14 Endocrine system
Hormones are chemical messengers (peptides, amino acids, steroids, catecholamines) produced by endocrine glands that may act locally on adjacent cells (paracrine action), on target cells at a distance (endocrine action) or, on the secretory cell itself (autocrine action). Hormones act by binding to specific target cell receptor proteins on the cell membrane (insulin, adrenaline) or cytoplasmic/nuclear receptors within the cell (thyroxine, steroid hormones). As a result of receptor activation and signalling, cellular growth/metabolism is modified.
THYROID
EMBRYOLOGY
The gland develops as an endodermal down growth from the tongue before the end of the third week that migrates in front of the developing trachea to its permanent site in the base of the neck. The tube of cells, the thyroglossal tract, associated with thyroid migration atrophies and disappears by about six weeks. The ultimobranchial bodies developing from the fourth pharyngeal pouches become incorporated into the developing thyroid. These are the origin of calcitonin secreting C cells. By ten weeks thyroid follicles are present.
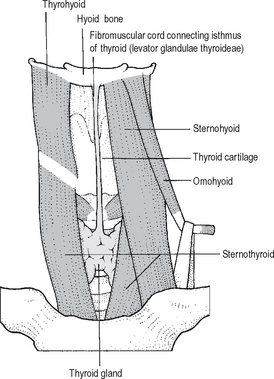
A thyroglossal duct is a remnant of the developing cord or tube of cells associated with thyroid descent. It lies in the midline from the foramen caecum of the tongue and passes via the hyoid bone to the pyramidal lobe of the thyroid, inferiorly a fibrous cord – sometimes known as the ‘levator glandulae thyroideae’ (Fig. 14.1). Epithelial remnants of the duct may proliferate and become filled with mucus i.e. thyroglossal cysts. Normally the cyst, lined by respiratory type or squamous epithelium lies above the thyroid cartilage – it may even lie at the level of or above the hyoid bone. Thyroglossal cysts elevate on protrusion of the tongue because of their association with the levator glandulae thyroideae and the hyoid bone.
BLOOD SUPPLY AND IMPORTANT RELATIONS
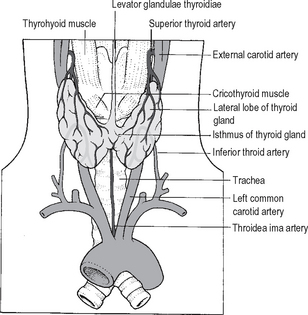
The normal thyroid gland has a very rich blood supply (5 ml per gram each minute). The blood flow through the thyroid may be markedly increased in thyrotoxicosis. There are four main thyroid arteries. The superior thyroid arteries, each arising from the external carotid artery, branch as they enter the upper poles of the gland and are intimately associated with a leash of veins. Together these vessels form the superior thyroidpedicles. The inferior thyroid arteries enter theposterior aspect of the mid-region of each thyroid lobe; they are branches of the thyrocervical trunk of the subclavian arteries. There are rich anastomoses between these vessels. A fifth small artery is sometimes present that enters the thyroid isthmus from below –the thyroidea ima artery arising from the brachiocephalic artery or the arch of the aorta (Fig. 14.2).
The venous drainage of the thyroid is of surgical importance. It is extensive and variable, but generally three groups are recognised. The superior thyroid veins tend to coalesce around the region of the superior thyroid artery and are ligated by the surgeon with the same tie used for the branches of the artery. Multiple inferior thyroid veins drain into the brachiocephalic veins. Veins draining the mid portion of the thyroid lobes drain either to the superior and inferior thyroid veins, or form a short venous trunk that drains directly into the internal jugular vein. This middlethyroid vein is not always present, and may sometimes be found only on one side of the neck. Careless handling of the vein during surgery can result in damage to the internal jugular vein and serious haemorrhage; the surgeon should identify and control the middle thyroidvein/s before the other thyroid blood vessels.
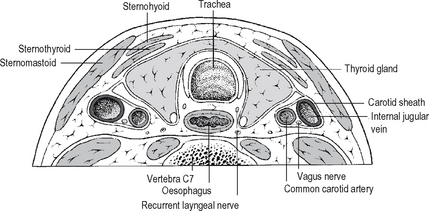
The recurrent laryngeal nerves (arising from the vagus) lie in the groove between trachea and oesophagus (Fig. 14.3) in close relationship to the inferior thyroid artery, parathyroid glands and the capsule of the thyroid gland. The position of each nerve may be anomalous due to ‘normal’ anatomical variation or thyroid gland pathology displacing the nerve. Their relationship to the inferior thyroid artery is variable –posterior or anterior to the artery, or between its branches. The nerve is also vulnerable to injury just before it enters the larynx below the inferior constrictormuscle: here it lies very close to the thyroid, often within a dense condensation of fascia binding the thyroid gland to the trachea – the ligament of Berry. The recurrent nerves must be regarded as vulnerable during thyroid surgery, at the very least an attempt should be made to identify the nerve and protect it at all thyroid procedures. The recurrent nerves supply the muscles of the larynx except cricothyroid, and sensation in the airway below the vocal folds. Injury to a nerve results in reduced mobility or paralysis of the vocal cord on that side as well as a sensory deficit in the larynx. Symptoms and signs that arise from unilateral nerve injury will depend upon the position of the affected vocal cord (midline or lateral) and the degree of compensation by the contralateral normal cord. The patient and the surgeon may sometimes be unaware of voice change. If the cord is paralysed in the lateral position and the gap between the cords is significant, there is hoarseness, rapid tiring, reduced voice power and a weak cough. Bilateral nerve damage affects the airway rather than the voice. If the cords lie in an adducted position there is dyspnoea and stridor. Urgent tracheostomy is required; this may be temporary or permanent depending upon the degree of recovery of nerve function.
Closely related to the superior thyroid vascular pedicle on each side of the neck is the external laryngeal branch of the superior laryngeal nerve. This may be identified on the surface of the cricothyroid muscle but in 20% of cases it lies within the muscle and in 20% intimately related to the superior thyroid artery or its branches. This nerve supplies the cricothyroid muscle which alters the tension of the vocal cord. Damage to the nerve may result in subtle changes that include voice fatigue or, a sudden decrease in the strength of the voice. The nerve should be protected as equally as the recurrent laryngeal nerve by ligation of the superior pole vessels on the capsule of the gland.
STRUCTURE AND FUNCTION
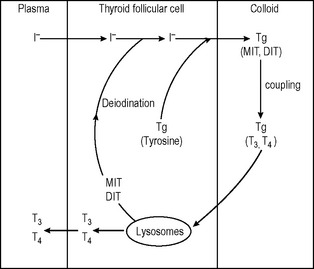
In each follicle, the epithelial cells are cuboidal when ‘resting’ and ‘columnar’ when under TSH stimulation (Fig. 14.4).
Thyroid follicular cells
The thyroid gland incorporates iodide into its cells from plasma by an active transport mechanism in which I− follows Na+. This ‘pump’ is influenced positively by TSH (thyroid-stimulating hormone) and TSH receptor antibodies (in Graves’ disease). Iodine incorporation into the cell can be blocked or inhibited by an excess of iodide, perchlorate and thiocyanate ions and some drugs – for example, digoxin.
CONTROL OF THYROID FUNCTION
Thyroid-stimulating hormone (TSH), from the anterior pituitary, has a stimulating effect on T3/T4 production. Thyrotrophin-releasing hormone (TRH), which is stored in the hypothalamus, stimulates TSH production and release. TRH reaches the anterior pituitary via the pituitary portal venous system. Therefore, with increased TRH stimulation, TSH production is raised, with a consequent rise in T3/T4 output from the thyroid. Rising levels of T3/T4 (primarily a rising T3 concentration) have an inhibitory effect on the TRH/TSH axis. Thus there is an elegant mechanism in place for the precise control of the production and release of thyroid hormones (Fig. 14.6).
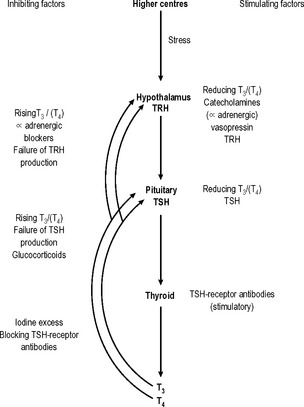
Fig. 14.6 Control of T3/T4 secretion. Feedback control of TSH production is predominantly T3 dependent.
MECHANISM OF ACTION OF THYROID HORMONES
INVESTIGATION OF THYROID FUNCTION
Thyroid function tests in routine use include TSH, free T4 and free T3. Measurement of TSH by a sensitive immunometric technique is the best single test to evaluate thyroid function. A low level of TSH and a high level of free T4 and/or free T3 will ordinarily indicate thyrotoxicosis. A high TSH combined with a low level of circulating thyroid hormone indicate a hypothyroid state.
DISORDERS OF THYROID FUNCTION
Hypothyroidism
This is defined as a hypometabolic disorder caused by a deficiency of or resistance to thyroid hormone. The many causes of hypothyroidism may be congenital or acquired. Primary hypothyroidism is the cause of 95% of adult cases; Hashimoto’s disease (chronic lymphocytic thyroiditis) is responsible for 70% of these. Myxoedema, the end result of severe long standing hypothyroidism, is associated with marked symptoms and signs, characteristic skin changes and in extreme cases, confusion and coma associated with a very high mortality. The patient has profound hypothermia, and may demonstrate hypoglycaemia, water retention, and hypoventilation. In generalised myxoedema there is accumulation of glycosaminoglycans within soft tissues, and facial and cutaneous oedema (containing mucopolysaccarides, hyaluronic acid and chondroitin sulphate). Patients with hypothyroidism sometimes present with a goitre to surgeons. The combination of abnormal thyroid function tests, positive TPO autoantibodies and sometimes aspiration cytologyis sufficient to confirm the diagnosis. Lifelong thyroxine is the treatment of choice and in most cases is associated with a reduction in size of the goitre as TSH levels fall. Thyroid lymphoma is more common in patients with lymphocytic thyroiditis. A nodule or continued enlargement of the thyroid in a patient with Hashimoto’s disease despite thyroxine treatment must be viewed with suspicion and aggressively investigated.
Hyperthyroidism
Treatment options for Graves’ disease include:
Antithyroid drugs
Patients with Graves’ disease who relapse after a course of antithyroid drugs or who cannot tolerate them because of side effects require some form of definitive treatment. Patients with toxic multinodular goitre or toxic adenoma become euthyroid with thionamides, but these drugs do not alter the natural history of the disease.
DISORDERS OF THYROID MORPHOLOGY
Malignant thyroid disease
Approximately 1% of all malignant disease arises in the thyroid.
Firstly, differentiated thyroid cancer cells, in common with normal thyroid cells, usually take up iodine – particularly when TSH levels are markedly increased. After total thyroidectomy, the patient is given T3 as thyroid hormone replacement (it has a shorter half life than T4). This is stopped and two weeks later, TSH levels are checked. If the TSH is markedly elevated an ablation dose of 131I is given whilst the TSH drive is high. The ß-particles emitted by the radio-active iodine will destroy residual thyroid and thyroid cancer cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree