone. However, in insulin-treated diabetics receiving subcutaneous insulin injections, this ratio is reversed, with as much as 60% of exogenous insulin being cleared by the kidney and the liver removing no more than 30–40%. The half-life of circulating insulin is 3–5 minutes.
Circulating Insulin
Basal serum insulin values of 5–15 μU/mL (30–90 pmol/L) are found in normal humans, with a peak rise to 60–90 μU/mL (360–540 pmol/L) during meals.
The Insulin Receptor
After insulin has entered the circulation, it diffuses into tissues, where it is bound by specialized receptors that are found on the membranes of most tissues. The biologic responses promoted by these insulin-receptor complexes have been identified in the primary target tissues regulating energy metabolism, ie, liver, muscle, and adipose tissue. The receptors bind insulin with high specificity and affinity in the picomolar range. The full insulin receptor consists of two covalently linked heterodimers, each containing an α subunit, which is entirely extracellular and constitutes the recognition site, and a β subunit that spans the membrane (Figure 41–3). The β subunit contains a tyrosine kinase. The binding of an insulin molecule to the α subunits at the outside surface of the cell activates the receptor and through a conformational change brings the catalytic loops of the opposing cytoplasmic β subunits into closer proximity. This facilitates mutual phosphorylation of tyrosine residues on the β subunits and tyrosine kinase activity directed at cytoplasmic proteins.
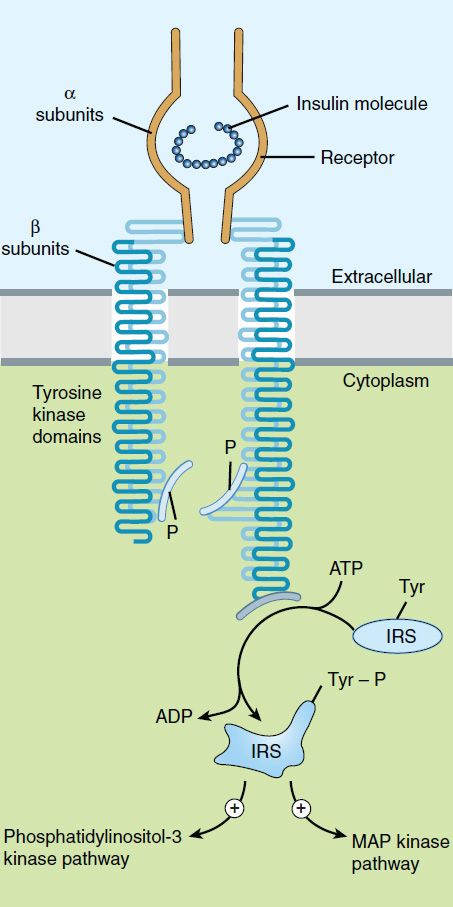
FIGURE 41–3 Schematic diagram of the insulin receptor heterodimer in the activated state. IRS, insulin receptor substrate; MAP, mitogen-activated protein; P, phosphate; Tyr, tyrosine.
The first proteins to be phosphorylated by the activated receptor tyrosine kinases are the docking proteins, insulin receptor substrates (IRS). After tyrosine phosphorylation at several critical sites, the IRS molecules bind to and activate other kinases subserving energy metabolism—most significantly phosphatidylinositol-3-kinase—which produce further phosphorylations. Alternatively, they may stimulate a mitogenic pathway and bind to an adaptor protein such as growth factor receptor-binding protein 2, which translates the insulin signal to a guanine nucleotide-releasing factor that ultimately activates the GTP binding protein, Ras, and the mitogen-activated protein kinase (MAPK) system. The particular IRS-phosphorylated tyrosine kinases have binding specificity with downstream molecules based on their surrounding 4–5 amino acid sequences or motifs that recognize specific Src homology 2 (SH2) domains on the other protein. This network of phosphorylations within the cell represents insulin’s second message and results in multiple effects, including translocation of glucose transporters (especially GLUT 4, Table 41–2) to the cell membrane with a resultant increase in glucose uptake; increased glycogen synthase activity and increased glycogen formation; multiple effects on protein synthesis, lipolysis, and lipogenesis; and activation of transcription factors that enhance DNA synthesis and cell growth and division.
TABLE 41–2 Glucose transporters.
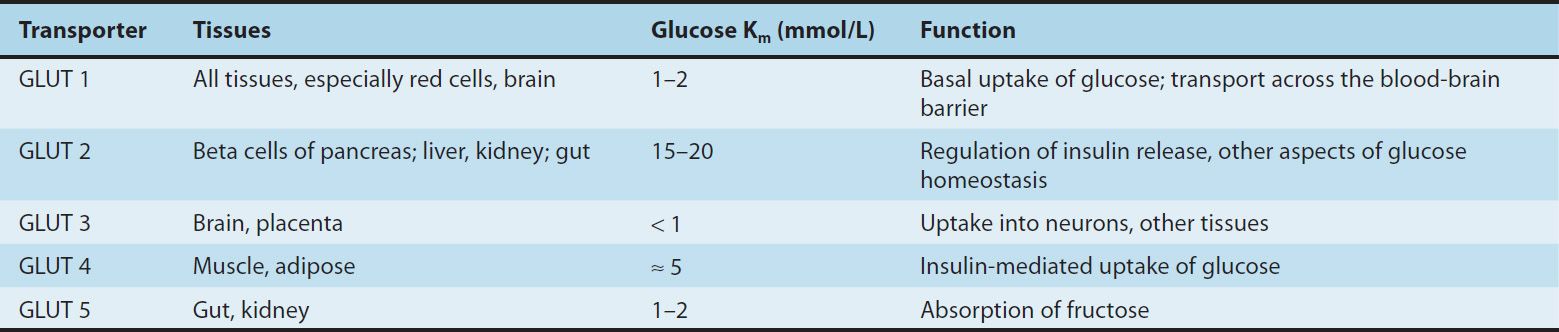
Various hormonal agents (eg, glucocorticoids) lower the affinity of insulin receptors for insulin; growth hormone in excess increases this affinity slightly. Aberrant serine and threonine phosphorylation of the insulin receptor β subunits or IRS molecules may result in insulin resistance and functional receptor down-regulation.
Effects of Insulin on Its Targets
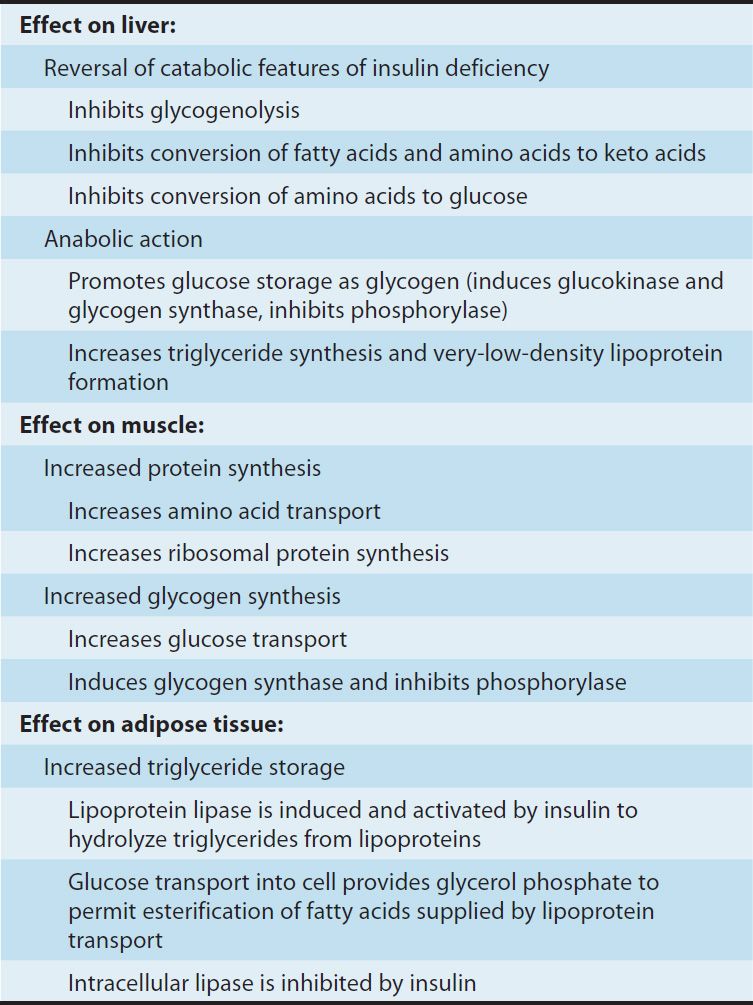
Insulin promotes the storage of fat as well as glucose (both sources of energy) within specialized target cells (Figure 41–4) and influences cell growth and the metabolic functions of a wide variety of tissues (Table 41–3).
