58
Management of the Poisoned Patient
CASE STUDY
A 62-year-old woman with a history of depression is found in her apartment in a lethargic state. An empty bottle of bupropion is on the bedside table. In the emergency department, she is unresponsive to verbal and painful stimuli. She has a brief generalized seizure, followed by a respiratory arrest. The emergency physician performs endotracheal intubation and administers a drug intravenously, followed by another substance via a nasogastric tube. The patient is admitted to the intensive care unit for continued supportive care and recovers the next morning. What drug might be used intravenously to prevent further seizures? What substance is commonly used to adsorb drugs still present in the gastrointestinal tract?
Over 1 million cases of acute poisoning occur in the USA each year, although only a small number are fatal. Most deaths are due to intentional suicidal overdose by an adolescent or adult. Childhood deaths due to accidental ingestion of a drug or toxic household product have been markedly reduced in the last 40 years as a result of safety packaging and effective poisoning prevention education.
Even with a serious exposure, poisoning is rarely fatal if the victim receives prompt medical attention and good supportive care. Careful management of respiratory failure, hypotension, seizures, and thermoregulatory disturbances has resulted in improved survival of patients who reach the hospital alive.
This chapter reviews the basic principles of poisoning, initial management, and specialized treatment of poisoning, including methods of increasing the elimination of drugs and toxins.
 TOXICOKINETICS & TOXICODYNAMICS
TOXICOKINETICS & TOXICODYNAMICS
The term toxicokinetics denotes the absorption, distribution, excretion, and metabolism of toxins, toxic doses of therapeutic agents, and their metabolites. The term toxicodynamics is used to denote the injurious effects of these substances on body functions. Although many similarities exist between the pharmacokinetics and toxicokinetics of most substances, there are also important differences. The same caution applies to pharmacodynamics and toxicodynamics.
SPECIAL ASPECTS OF TOXICOKINETICS
Volume of Distribution
The volume of distribution (Vd) is defined as the apparent volume into which a substance is distributed in the body (see Chapter 3). A large V implies that the drug is not readily accessible to measures aimed at purifying the blood, such as hemodialysis. Examples of drugs with large volumes of distribution (> 5 L/kg) include antidepressants, antipsychotics, antimalarials, opioids, propranolol, and verapamil. Drugs with a relatively small V (< 1 L/kg) include salicylate, ethanol, phenobarbital, lithium, valproic acid, and phenytoin (see Table 3–1).
Clearance
Clearance is a measure of the volume of plasma that is cleared of drug per unit time (see Chapter 3). The total clearance for most drugs is the sum of clearances via excretion by the kidneys and metabolism by the liver. In planning a detoxification strategy, it is important to know the contribution of each organ to total clearance. For example, if a drug is 95% cleared by liver metabolism and only 5% cleared by renal excretion, even a dramatic increase in urinary concentration of the drug will have little effect on overall elimination.
Overdosage of a drug can alter the usual pharmacokinetic processes, and this must be considered when applying kinetics to poisoned patients. For example, dissolution of tablets or gastric emptying time may be slowed so that absorption and peak toxic effects are delayed. Drugs may injure the epithelial barrier of the gastrointestinal tract and thereby increase absorption. If the capacity of the liver to metabolize a drug is exceeded, the first-pass effect will be reduced and more drug will be delivered to the circulation. With a dramatic increase in the concentration of drug in the blood, protein-binding capacity may be exceeded, resulting in an increased fraction of free drug and greater toxic effect. At normal dosage, most drugs are eliminated at a rate proportional to the plasma concentration (first-order kinetics). If the plasma concentration is very high and normal metabolism is saturated, the rate of elimination may become fixed (zero-order kinetics). This change in kinetics may markedly prolong the apparent serum half-life and increase toxicity.
SPECIAL ASPECTS OF TOXICODYNAMICS
The general dose-response principles described in Chapter 2 are relevant when estimating the potential severity of an intoxication. When considering quantal dose-response data, both the therapeutic index and the overlap of therapeutic and toxic response curves must be considered. For instance, two drugs may have the same therapeutic index but unequal safe dosing ranges if the slopes of their dose-response curves are not the same. For some drugs, eg, sedative-hypnotics, the major toxic effect is a direct extension of the therapeutic action, as shown by their graded dose-response curve (see Figure 22–1). In the case of a drug with a linear dose-response curve (drug A), lethal effects may occur at 10 times the normal therapeutic dose. In contrast, a drug with a curve that reaches a plateau (drug B) may not be lethal at 100 times the normal dose.
