51
Clinical Use of Antimicrobial Agents
CASE STUDY
A 51-year-old alcoholic patient presents to the emergency department with fever, headache, neck stiffness, and altered mental status for 12 hours. Vital signs are blood pressure 90/55 mm Hg, pulse 120/min, respirations 30/min, temperature 40°C [104°F] rectal. The patient is minimally responsive to voice and does not follow commands. Examination is significant for a right third cranial nerve palsy and nuchal rigidity. Laboratory results show a white blood cell count of 24,000/mm3 with left shift, but other hematologic and chemistry values are within normal limits. An emergency CT scan of the head is normal. Blood cultures are obtained, and a lumbar puncture reveals the following cerebrospinal fluid (CSF) values: white blood cells 5000/mm3, red blood cells 10/mm3, protein 200 mg/dL, glucose 15 mg/dL (serum glucose 96 taken at same time). CSF Gram stain reveals gram-positive cocci in pairs. What is the most likely diagnosis in this patient? What organisms should be treated empirically? Are there other pharmacologic interventions to consider before initiating antimicrobial therapy?
The development of antimicrobial drugs represents one of the most important advances in therapeutics, both in the control or cure of serious infections and in the prevention and treatment of infectious complications of other therapeutic modalities such as cancer chemotherapy, immunosuppression, and surgery. However, evidence is overwhelming that antimicrobial agents are vastly overprescribed in outpatient settings in the United States, and the availability of antimicrobial agents without prescription in many developing countries has—by facilitating the development of resistance—already severely limited therapeutic options in the treatment of life-threatening infections. Therefore, the clinician should first determine whether antimicrobial therapy is warranted for a given patient. The specific questions one should ask include the following:
1. Is an antimicrobial agent indicated on the basis of clinical findings? Or is it prudent to wait until such clinical findings become apparent?
2. Have appropriate clinical specimens been obtained to establish a microbiologic diagnosis?
3. What are the likely etiologic agents for the patient’s illness?
4. What measures should be taken to protect individuals exposed to the index case to prevent secondary cases, and what measures should be implemented to prevent further exposure?
5. Is there clinical evidence (eg, from well-executed clinical trials) that antimicrobial therapy will confer clinical benefit for the patient?
Once a specific cause is identified based on specific microbiologic tests, the following further questions should be considered:
1. If a specific microbial pathogen is identified, can a narrower-spectrum agent be substituted for the initial empiric drug?
2. Is one agent or a combination of agents necessary?
3. What are the optimal dose, route of administration, and duration of therapy?
4. What specific tests (eg, susceptibility testing) should be undertaken to identify patients who will not respond to treatment?
5. What adjunctive measures can be undertaken to eradicate the infection? For example, is surgery feasible for removal of devitalized tissue or foreign bodies—or drainage of an abscess—into which antimicrobial agents may be unable to penetrate? Is it possible to decrease the dosage of immunosuppressive therapy in patients who have undergone organ transplantation? Is it possible to reduce morbidity or mortality due to the infection by reducing host immunologic response to the infection (eg, by the use of corticosteroids for the treatment of severe Pneumocystis jiroveci pneumonia or meningitis due to Streptococcus pneumoniae)?
 EMPIRIC ANTIMICROBIAL THERAPY
EMPIRIC ANTIMICROBIAL THERAPY
Antimicrobial agents are frequently used before the pathogen responsible for a particular illness or the susceptibility to a particular antimicrobial agent is known. This use of antimicrobial agents is called empiric (or presumptive) therapy and is based on experience with a particular clinical entity. The usual justification for empiric therapy is the hope that early intervention will improve the outcome; in the best cases, this has been established by placebo-controlled, double-blind prospective clinical trials. For example, treatment of febrile episodes in neutropenic cancer patients with empiric antimicrobial therapy has been demonstrated to have impressive morbidity and mortality benefits even though the specific bacterial agent responsible for fever is determined for only a minority of such episodes.
Finally, there are many clinical entities, such as certain episodes of community-acquired pneumonia, in which it is difficult to identify a specific pathogen. In such cases, a clinical response to empiric therapy may be an important clue to the likely pathogen.
Frequently, the signs and symptoms of infection diminish as a result of empiric therapy, and microbiologic test results become available that establish a specific microbiologic diagnosis. At the time that the pathogenic organism responsible for the illness is identified, empiric therapy is optimally modified to definitive therapy, which is typically narrower in coverage and is given for an appropriate duration based on the results of clinical trials or experience when clinical trial data are not available.
Approach to Empiric Therapy
Initiation of empiric therapy should follow a specific and systematic approach.