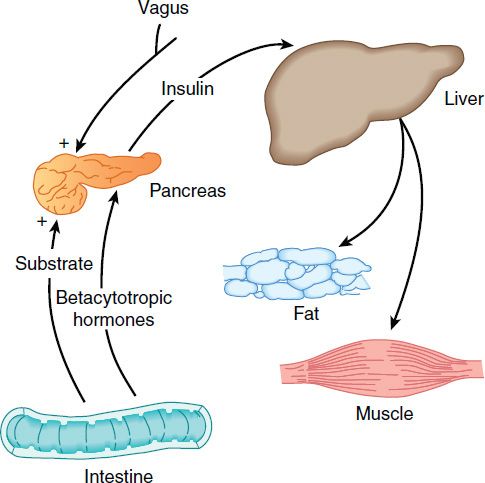
FIGURE 41–4 Insulin promotes synthesis (from circulating nutrients) and storage of glycogen, triglycerides, and protein in its major target tissues: liver, fat, and muscle. The release of insulin from the pancreas is stimulated by increased blood glucose, incretins, vagal nerve stimulation, and other factors (see text).
TABLE 41–3 Endocrine effects of insulin.
Characteristics of Available Insulin Preparations
Commercial insulin preparations differ in a number of ways, such as differences in the recombinant DNA production techniques, amino acid sequence, concentration, solubility, and the time of onset and duration of their biologic action.
A. Principal Types and Duration of Action of Insulin Preparations
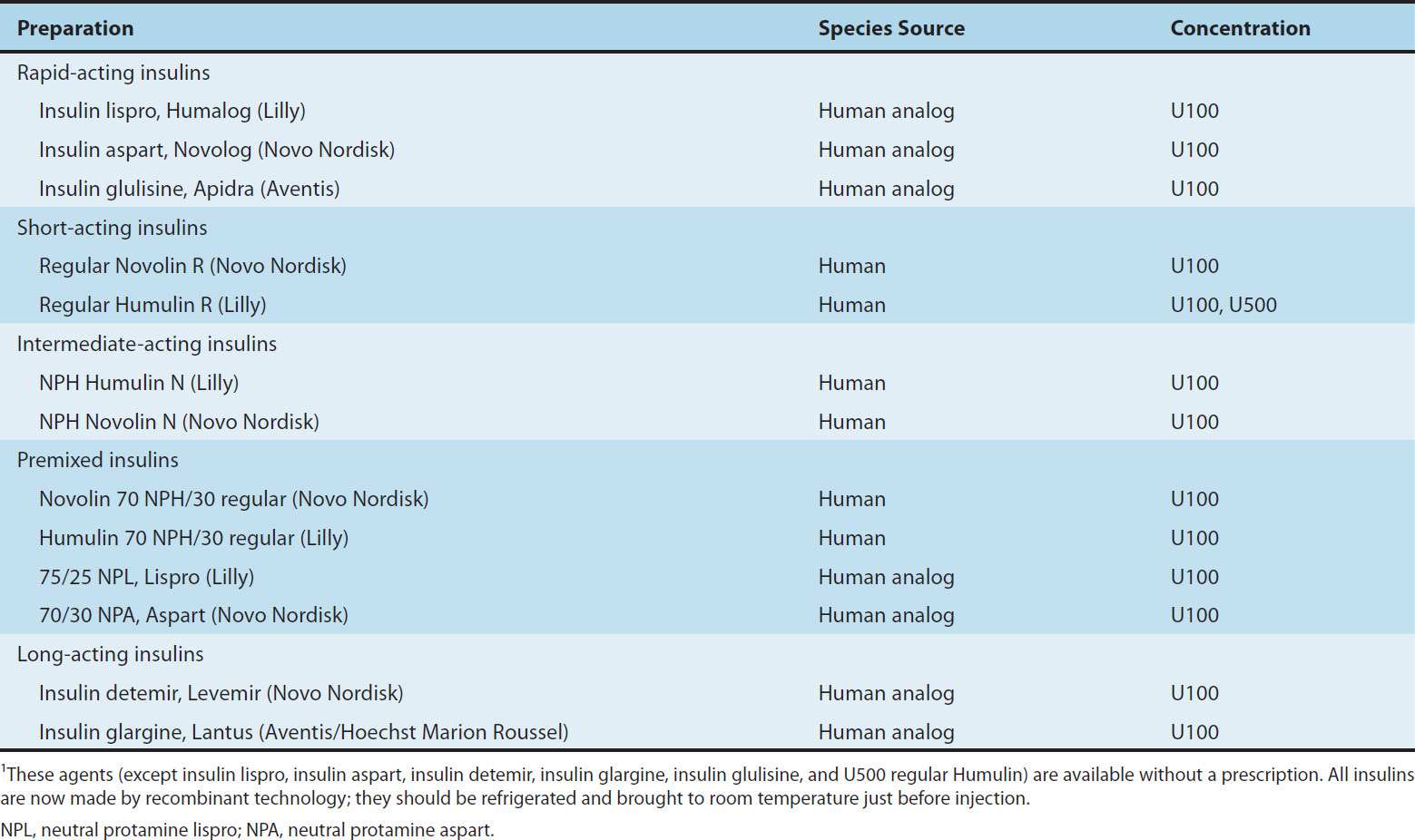
Four principal types of injected insulins are available: (1) rapid-acting, with very fast onset and short duration; (2) short-acting, with rapid onset of action; (3) intermediate-acting; and (4) long-acting, with slow onset of action (Figure 41–5, Table 41–4). Injected rapid-acting and short-acting insulins are dispensed as clear solutions at neutral pH and contain small amounts of zinc to improve their stability and shelf life. Injected intermediate-acting NPH insulins have been modified to