For many drugs, at least part of the toxic effect may be different from the therapeutic action. For example, intoxication with drugs that have atropine-like effects (eg, tricyclic antidepressants) reduces sweating, making it more difficult to dissipate heat. In tricyclic antidepressant intoxication, there may also be increased muscular activity or seizures; the body’s production of heat is thus enhanced, and lethal hyperpyrexia may result. Overdoses of drugs that depress the cardiovascular system, eg, β blockers or calcium channel blockers, can profoundly alter not only cardiac function but all functions that are dependent on blood flow. These include renal and hepatic elimination of the toxin and that of any other drugs that may be given.
 APPROACH TO THE POISONED PATIENT
APPROACH TO THE POISONED PATIENT
HOW DOES THE POISONED PATIENT DIE?
An understanding of common mechanisms of death due to poisoning can help prepare the care-giver to treat patients effectively. Many toxins depress the central nervous system (CNS), resulting in obtundation or coma. Comatose patients frequently lose their airway protective reflexes and their respiratory drive. Thus, they may die as a result of airway obstruction by the flaccid tongue, aspiration of gastric contents into the tracheobronchial tree, or respiratory arrest. These are the most common causes of death due to overdoses of narcotics and sedative-hypnotic drugs (eg, barbiturates and alcohol).
Cardiovascular toxicity is also frequently encountered in poisoning. Hypotension may be due to depression of cardiac contractility; hypovolemia resulting from vomiting, diarrhea, or fluid sequestration; peripheral vascular collapse due to blockade of α-adrenoceptor-mediated vascular tone; or cardiac arrhythmias. Hypothermia or hyperthermia due to exposure as well as the temperature-dysregulating effects of many drugs can also produce hypotension. Lethal arrhythmias such as ventricular tachycardia and fibrillation can occur with overdoses of many cardioactive drugs such as ephedrine, amphetamines, cocaine, digitalis, and theophylline; and drugs not usually considered cardioactive, such as tricyclic antidepressants, antihistamines, and some opioid analogs.
Cellular hypoxia may occur in spite of adequate ventilation and oxygen administration when poisoning is due to cyanide, hydrogen sulfide, carbon monoxide, and other poisons that interfere with transport or utilization of oxygen. Such patients may not be cyanotic, but cellular hypoxia is evident by the development of tachycardia, hypotension, severe lactic acidosis, and signs of ischemia on the electrocardiogram.
Seizures, muscular hyperactivity, and rigidity may result in death. Seizures may cause pulmonary aspiration, hypoxia, and brain damage. Hyperthermia may result from sustained muscular hyperactivity and can lead to muscle breakdown and myoglobinuria, renal failure, lactic acidosis, and hyperkalemia. Drugs and poisons that often cause seizures include antidepressants, isoniazid (INH), diphenhydramine, cocaine, and amphetamines.
Other organ system damage may occur after poisoning and is sometimes delayed in onset. Paraquat attacks lung tissue, resulting in pulmonary fibrosis, beginning several days after ingestion. Massive hepatic necrosis due to poisoning by acetaminophen or certain mushrooms results in hepatic encephalopathy and death 48–72 hours or longer after ingestion.
Finally, some patients may die before hospitalization because the behavioral effects of the ingested drug may result in traumatic injury. Intoxication with alcohol and other sedative-hypnotic drugs is a common contributing factor to motor vehicle accidents. Patients under the influence of hallucinogens such as phencyclidine (PCP) or lysergic acid diethylamide (LSD) may suffer trauma when they become combative or fall from a height.
 INITIAL MANAGEMENT OF THE POISONED PATIENT
INITIAL MANAGEMENT OF THE POISONED PATIENT
The initial management of a patient with coma, seizures, or otherwise altered mental status should follow the same approach regardless of the poison involved: supportive measures are the basics (“ABCDs”) of poisoning treatment.
