DRUG CLASSES
Primary antitubercular
Secondary antitubercular
PHARMACOLOGY IN PRACTICE
Betty Peterson has come into the clinic with complaints of a chronic cough. Ms. Peterson requests that a TB skin test be performed as well as a chest x-ray. She is concerned that she will be diagnosed with TB, telling you that she watched a TV show about the rise in TB in crowded areas. When questioned, she states that “a lot of people” live in the apartment next door. Should Betty Peterson be assessed for TB?
Tuberculosis (TB) is a major health problem throughout the world, especially in Asia and Sub-Saharan Africa; almost 1.5 million deaths each year are caused by TB. The World Health Organization (WHO) predicts that 8 million to 9 million people worldwide will contract this disease each year. Individuals living in crowded conditions, those with compromised immune systems, and those with debilitative conditions are especially susceptible to TB. People with human immunodeficiency virus (HIV) infection are especially at risk for TB because of their compromised immune systems.
Tuberculosis is an infectious disease caused by the Mycobacterium tuberculosis bacterium. The pathogen is also referred to as the tubercle bacillus. The disease is transmitted from one person to another by droplets dispersed in the air when an infected person coughs or sneezes. These droplets are then inhaled by noninfected persons. Although TB primarily affects the lungs, other organs may be involved.
 NURSING ALERT
NURSING ALERT
One quarter of all HIV-related deaths are caused by active TB. Yet, TB in patients infected with HIV can be difficult to diagnose. HIV patients’ immune systems are deficient, so TB skin tests may not show a reaction even when the disease is present. X-ray studies, sputum analyses, or physical examinations may be needed to diagnose M. tuberculosis infection accurately in patients with HIV infection.
Extrapulmonary (outside of the lungs) tuberculosis is the term used to distinguish TB affecting other organs of the body from the infection located only in the lungs. Organs that can be affected include the liver, bones, spleen, and adrenal glands. Figure 10.1 illustrates the areas affected by TB and the drugs used in treatment.
Antitubercular drugs are classified as primary (first-line) and secondary (second-line) drugs. Primary drugs provide the foundation for treatment. Tuberculosis responds well to long-term treatment with a combination of three or more antitubercular drugs. Antitubercular drugs are also used as prophylactic therapy to prevent the spreading of TB. Secondary drugs are used for multidrug-resistant TB (MDR-TB), which are more costly and toxic than primary drugs.
Secondary drugs are also used to treat extrapulmonary TB. The primary antitubercular drugs are discussed in this chapter. Both primary and secondary antitubercular drugs are listed in the Summary Drug Table: Antitubercular Drugs. Certain fluoroquinolones such as ciprofloxacin, ofloxacin, and levofloxacin have proven effective against TB and are considered secondary drugs (see Chapter 9).
Actions
Many antitubercular drugs are bacteriostatic against the M. tuberculosis bacillus. These drugs usually act to inhibit bacterial cell wall synthesis, which slows the multiplication rate of the bacteria. Isoniazid is bactericidal, with rifampin and streptomycin having some bactericidal activity.
Uses
Antitubercular drugs are used in a protocol, called Standard Treatment, to treat active TB. Isoniazid (INH), however, may be used alone in preventive therapy (prophylaxis). For example, when a person is diagnosed with TB, family members of the infected individual must be given prophylactic treatment with isoniazid for 6 to 9 months. Display 10.1 identifies prophylactic uses for isoniazid.
Display 10.1 Prophylactic Uses for Isoniazid (INH)
Isoniazid may be used in the following situations:
• Household members and other close associates of those recently diagnosed as having tuberculosis
• Those whose tuberculin skin test has become positive in the last 2 years
• Those with positive skin tests whose radiographic (x-ray) findings indicate nonprogressive, healed, or quiescent (causing no symptoms) tubercular lesions
• Those at risk for developing tuberculosis (e.g., those with Hodgkin’s disease, severe diabetes mellitus, leukemia, and other serious illnesses and those receiving corticosteroids or drug therapy for a malignancy)
• All patients younger than 35 years (primarily children to age 7) who have a positive skin test
• Persons with acquired immunodeficiency syndrome (AIDS) or those who are positive for HIV and have a positive tuberculosis skin test or a negative tuberculosis skin test but have a history of a prior significant reaction to purified protein derivative (a skin test for tuberculosis)
Standard Treatment
Standard treatment for TB is divided into two phases: the initial phase, followed by a continuing phase. During the initial phase, drugs are used to kill the rapidly multiplying M. tuberculosis and to prevent drug resistance. The initial phase lasts approximately 2 months and the continuing phase approximately 4 months, with the total treatment regimen lasting for 6 to 9 months, depending on the patient’s response to therapy.
The Centers for Disease Control and Prevention (CDC) recommends that treatment begin as soon as possible after the diagnosis of TB. The recommended treatment regimen is for the administration of the primary drugs—rifampin (Rifadin), isoniazid (INH), pyrazinamide, and ethambutol (Myambutol)—for a minimum of 2 months. The second or continuation phase includes only the drugs rifampin and isoniazid. The CDC recommends this phase for 4 months or up to 7 months in special populations. These special circumstances include the following:
• Positive sputum culture after completion of initial treatment
• Cavitary (hole or pocket of) disease and positive sputum culture after initial treatment
• When pyrazinamide was not included in the initial treatment
• Positive sputum culture after initial treatment in a patient with previously diagnosed HIV infection
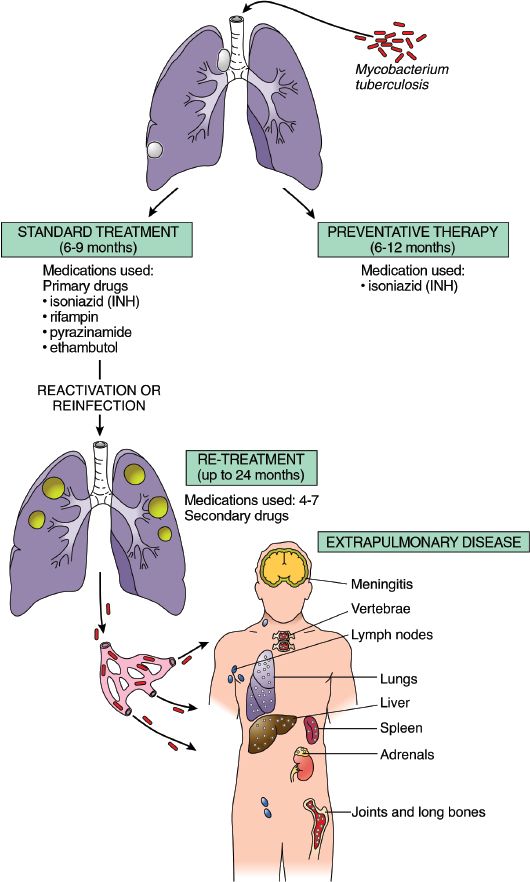
Figure 10.1 Sites of tuberculosis infection and treatment options. (Adapted from Rubin, E., & Farber, J. L. [1999]. Pathology [3rd ed.]. Philadelphia: Lippincott Williams & Wilkins.)
Retreatment
At times, treatment fails because of inadequate initial drug treatment or noncompliance with the drug regimen. When treatment fails, retreatment is necessary using the secondary drugs. Retreatment generally includes the use of four or more antitubercular drugs. Retreatment drug regimens most often consist of ethionamide (Trecator), aminosalicylic acid (Paser), cycloserine (Seromycin), and capreomycin (Capastat). Ofloxacin and ciprofloxacin (Cipro) may also be used in retreatment. Sometimes during retreatment, seven or more drugs may be used, with the ineffective drugs discontinued when susceptibility test results are available. Treatment is individualized based on the susceptibility of the microorganism. Up to 24 months of continued treatment after sputum cultures are no longer positive for TB can be part of the plan.
Resistance to the Antitubercular Drugs
Of increasing concern is MDR-TB. Bacterial resistance develops, sometimes rapidly, because of lack of adherence to lengthy drug dosing schedules. Treatment is individualized and based on laboratory studies that identify the drugs to which the organism is susceptible. The CDC recommends using three or more drugs with initial therapy, as well as in retreatment, because using a combination of drugs slows the development of bacterial resistance. Tuberculosis caused by drug-resistant organisms should be considered in patients who have no response to therapy and in patients who have been treated in the past.
This chapter discusses the following primary antitubercular drugs: ethambutol, isoniazid, pyrazinamide, and rifampin. Other primary and secondary drugs are listed in the Summary Drug Table: Antitubercular Drugs.
ETHAMBUTOL
Adverse Reactions
Generalized Reactions
• Dermatitis and pruritus (itching)
• Joint pain
• Anorexia
• Nausea and vomiting
Severe Reactions
• Anaphylactoid reactions (unusual or exaggerated allergic reactions)
• Optic neuritis (a decrease in visual acuity and changes in color perception); optic neuritis is dose related.
Contraindications, Precautions, and Interactions
Ethambutol is not recommended for patients with a history of hypersensitivity to the drug or children younger than 13 years. The drug is used with caution during pregnancy (category B), in patients with hepatic or renal impairment, and in patients with diabetic retinopathy or cataracts.
ISONIAZID
Adverse Reactions
The incidence of adverse reactions appears to be higher when larger doses of isoniazid are prescribed.
Generalized Reactions
• Nausea and vomiting
• Epigastric distress
• Fever
• Skin eruptions
• Hematologic changes
• Jaundice
• Hypersensitivity
Toxicity
• Peripheral neuropathy (numbness and tingling of the extremities) is the most common symptom of toxicity.
• Severe hepatitis has been associated with isoniazid therapy and may appear after many months of treatment and be fatal.
Contraindications and Precautions
Isoniazid is contraindicated in patients with a history of hypersensitivity to the drug. The drug is used with caution during pregnancy (category C) or lactation and in patients with hepatic and renal impairment.
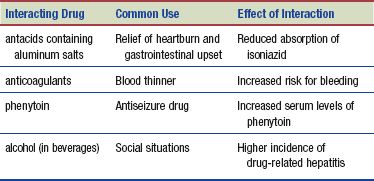
Interactions
The following interactions may occur when isoniazid is administered with another agent:
PYRAZINAMIDE
Adverse Reactions
Generalized Reactions
• Nausea and vomiting
• Diarrhea
• Myalgia (aches)
• Rashes
Hepatotoxicity
Hepatotoxicity is the principal adverse reaction seen with pyrazinamide use. Symptoms of hepatotoxicity may range from none (except for slightly abnormal hepatic function test results) to a more severe reaction such as jaundice.
Contraindications and Precautions
Pyrazinamide is contraindicated in patients with a history of hypersensitivity to the drug, acute gout (a metabolic disorder resulting in increased levels of uric acid and causing severe joint pain), or severe hepatic damage.
Pyrazinamide should be used cautiously in patients during pregnancy (category C) and lactation and in patients with hepatic and renal impairment, HIV infection, and diabetes mellitus.
Interactions
When pyrazinamide is administered with the antigout medications allopurinol (Zyloprim), colchicine, or probenecid, its effectiveness decreases.
RIFAMPIN
Adverse Reactions
Generalized reactions include the following:
• Nausea and vomiting
• Epigastric distress, heartburn, fatigue
• Vertigo (dizziness)
• Rash
• Reddish-orange discoloration of body fluids (urine, tears, saliva, sweat, and sputum)
• Hematologic changes, renal insufficiency
Contraindications and Precautions
Rifampin is contraindicated in patients with a history of hypersensitivity to the drug. The drug is used with caution during pregnancy (category C) and lactation and in patients with hepatic or renal impairment.
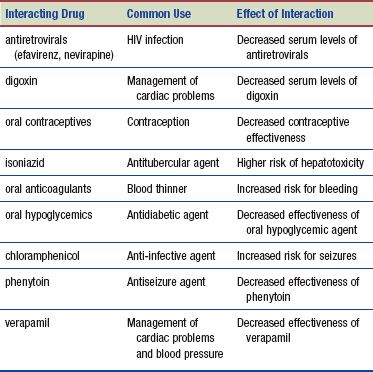
Interactions
The following interactions may occur when rifampin is administered with another agent:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