provide prolonged action and are dispensed as a turbid suspension at neutral pH with protamine in phosphate buffer (neutral protamine Hagedorn [NPH] insulin). Insulin glargine and insulin detemir are clear, soluble long-acting insulins.
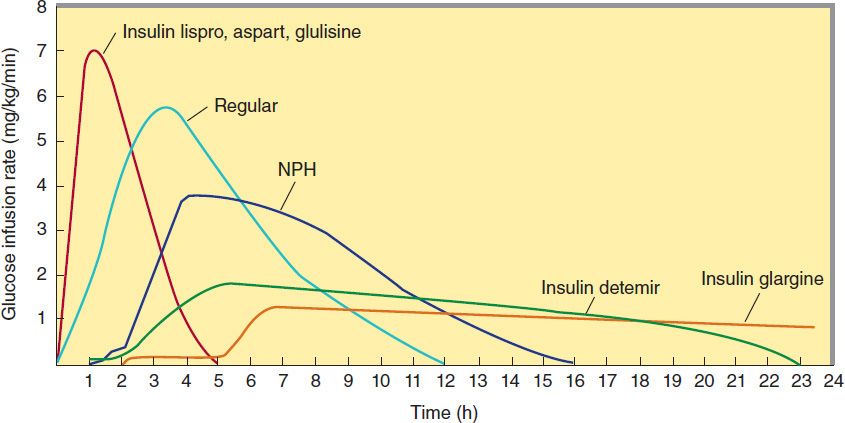
FIGURE 41–5 Extent and duration of action of various types of insulin as indicated by the glucose infusion rates (mg/kg/min) required to maintain a constant glucose concentration. The durations of action shown are typical of an average dose of 0.2–0.3 U/kg. The durations of regular and NPH insulin increase considerably when dosage is increased.
TABLE 41–4 Some insulin preparations available in the USA.1
The goal of subcutaneous insulin therapy is to replicate normal physiologic insulin secretion and replace the background or basal (overnight, fasting, and between-meal) as well as bolus or prandial (mealtime) insulin. An exact reproduction of the normal glycemic profile is not technically possible because of the limitations inherent in subcutaneous administration of insulin. Current regimens generally use insulin analogs because of their more predictable action. Intensive therapy (“tight control”) attempts to restore near-normal glucose patterns throughout the day while minimizing the risk of hypoglycemia.