First, the airway should be cleared of vomitus or any other obstruction and an oral airway or endotracheal tube inserted if needed. For many patients, simple positioning in the lateral, leftside-down position is sufficient to move the flaccid tongue out of the airway. Breathing should be assessed by observation and pulse oximetry and, if in doubt, by measuring arterial blood gases. Patients with respiratory insufficiency should be intubated and mechanically ventilated. The circulation should be assessed by continuous monitoring of pulse rate, blood pressure, urinary output, and evaluation of peripheral perfusion. An intravenous line should be placed and blood drawn for serum glucose and other routine determinations.
At this point, every patient with altered mental status should receive a challenge with concentrated dextrose, unless a rapid bedside blood glucose test demonstrates that the patient is not hypoglycemic. Adults are given 25 g (50 mL of 50% dextrose solution) intravenously, children 0.5 g/kg (2 mL/kg of 25% dextrose). Hypoglycemic patients may appear to be intoxicated, and there is no rapid and reliable way to distinguish them from poisoned patients. Alcoholic or malnourished patients should also receive 100 mg of thiamine intramuscularly or in the intravenous infusion solution at this time to prevent Wernicke’s syndrome.
The opioid antagonist naloxone may be given in a dose of 0.4–2 mg intravenously. Naloxone reverses respiratory and CNS depression due to all varieties of opioid drugs (see Chapter 31). It is useful to remember that these drugs cause death primarily by respiratory depression; therefore, if airway and breathing assistance have already been instituted, naloxone may not be necessary. Larger doses of naloxone may be needed for patients with overdose involving propoxyphene, codeine, and some other opioids. The benzodiazepine antagonist flumazenil (see Chapter 22) may be of value in patients with suspected benzodiazepine overdose, but it should not be used if there is a history of tricyclic antidepressant overdose or a seizure disorder, as it can induce convulsions in such patients.
History & Physical Examination
Once the essential initial ABCD interventions have been instituted, one can begin a more detailed evaluation to make a specific diagnosis. This includes gathering any available history and performing a toxicologically oriented physical examination. Other causes of coma or seizures such as head trauma, meningitis, or metabolic abnormalities should be sought and treated. Some common intoxications are described under Common Toxic Syndromes.
A. History
Oral statements about the amount and even the type of drug ingested in toxic emergencies may be unreliable. Even so, family members, police, and fire department or paramedical personnel should be asked to describe the environment in which the toxic emergency occurred and should bring to the emergency department any syringes, empty bottles, household products, or over-the-counter medications in the immediate vicinity of the possibly poisoned patient.
B. Physical Examination
A brief examination should be performed, emphasizing those areas most likely to give clues to the toxicologic diagnosis. These include vital signs, eyes and mouth, skin, abdomen, and nervous system.
1. Vital signs—Careful evaluation of vital signs (blood pressure, pulse, respirations, and temperature) is essential in all toxicologic emergencies. Hypertension and tachycardia are typical with amphetamines, cocaine, and antimuscarinic (anticholinergic) drugs. Hypotension and bradycardia are characteristic features of overdose with calcium channel blockers, β blockers, clonidine, and sedative hypnotics. Hypotension with tachycardia is common with tricyclic antidepressants, trazodone, quetiapine, vasodilators, and β agonists. Rapid respirations are typical of salicylates, carbon monoxide, and other toxins that produce metabolic acidosis or cellular asphyxia. Hyperthermia may be associated with sympathomimetics, anticholinergics, salicylates, and drugs producing seizures or muscular rigidity. Hypothermia can be caused by any CNS-depressant drug, especially when accompanied by exposure to a cold environment.
2. Eyes—The eyes are a valuable source of toxicologic information. Constriction of the pupils (miosis) is typical of opioids, clonidine, phenothiazines, and cholinesterase inhibitors (eg, organophosphate insecticides), and deep coma due to sedative drugs. Dilation of the pupils (mydriasis) is common with amphetamines, cocaine, LSD, and atropine and other anticholinergic drugs. Horizontal nystagmus is characteristic of intoxication with phenytoin, alcohol, barbiturates, and other sedative drugs. The presence of both vertical and horizontal nystagmus is strongly suggestive of phencyclidine poisoning. Ptosis and ophthalmoplegia are characteristic features of botulism.
3. Mouth—The mouth may show signs of burns due to corrosive substances, or soot from smoke inhalation. Typical odors of alcohol, hydrocarbon solvents, or ammonia may be noted. Poisoning due to cyanide can be recognized by some examiners as an odor like bitter almonds.
4. Skin—The skin often appears flushed, hot, and dry in poisoning with atropine and other antimuscarinics. Excessive sweating occurs with organophosphates, nicotine, and sympathomimetic drugs. Cyanosis may be caused by hypoxemia or by methemoglobinemia. Icterus may suggest hepatic necrosis due to acetaminophen or Amanita phalloides mushroom poisoning.
5. Abdomen—Abdominal examination may reveal ileus, which is typical of poisoning with antimuscarinic, opioid, and sedative drugs. Hyperactive bowel sounds, abdominal cramping, and diarrhea are common in poisoning with organophosphates, iron, arsenic, theophylline, A phalloides, and A muscaria.
6. Nervous system—A careful neurologic examination is essential. Focal seizures or motor deficits suggest a structural lesion (eg, intracranial hemorrhage due to trauma) rather than toxic or metabolic encephalopathy. Nystagmus, dysarthria, and ataxia are typical of phenytoin, carbamazepine, alcohol, and other sedative intoxication. Twitching and muscular hyperactivity are common with atropine and other anticholinergic agents, and cocaine and other sympathomimetic drugs. Muscular rigidity can be caused by haloperidol and other antipsychotic agents, and by strychnine or by tetanus. Generalized hypertonicity of muscles and lower extremity clonus are typical of serotonin syndrome. Seizures are often caused by overdose with antidepressants (especially tricyclic antidepressants and bupropion [as in the case study]), cocaine, amphetamines, theophylline, isoniazid, and diphenhydramine. Flaccid coma with absent reflexes and even an isoelectric electroencephalogram may be seen with deep coma due to sedative-hypnotic or other CNS depressant intoxication and may be mistaken for brain death.
Laboratory & Imaging Procedures
A. Arterial Blood Gases
Hypoventilation results in an elevated PCO2 (hypercapnia) and a low PO2 (hypoxia). The PO2 may also be low in a patient with aspiration pneumonia or drug-induced pulmonary edema. Poor tissue oxygenation due to hypoxia, hypotension, or cyanide poisoning will result in metabolic acidosis. The PO2 measures only oxygen dissolved in the plasma and not total blood oxygen content or oxyhemoglobin saturation and may appear normal in patients with severe carbon monoxide poisoning. Pulse oximetry may also give falsely normal results in carbon monoxide intoxication.
B. Electrolytes
Sodium, potassium, chloride, and bicarbonate should be measured. The anion gap is then calculated by subtracting the measured anions from cations:
Anion gap = (Na+ + K+) – (HCO3− + Cl−)
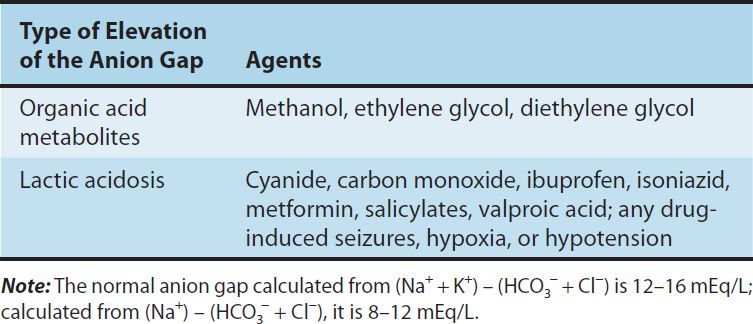
Normally, the sum of the cations exceeds the sum of the anions by no more than 12–16 mEq/L (or 8–12 mEq/L if the formula used for estimating the anion gap omits the potassium level). A larger than expected anion gap is caused by the presence of unmeasured anions (lactate, etc) accompanying metabolic acidosis. This may occur with numerous conditions, such as diabetic ketoacidosis, renal failure, or shock-induced lactic acidosis. Drugs that may induce an elevated anion gap metabolic acidosis (Table 58–1) include aspirin, metformin, methanol, ethylene glycol, isoniazid, and iron.
TABLE 58–1 Examples of drug-induced anion gap acidosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