A. Formulate a Clinical Diagnosis of Microbial Infection
Using all available data, the clinician should determine that there is a clinical syndrome compatible with infection (eg, pneumonia, cellulitis, sinusitis).
B. Obtain Specimens for Laboratory Examination
Examination of stained specimens by microscopy or simple examination of an uncentrifuged sample of urine for white blood cells and bacteria may provide important immediate etiologic clues. Cultures of selected anatomic sites (blood, sputum, urine, cerebrospinal fluid, and stool) and nonculture methods (antigen testing, polymerase chain reaction, and serology) may also confirm specific etiologic agents.
C. Formulate a Microbiologic Diagnosis
The history, physical examination, and immediately available laboratory results (eg, Gram stain of urine or sputum) may provide highly specific information. For example, in a young man with urethritis and a Gram-stained smear from the urethral meatus demonstrating intracellular gram-negative diplococci, the most likely pathogen is Neisseria gonorrhoeae. In the latter instance, however, the clinician should be aware that a significant number of patients with gonococcal urethritis have negative Gram stains for the organism and that a significant number of patients with gonococcal urethritis harbor concurrent chlamydial infection that is not demonstrated on the Gram-stained smear.
D. Determine the Necessity for Empiric Therapy
Whether or not to initiate empiric therapy is an important clinical decision based partly on experience and partly on data from clinical trials. Empiric therapy is indicated when there is a significant risk of serious morbidity or mortality if therapy is withheld until a specific pathogen is detected by the clinical laboratory.
In other settings, empiric therapy may be indicated for public health reasons rather than for demonstrated superior outcome of therapy in a specific patient. For example, urethritis in a young sexually active man usually requires treatment for N gonorrhoeae and Chlamydia trachomatis despite the absence of microbiologic confirmation at the time of diagnosis. Because the risk of noncompliance with follow-up visits in this patient population may lead to further transmission of these sexually transmitted pathogens, empiric therapy is warranted.
E. Institute Treatment
Selection of empiric therapy may be based on the microbiologic diagnosis or a clinical diagnosis without available microbiologic clues. If no microbiologic information is available, the antimicrobial spectrum of the agent or agents chosen must necessarily be broader, taking into account the most likely pathogens responsible for the patient’s illness.
Choice of Antimicrobial Agent
Selection from among several drugs depends on host factors that include the following: (1) concomitant disease states (eg, AIDS, neutropenia due to the use of cytotoxic chemotherapy, organ transplantation, severe chronic liver or kidney disease) or the use of immunosuppressive medications; (2) prior adverse drug effects; (3) impaired elimination or detoxification of the drug (may be genetically predetermined but more frequently is associated with impaired renal or hepatic function due to underlying disease); (4) age of the patient; (5) pregnancy status; and (6) epidemiologic exposure (eg, exposure to a sick family member or pet, recent hospitalization, recent travel, occupational exposure, or new sexual partner).
Pharmacologic factors include (1) the kinetics of absorption, distribution, and elimination; (2) the ability of the drug to be delivered to the site of infection; (3) the potential toxicity of an agent; and (4) pharmacokinetic or pharmacodynamic interactions with other drugs.
Knowledge of the susceptibility of an organism to a specific agent in a hospital or community setting is important in the selection of empiric therapy. Pharmacokinetic differences among agents with similar antimicrobial spectrums may be exploited to reduce the frequency of dosing (eg, ceftriaxone, ertapenem, or daptomycin may be conveniently given once every 24 hours). Finally, increasing consideration is being given to the cost of antimicrobial therapy, especially when multiple agents with comparable efficacy and toxicity are available for a specific infection. Changing from intravenous to oral antibiotics for prolonged administration can be particularly cost-effective.
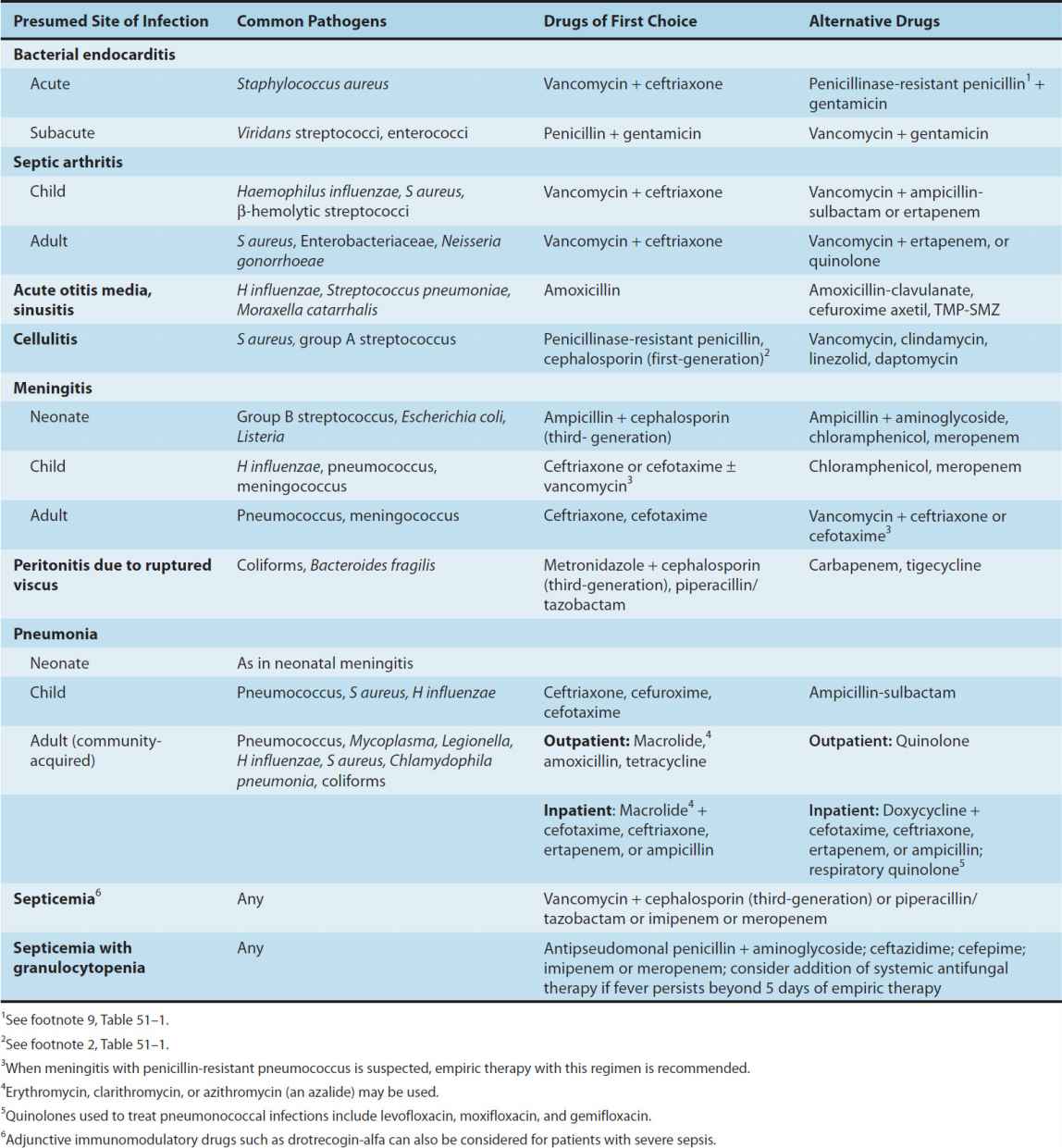
Brief guides to empiric therapy based on presumptive microbial diagnosis and site of infection are given in Tables 51–1 and 51–2.
TABLE 51–1 Empiric antimicrobial therapy based on microbiologic etiology.
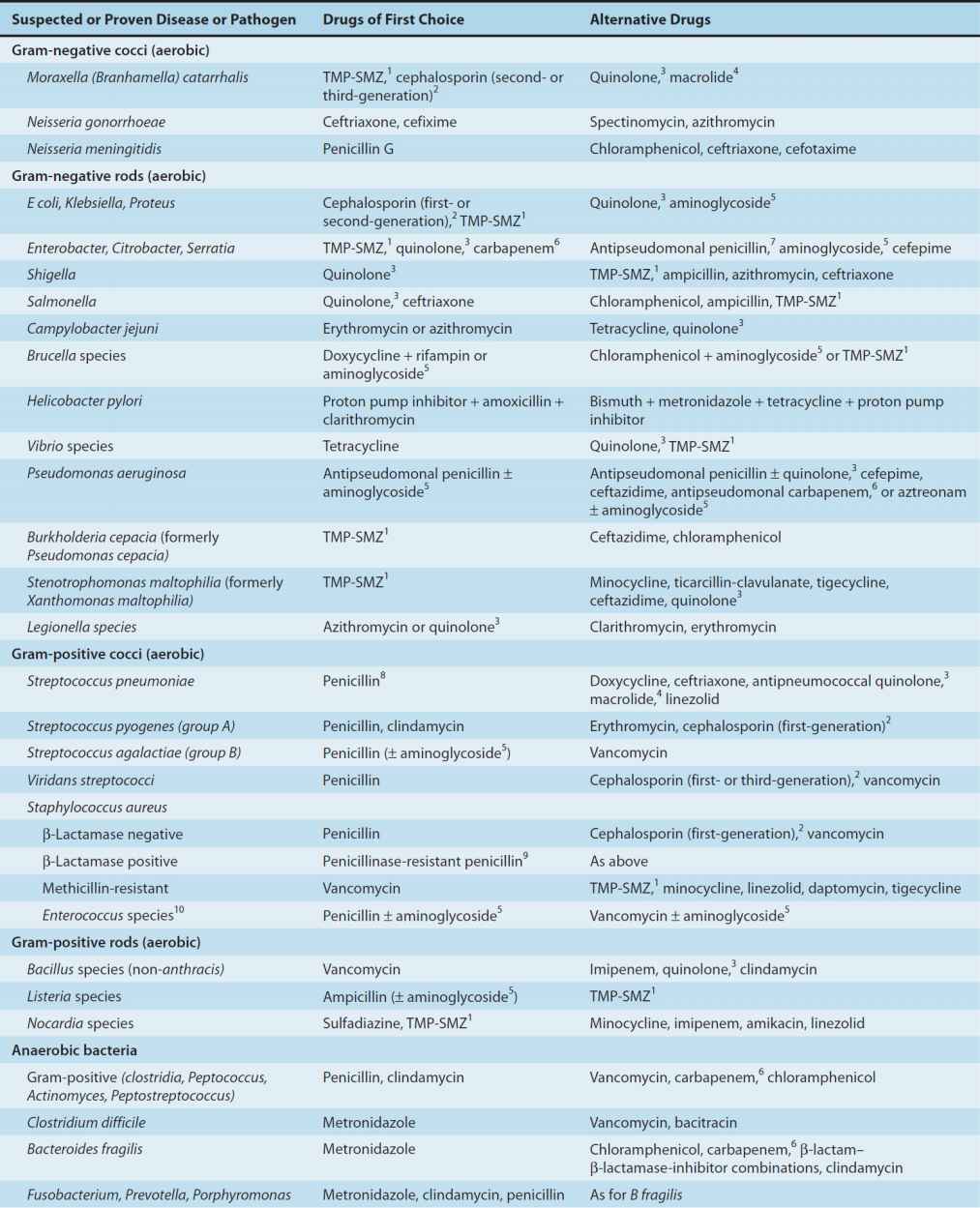
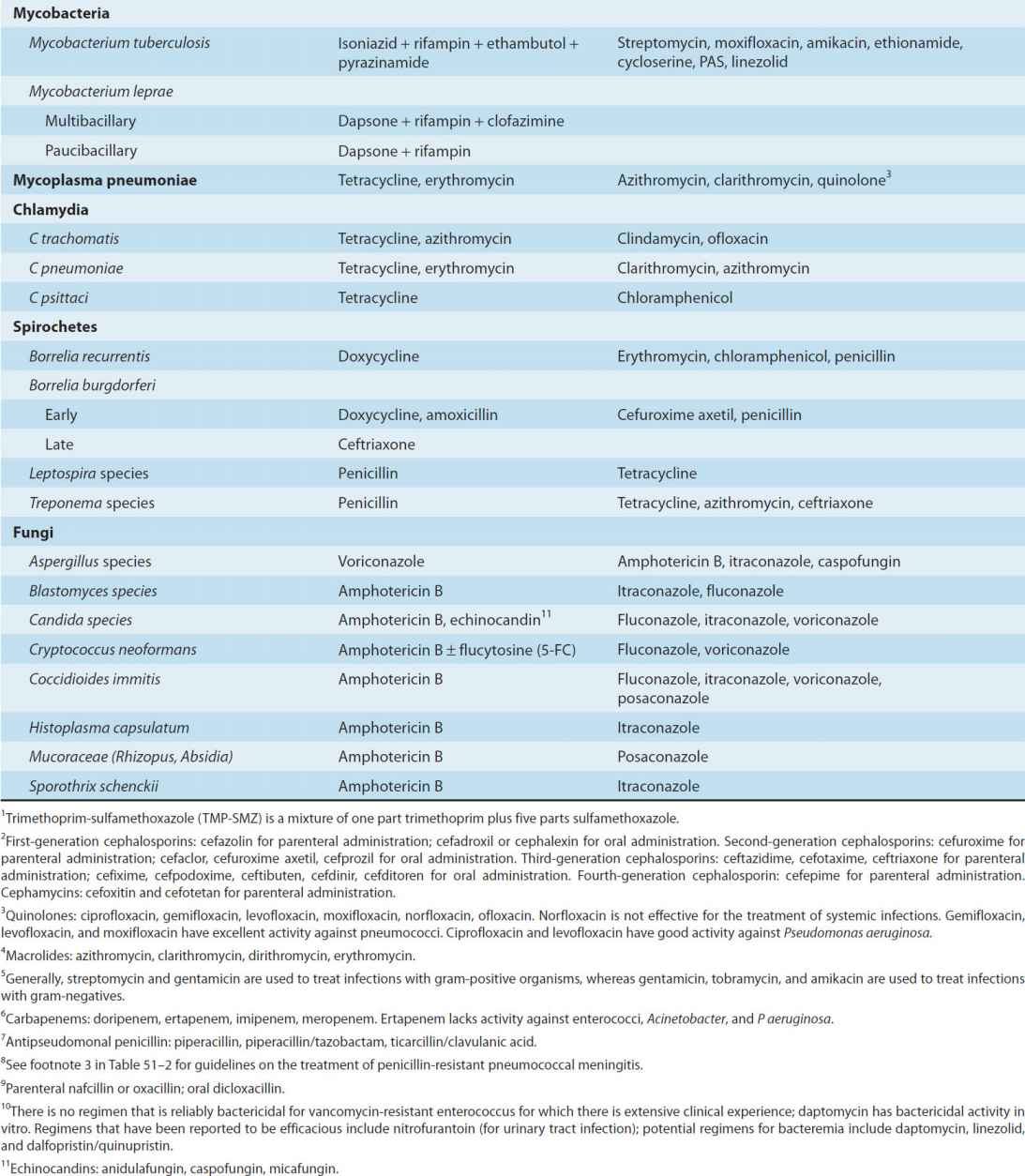
TABLE 51–2 Empiric antimicrobial therapy based on site of infection.
 ANTIMICROBIAL THERAPY OF INFECTIONS WITH KNOWN ETIOLOGY
ANTIMICROBIAL THERAPY OF INFECTIONS WITH KNOWN ETIOLOGY
INTERPRETATION OF CULTURE RESULTS
Properly obtained and processed specimens for culture frequently yield reliable information about the cause of infection. The lack of a confirmatory microbiologic diagnosis may be due to the following:
1. Sample error, eg, obtaining cultures after antimicrobial agents have been administered, inadequate volume or quantity of specimen obtained, or contamination of specimens sent for culture
2. Noncultivable or slow-growing organisms (Histoplasma capsulatum, Bartonella or Brucella species), in which cultures are often discarded before sufficient growth has occurred for detection
3. Requesting bacterial cultures when infection is due to other organisms
4. Not recognizing the need for special media or isolation techniques (eg, charcoal yeast extract agar for isolation of Legionella species, shell-vial tissue culture system for rapid isolation of cytomegalovirus)
Even in the setting of a classic infectious disease for which isolation techniques have been established for decades (eg, pneumococcal pneumonia, pulmonary tuberculosis, streptococcal pharyngitis), the sensitivity of the culture technique may be inadequate to identify all cases of the disease.
GUIDING ANTIMICROBIAL THERAPY OF ESTABLISHED INFECTIONS
Susceptibility Testing
Testing bacterial pathogens in vitro for their susceptibility to antimicrobial agents is extremely valuable in confirming susceptibility, ideally to a narrow-spectrum nontoxic antimicrobial drug. Tests measure the concentration of drug required to inhibit growth of the organism (minimal inhibitory concentration [MIC]) or to kill the organism (minimal bactericidal concentration [MBC]). The results of these tests can then be correlated with known drug concentrations in various body compartments. Only MICs are routinely measured in most infections, whereas in infections in which bactericidal therapy is required for eradication of infection (eg, meningitis, endocarditis, sepsis in the granulocytopenic host), MBC measurements occasionally may be useful.
Specialized Assay Methods
A. Beta-Lactamase Assay
For some bacteria (eg, Haemophilus species), the susceptibility patterns of strains are similar except for the production of β lactamase. In these cases, extensive susceptibility testing may not be required, and a direct test for β lactamase using a chromogenic β-lactam substrate (nitrocephin disk) may be substituted.
B. Synergy Studies
Synergy studies are in vitro tests that attempt to measure synergistic, additive, indifferent, or antagonistic drug interactions. In general, these tests have not been standardized and have not correlated well with clinical outcome. (See section on Antimicrobial Drug Combinations for details.)
MONITORING THERAPEUTIC RESPONSE: DURATION OF THERAPY
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