Intensive regimens involving multiple daily injections (MDI) use long-acting insulin analogs to provide basal or background coverage, and rapid-acting insulin analogs to meet the mealtime requirements. The latter insulins are given as supplemental doses to correct transient hyperglycemia. The most sophisticated insulin regimen delivers rapid-acting insulin analogs through a continuous subcutaneous insulin infusion device. Conventional therapy consists of split-dose injections of mixtures of rapid- or short-acting and intermediate-acting insulins.
1. Rapid-acting insulin—Three injected rapid-acting insulin analogs—insulin lispro, insulin aspart, and insulin glulisine—are commercially available. The rapid-acting insulins permit more physiologic prandial insulin replacement because their rapid onset and early peak action more closely mimic normal endogenous prandial insulin secretion than regular insulin, and they have the additional benefit of allowing insulin to be taken immediately before the meal without sacrificing glucose control. Their duration of action is rarely more than 4–5 hours, which decreases the risk of late postmeal hypoglycemia. The injected rapid-acting insulins have the lowest variability of absorption (approximately 5%) of all available commercial insulins (compared with 25% for regular insulin and 25% to over 50% for long-acting analog formulations and intermediate insulin, respectively). They are the preferred insulins for use in continuous subcutaneous insulin infusion devices.
Insulin lispro, the first monomeric insulin analog to be marketed, is produced by recombinant technology wherein two amino acids near the carboxyl terminal of the B chain have been reversed in position: Proline at position B28 has been moved to B29, and lysine at position B29 has been moved to B28 (Figure 41–1). Reversing these two amino acids does not interfere in any way with insulin lispro’s binding to the insulin receptor, its circulating half-life, or its immunogenicity, which are similar to those of human regular insulin. However, the advantage of this analog is its very low propensity—in contrast to human insulin—to self-associate in antiparallel fashion and form dimers. To enhance the shelf life of insulin in vials, insulin lispro is stabilized into hexamers by a cresol preservative. When injected subcutaneously, the drug quickly dissociates into monomers and is rapidly absorbed with onset of action within 5–15 minutes and peak activity as early as 1 hour. The time to peak action is relatively constant, regardless of the dose.
Insulin aspart is created by the substitution of the B28 proline with a negatively charged aspartic acid (Figure 41–1). This modification reduces the normal ProB28 and GlyB23 monomer-monomer interaction, thereby inhibiting insulin self-aggregation. Its absorption and activity profile are similar to those of insulin lispro, and it is more reproducible than regular insulin, but it has binding properties, activity, and mitogenicity characteristics similar to those of regular insulin in addition to equivalent immunogenicity.
Insulin glulisine is formulated by substituting a lysine for asparagine at B3 and glutamic acid for lysine at B29. Its absorption, action, and immunologic characteristics are similar to those of other injected rapid-acting insulins. After high-dose insulin glulisine interaction with the insulin receptor, there may be downstream differences in IRS-2 pathway activation relative to native insulin. The clinical significance of such differences is unclear.
2. Short-acting insulin—Regular insulin is a short-acting soluble crystalline zinc insulin that is now made by recombinant DNA techniques to produce a molecule identical to human insulin. Its effect appears within 30 minutes, peaks between 2 and 3 hours after subcutaneous injection, and generally lasts 5–8 hours. In high concentrations, eg, in the vial, regular insulin molecules self-aggregate in antiparallel fashion to form dimers that stabilize around zinc ions to create insulin hexamers. The hexameric nature of regular insulin causes a delayed onset and prolongs the time to peak action. After subcutaneous injection, the insulin hexamers are too large and bulky to be transported across the vascular endothelium into the bloodstream. As the insulin depot is diluted by interstitial fluid and the concentration begins to fall, the hexamers break down into dimers and finally monomers. This results in three rates of absorption of the injected insulin, with the final monomeric phase having the fastest uptake out of the injection site.
The clinical consequence is that when regular insulin is administered at mealtime, the blood glucose rises faster than the insulin with resultant early postprandial hyperglycemia and an increased risk of late postprandial hypoglycemia. Therefore, regular insulin should be injected 30–45 or more minutes before the meal to minimize the mismatching. As with all older insulin formulations, the duration of action as well as the time of onset and the intensity of peak action increase with the size of the dose. Clinically, this is a critical issue because the pharmacokinetics and pharmacodynamics of small doses of regular and NPH insulins differ greatly from those of large doses. The delayed absorption, dose-dependent duration of action, and variability of absorption (˜ 25%) of regular human insulin frequently results in a mismatching of insulin availability with need, and its use is declining.
However, short-acting, regular soluble insulin is the only type that should be administered intravenously because the dilution causes the hexameric insulin to immediately dissociate into monomers. It is particularly useful for intravenous therapy in the management of diabetic ketoacidosis and when the insulin requirement is changing rapidly, such as after surgery or during acute infections.
3. Intermediate-acting and long-acting insulins
a. NPH (neutral protamine Hagedorn, or isophane) insulin—NPH insulin is an intermediate-acting insulin whose absorption and onset of action are delayed by combining appropriate amounts of insulin and protamine so that neither is present in an uncomplexed form (“isophane”). After subcutaneous injection, proteolytic tissue enzymes degrade the protamine to permit absorption of insulin. NPH insulin has an onset of approximately 2–5 hours and duration of 4–12 hours (Figure 41–5); it is usually mixed with regular, lispro, aspart, or glulisine insulin and given two to four times daily for insulin replacement. The dose regulates the action profile; specifically, small doses have lower, earlier peaks and a short duration of action with the converse true for large doses. The action of NPH is highly unpredictable, and its variability of absorption is over 50%. The clinical use of NPH is waning because of its adverse pharmacokinetics combined with the availability of long-acting insulin analogs that have a more predictable and physiologic action.
b. Insulin glargine—Insulin glargine is a soluble, “peakless” (ie, having a broad plasma concentration plateau), long-acting insulin analog. This product was designed to provide reproducible, convenient, background insulin replacement. The attachment of two arginine molecules to the B-chain carboxyl terminal and substitution of a glycine for asparagine at the A21 position created an analog that is soluble in an acidic solution but precipitates in the more neutral body pH after subcutaneous injection. Individual insulin molecules slowly dissolve away from the crystalline depot and provide a low, continuous level of circulating insulin. Insulin glargine has a slow onset of action (1–1.5 hours) and achieves a maximum effect after 4–6 hours. This maximum activity is maintained for 11–24 hours or longer. Glargine is usually given once daily, although some very insulin-sensitive or insulin-resistant individuals benefit from split (twice a day) dosing. To maintain solubility, the formulation is unusually acidic (pH 4.0), and insulin glargine should not be mixed with other insulins. Separate syringes must be used to minimize the risk of contamination and subsequent loss of efficacy. The absorption pattern of insulin glargine appears to be independent of the anatomic site of injection, and this drug is associated with less immunogenicity than human insulin in animal studies. Glargine’s interaction with the insulin receptor is similar to that of native insulin and shows no increase in mitogenic activity in vitro. It has sixfold to sevenfold greater binding than native insulin to the insulin-like growth factor-1 (IGF-1) receptor, but the clinical significance of this is unclear.
c. Insulin detemir—This insulin is the most recently developed long-acting insulin analog. The terminal threonine is dropped from the B30 position and myristic acid (a C-14 fatty acid chain) is attached to the terminal B29 lysine. These modifications prolong the availability of the injected analog by increasing both self-aggregation in subcutaneous tissue and reversible albumin binding. Insulin detemir has the most reproducible effect of the intermediate- and long-acting insulins, and its use is associated with less hypoglycemia than NPH insulin. Insulin detemir has a dose-dependent onset of action of 1–2 hours and duration of action of more than 12 hours. It is given twice daily to obtain a smooth background insulin level.
4. Mixtures of insulins—Because intermediate-acting NPH insulins require several hours to reach adequate therapeutic levels, their use in diabetic patients usually requires supplements of rapid-or short-acting insulin before meals. For convenience, these are often mixed together in the same syringe before injection. Insulin lispro, aspart, and glulisine can be acutely mixed (ie, just before injection) with NPH insulin without affecting their rapid absorption. However, premixed preparations have thus far been unstable. To remedy this, intermediate insulins composed of isophane complexes of protamine with insulin lispro and insulin aspart have been developed. These intermediate insulins have been designated as “NPL” (neutral protamine lispro) and “NPA” (neutral protamine aspart) and have the same duration of action as NPH insulin. They have the advantage of permitting formulation as premixed combinations of NPL and insulin lispro, and as NPA and insulin aspart, and they have been shown to be safe and effective in clinical trials. The FDA has approved 75%/25% NPL/insulin lispro and 70%/30% NPA/insulin aspart premixed formulations. Additional ratios are available abroad. Insulin glargine and detemir must be given as separate injections. They are not miscible acutely or in a premixed preparation with any other insulin formulation.
Premixed formulations of 70%/30% NPH/regular continue to be available. These preparations have all the limitations of regular insulin, namely, highly dose-dependent pharmacokinetic and pharmacodynamic profiles, and variability in absorption.
B. Insulin Production
Mass production of human insulin and insulin analogs by recombinant DNA techniques is carried out by inserting the human or a modified human proinsulin gene into Escherichia coli or yeast and treating the extracted proinsulin to form the insulin or insulin analog molecules.
C. Concentration
All insulins in the USA and Canada are available in a concentration of 100 U/mL (U100). A limited supply of U500 regular human insulin is available for use in rare cases of severe insulin resistance in which larger doses of insulin are required.
Insulin Delivery Systems
A. Standard Delivery
The standard mode of insulin therapy is subcutaneous injection using conventional disposable needles and syringes.
B. Portable Pen Injectors
To facilitate multiple subcutaneous injections of insulin, particularly during intensive insulin therapy, portable pen-sized injectors have been developed. These contain cartridges of insulin and replaceable needles.
Disposable insulin pens are also available for selected formulations. These are regular insulin, insulin lispro, insulin aspart, insulin glulisine, insulin glargine, insulin detemir, and several mixtures of NPH with regular, lispro, or aspart insulin (Table 41–4). They have been well accepted by patients because they eliminate the need to carry syringes and bottles of insulin to the workplace and while traveling.
C. Continuous Subcutaneous Insulin Infusion Devices (CSII, Insulin Pumps)
Continuous subcutaneous insulin infusion devices are external open-loop pumps for insulin delivery. The devices have a user-programmable pump that delivers individualized basal and bolus insulin replacement doses based on blood glucose self-monitoring results.
Normally, the 24-hour background basal rates are preprogrammed and relatively constant from day to day, although temporarily altered rates can be superimposed to adjust for a short-term change in requirement. For example, the basal delivery rate might need to be decreased for several hours because of the increased insulin sensitivity associated with strenuous activity.
Boluses are used to correct high blood glucose levels and to cover mealtime insulin requirements based on the carbohydrate content of the food and concurrent activity. Bolus amounts are either dynamically programmed or use preprogrammed algorithms. When the boluses are dynamically programmed, the user calculates the dose based on the amount of carbohydrate consumed and the current blood glucose level. Alternatively, the meal or snack dose algorithm (grams of carbohydrate covered by a unit of insulin) and insulin sensitivity or blood glucose correction factor (fall in blood glucose level in response to a unit of insulin) can be preprogrammed into the pump. If the user enters the carbohydrate content of the food and current blood glucose value, the insulin pump will calculate the most appropriate dose of insulin. Advanced insulin pumps also have an “insulin on board” feature that adjusts a high blood glucose correction dose to correct for residual activity of previous bolus doses.
The traditional pump—which contains an insulin reservoir, the program chip, the keypad, and the display screen—is about the size of a pager. It is usually placed on a belt or in a pocket, and the insulin is infused through thin plastic tubing that is connected to the subcutaneously inserted infusion set. The abdomen is the favored site for the infusion set, although flanks and thighs are also used. The insulin reservoir, tubing, and infusion set need to be changed using sterile techniques every 2 or 3 days. Currently, only one pump does not require tubing. In this model, the pump is attached directly to the infusion set. Programming is done through a hand-held unit that communicates wirelessly with the pump. CSII delivery is regarded as the most physiologic method of insulin replacement.
Use of these continuous infusion devices is encouraged for people who are unable to obtain target control while on multiple injection regimens and in circumstances in which excellent glycemic control is desired, such as during pregnancy. Optimal use of these devices requires responsible involvement and commitment by the patient. Insulin aspart, lispro, and glulisine all are specifically approved for pump use and are preferred pump insulins because their favorable pharmacokinetic attributes allow glycemic control without increasing the risk of hypoglycemia.
D. Inhaled Insulin
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


