Fig. 28.1
Symmetric, erythematous morbilliform drug reaction in a patient with DRESS syndrome
Drug is high risk, such as an aromatic AED
Fever
Liver abnormalities or other organ involvement
Leukocyte abnormalities: eosinophilia, atypical lymphocytosis, leukocytosis
Lymphadenopathy
Potential diagnostic testing includes patch testing and lymphocyte toxicity assay/lymphocyte transformation test. Patch testing is not useful for immediate diagnostic purposes because it must be done at least 6 weeks after complete recovery; in addition, its diagnostic accuracy in the case of DRESS/DIHS is not well established. The lymphocyte toxicity assay is used to predict susceptibility of patients to DRESS/DIHS; the transformation test is used to confirm diagnosis by detection of peripherally circulating drug-specific T-cells. Neither of these tests has been established as appropriate and valid in the diagnosis of DRESS/DIHS at this stage.
Testing for HHV-6 reactivation through detection of a rise in anti-HHV-6 IgG titers and/or HHV-6 DNA levels 2–3 weeks after the onset of the cutaneous reaction may be considered. HHV-6 reactivation is not detected in all DRESS/DIHS patients; this may be due to the complex interplay of immune suppression by AEDs and the anti-viral response. In a study of 100 patients, a rise in HHV-6 IgG titers was detected in 62. HHV-6 reactivation seems to be more common in more severe cases, so it may be used as a marker of prognosis.
The first step in suspected AED-induced DRESS/DIHS is to create a medication timeline to confirm timing after drug initiation and to identify any other potential culprits. The implicated drug should be stopped immediately. If the implicated drug is an aromatic AED, other aromatic AEDs should also be avoided even if they have been tolerated in the past. Cross-reactivity among aromatic AEDs is 80 %. Valproic acid, gabapentin, benzodiazepines, and levetiracetam have been suggested as safer alternatives. Cross-reactivity between aromatic and non-aromatic AEDs is less commonly reported (e.g., carbamazepine and valproic acid). Note that cross-reactivity among aromatic AEDs occurs in DRESS/DIHS, but not necessarily in other serious adverse cutaneous reactions such as SJS/TEN.
Systemic corticosteroids are often used to treat DRESS/DIHS, but efficacy has not been demonstrated by randomized controlled trials. Supportive treatment includes antipyretics, antihistamines, and topical corticosteroids and moisturizers. Symptoms may persist for weeks after drug withdrawal. Because of the possible genetic component of DRESS/DIHS, the patient should be encouraged to notify first-degree relatives.
AEDs and SJS/TEN
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening cutaneous reactions, most often drug-induced. They differ in the percent of the body surface area involved: SJS <10 %, SJS/TEN overlap 10–30 %, and TEN >30 %. More than 100 drugs have been associated with SJS/TEN; 80–95 % of cases are caused by a medication. The medications most strongly associated with SJS/TEN are AEDs, antibiotics, and xanthine oxidase inhibitors. The overall incidence of SJS, SJS/TEN overlap, and TEN is estimated to be 2–7 per million people per year; SJS is approximately three times more common than TEN. The mortality rate is substantial: approximately 1–10 % for SJS and 25–30 % for TEN.
This section will focus on AED-induced SJS/TEN; please see Chap. 24 for a discussion of SJS/TEN in more detail.
Epidemiology and Pathophysiology
AEDs considered high risk for SJS/TEN include carbamazepine, phenytoin, phenobarbital, valproic acid, and lamotrigine. Combining AEDs, in particular lamotrigine and valproic acid, increases the risk of SJS/TEN. The incidence of SJS/TEN caused by various AEDs varies significantly according to the population being studied. For example, Asian populations have a higher incidence of carbamazepine-induced SJS/TEN than non-Asian populations.
The pathophysiology of SJS/TEN is a CD8+ T-cell mediated immune reaction directed at keratinocytes. Either the offending drug induces this immune response or its metabolite binds to cellular peptides, forming an immunogenic hapten. The activation of CD8+ and other immune cells induces keratinocyte apoptosis. Particular human leukocyte antigen (HLA) allotypes confer a higher risk in some populations, and so may be involved in the pathogenesis.
There is a relationship between the HLA-B*1502 allele and carbamazepine-induced SJS/TEN in patients of Asian ancestry (specifically, Han-Chinese, Thai, