Fig. 34.1
Severe dryness and fissuring of lips and nose in a patient on isotretinoin
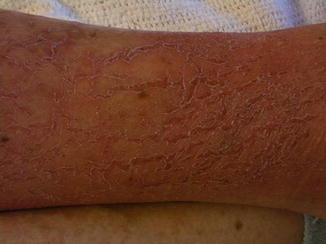
Fig. 34.2
Xerotic skin with reticulate fissuring seen in asteatotic dermatitis over the ankle in a patient on isotretinoin
There are many other rare, but reported, cutaneous side effects of isotretinoin therapy. Some patients experience an increase in Staphylococcus aureus colonization on the skin. Lip abscesses have formed as a result of bacterial colonization in patients with severe cheilitis. Some patients can experience the unfortunate event of worsening acne (Fig. 34.3) with isotretinoin. There have been case reports of patients developing acne fulminans during a course of isotretinoin, requiring cessation of therapy. Pyogenic granuloma formation is an uncommon but significantly distressing adverse effect for some patients taking isotretinoin. Normally the complication resolves after discontinuation of the medication. There are case reports of periungual pyogenic granulomas, though they can occur anywhere on the body. The mechanism by which isotretinoin causes pyogenic granuloma formation remains unclear.
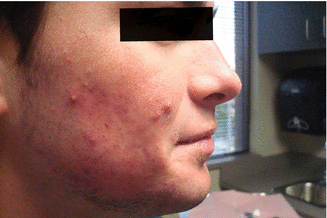

Fig. 34.3
Increased redness and inflammation of pustules after 1 month of isotretinoin therapy
Isotretinoin also has the ability to adversely affect the fingernails and toenails. Some reported adverse effects include nail fragility, median nail dystrophy, and nail brittleness. These are also transient and resolve after completion of isotretinoin course. Ingrown nails have been reported, but are rare. There are isolated reports of facial edema caused by isotretinoin, though other reports have lauded isotretinoin as a potential treatment for this type of edema.
Other rare adverse effects include increased sweating, bruising, hair abnormalities such as alopecia and telogen effluvium, flushing, excess granulation tissue, sun sensitivity, rectal bleeding with anal fissures, pseudoporphyria, and acute generalized exanthematous pustulosis.
The most common ocular side effect of isotretinoin is conjunctivitis, occurring in 20–50 % of patients during therapy. Other common complaints include hordeolum, chalazion, and blepharitis. Meibomian gland dysfunction is one mechanism that can lead to dry eye symptoms. Isotretinoin alters gene expression and cell proliferation in the meibomian glands, which could, in part, lead to dry eye symptoms. There are reports of sub-conjunctival hemorrhage associated with isotretinoin, but this does not appear to be a common finding, is asymptomatic, and self-resolves. Other more serious, but less common, ocular side effects include corneal opacities and decreased night vision.
Tretinoin (Retin-A®)
Topical tretinoin (13-cis-retinoic acid) has become the standard agent in the treatment of acne vulgaris, primarily due to its action on the initial part of the pathological process, the microcomedone. Tretinoin has shown to dramatically reduce lesion counts. One of the other most common usages of topical tretinoin is for photo-aging, with many studies showing improvements in fine and coarse wrinkling, roughness, and redness. More rarely, oral tretinoin has been used systemically for acute promyelocytic leukemia (APML).
Tretinoin, like other topical retinoids, has predictable adverse effects, including dryness and skin irritation, likely from the hydroalcoholic ingredients. These are dose-dependent and resolve after cessation of therapy. Pyogenic granulomas have been reported following tretinoin therapy, developing after initiation and disappearing after cessation of the drug. Many studies have shown that tretinoin is a photosensitizer; however, neither phototoxic nor photoallergic reactions are generally observed. This “photosensitivity” is most likely secondary to thinning of the stratum corneum with enhanced ultraviolet light susceptibility.
Acitretin (Soriatane®)
Previously known as etretin, acitretin is the active metabolite of etretinate and has basically supplanted etretin due to a better safety profile. Acitretin is most commonly used to treat psoriasis and has been found to be particularly effective in treating the pustular, nail, and erythrodermic variants. It is currently the only non-immunomodulatory FDA-approved systemic psoriasis treatment. Other indications include Darier’s disease and chemoprevention of non-melanoma skin cancers in organ transplant and immunosuppressed patients, hidradenitis suppurativa, and treatment of congenital disorders of keratinization.
Acitretin specifically inhibits the migration of polymorphonuclear cells into the epidermis by preventing release of toxic oxygen species, decreasing proliferation of lymphocytes, and decreasing antigen presentation of keratinocytes in vitro.
Pharmacologically, acitretin has a half-life of 1–2 days and is much less lipophilic than etreinate, allowing more rapid elimination from the body. Dosing for psoriasis and other disorders normally starts at 10–25 mg daily and is then escalated as tolerated and as needed, often reaching doses of 40–50 mg daily depending on disease control and patient tolerance.
Acitretin shares many of the same adverse effects of oral isotretinoin. Mucocutaneous adverse effects are common and include cheilitis, xerosis, nail dystrophy, and pyogenic granuloma. These mucocutaneous side effects respond to supportive measures, are mostly dose-dependent, and resolve once the medication is discontinued. Similar to isotretinoin, it is a significant teratogen and prescribers need to use caution when treating women of child-bearing age. Some authors recommend monthly pregnancy testing for those women taking acitretin. Indeed, the elimination time for acitretin is prolonged, meaning patients should not give blood or become pregnant for 3 years after treatment. Even when acitretin falls to undetectable levels in the blood, there is the potential for it to re-esterify into etretinate. Alcohol consumption appears to contribute to this re-esterification.
Etretinate
Etretinate (Tegison) is an aromatic retinoid known for its successful treatment of extensive plaque-like, pustular and erythrodermic psoriasis, Darier’s disease, lichen planus, oral leukoplakia, porokeratosis of Mibelli, lichen planus, and hereditary ichthyoses, with less success in the treatment of acne. Etretinate is deposited in the epidermis in 7–10 days and subsequently prevents epidermal accumulation. Peak plasma concentrations occur approximately 4 hours after administration, with a half-life of 10 days. After long-term administration, it is stored in adipose tissue, which is then eliminated slowly from the body, sometimes taking 2 years. Although elimination can be prolonged, toxicity after chronic administration is rare with a dose between 10 and 50 mg/day. Case reports have been published with harlequin ichthyosis infants who have survived on etretinate. A prophylactic effect was reported in patients with malignant degeneration of porokeratosis of Mibelli. In one patient, no new epithelial tumors developed while on a 2-year course of retinoids. Etretinate has also been useful in the treatment of epidermodysplasia verruciformis. Lichen planus is often self-remitting; however, chronic forms of the skin and mucous membranes can be severe and painful. Etretinate has been useful in the treatment of chronic lichen planus, with the best results in the erosive-atrophic form.
The most commonly reported side effects of etretinate include mucocutaneous dryness, and liver and triglyceride abnormalities. Colonization of the nares with Staphylococcus aureus has been reported. Patients on etretinate may feel excessively cold, presumably due to excessive heat loss through the erythematous skin. An increased photosensitivity has been reported with etretinate use in less than 13 % of patients. However, the combination of PUVA and etretinate has demonstrated success in the treatment of psoriasis, and due to the lack of evidence supporting this so-called photosensitivity when patients are subjected to phototesting, many patients are still encouraged to seek some sunlight in management of their psoriasis. Palmoplantar pustular and papular lesions have been reported in patients on etretinate therapy. Saurat et al. showed this was due to etretinate-induced miliaria in hyperhydrotic patients, and the papules could be early psoriatic lesions. Erythema multiforme was reported in two patients after etretinate therapy, with confirmation by re-challenge in one of the patients. Etretinate has also been reported to cause diffuse alopecia, specifically telogen effluvium, in 10–75 % of patients, and this is the most common cause of discontinuation of the medication, especially in women. This is directly proportional to the dose, with the scalp being the most commonly affected site, and eyebrows and eyelashes rarely affected. The hair shafts may be different upon re-growth, with curly, kinky, or twisted hairs replacing formerly straight hair shafts. Two cases of pyogenic granuloma formation have been reported. Nail changes can vary, with decreased shine, thinning, fragility, and/or loosening/loss of the nail entirely. These changes can occur alone or in combination with redness and edema of the nail bed as well as excess granulation tissue deposition.
Adapalene (Differin®)
Adapalene is a receptor-selective retinoid analog that binds with the highest affinity to members of the RAR family. Like topical tretinoin, adapalene is used as a topical treatment for acne vulgaris. The medication is available in 0.1 and 0.3 % gel and cream forms and is used daily for control of acne and some cases of pustular rosacea.
Numerous studies have compared adapalene with tretinoin regarding their respective efficacy in treating acne. A large meta-analysis concluded that topical adapalene 0.1 % was more effective than topical tretinoin 0.025 % and was better tolerated by patients. Other subsequent studies have mixed results, some demonstrating better efficacy with higher concentrations of tretinoin than 0.025 % but also suggesting that adapalene is better tolerated by patients, with less incidence of irritant dermatitis. In any case, it is an effective and sometimes better-tolerated alternative to tretinoin.
Adverse effects of adapalene are somewhat similar to tretinoin though, as discussed above, can be better tolerated. The most common side effects include skin irritation, xerosis, and mild exfoliation. These are controlled via supportive measures, with one study finding daily moisturizer use an effective means of considerably reducing skin irritation and dryness. Rarely, pyogenic granulomas have been associated with topical adapalene therapy.
Tazarotene (Tazorac®)
Tazarotene is available in concentrations of 0.05 and 0.1 % in gel and cream forms. A foam form is currently being evaluated in clinical trials. An oral form is not currently available in the United States and has not been approved by the FDA.
Tazarotene is indicated for use in patients with plaque-type psoriasis, acne vulgaris, and photo-aged skin. Studies have found that tazarotene performed favorably compared to all strengths of tretinoin in controlling inflammatory acne lesions. Studies inconsistently find tazarotene may be slightly more irritating than tretinoin.
As with other topical retinoids, the most common adverse effects include cheilitis and irritant dermatitis at the site of application. Similarly, the adverse effects are normally dose-dependent and resolve after discontinuation. As with other topical and systemic retinoids, there are cases of pyogenic granuloma formation after topical application of tazarotene, though this seems to be a rare adverse effect.
Oral tazarotene is atypical in that it is not associated with the adverse events typical for other systemic retinoids (hyperlipidemia, etc.). It is considered pregnancy category X and may be associated with more severe skeletal abnormalities.
Bexarotene
Bexarotene (Targretin®) is the first synthetic retinoid X receptor–selective retinoid that is FDA-approved (1999) for cutaneous T-cell lymphoma (CTCL) treatment, with a response rate of 50 % in all stages of the disease. The mechanism of action in CTCL is presumably via apoptosis, but remains largely unknown. Safety and effectiveness of bexarotene have been shown when used as both monotherapy and combination therapy, but therapeutic monitoring is still recommended. It is very expensive.
Although generally well tolerated, adverse cutaneous side effects have been reported, such as pruritus and rash. These effects are proportional to the bexarotene dose. Bagazgoitia et al. reported a 55-year-old woman with Sézary syndrome who developed a progressive generalized exfoliative erythroderma after initiation of bexarotene. Similarly, Ruiz-de-Casas et al. reported granulomatous papules and nodules on the face and left arm in a 39-year-old woman with Sézary syndrome after treatment with bexarotene. In both of these cases, the cutaneous reactions and lesions disappeared when bexarotene was discontinued.
Alitretinoin (Panretin®)
Alitretinoin (9-cis-retinoic acid,) is an isomer of tretinoin with significant anti-inflammatory and immunomodulatory activity. Alitretinoin can bind to all six known intracellular retinoid receptors (RAR-α, -β, -γ, and RXR-α, -β, -γ), with a slightly higher affinity for RAR receptors. Thus, alitretinoin can inhibit cellular proliferation, induce terminal cellular differentiation, and induce apoptosis (the latter of which is regulated by RXRs). Although priced over ten times higher than tretinoin and tazarotene, alitretinoin is currently the only licensed product for moderate to severe chronic hand eczema that is otherwise unresponsive to potent topical corticosteroids. Other indications for topical alitretinoin include photoaging, cutaneous T-cell lymphoma, pyogenic granulomas, and Kaposi sarcoma.
Alitretinoin is generally safe and well-tolerated. However, few mild-to-moderately severe cutaneous adverse events have been reported that are limited to the site of application. These include rash, erythema, pruritus, pain, exfoliative dermatitis, tingling, and edema, with rash and erythema being the most common. Walmesely et al. reported one patient who developed cellulitis and bacteremia after scratching a lesion that was treated with alitretinoin. These reactions were reversible on reduction in frequency or suspension of application. Although retinoids as a class have been associated with photosensitivity, alitretinoin has not been associated with this side effect. In vitro studies have demonstrated that alitretinoin exhibits a minimal photosensitizing effect; however, it is still recommended that patients minimize sun exposure during use. Due to the success of other retinoids (tazarotene and tretinoin) in the treatment of photoaging, Baumann et al. evaluated the efficacy of alitretinoin for this indication. Aliretinoin 0.1 % topical get was well-tolerated by 20 patients and both benign and precancerous skin lesions showed improvement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


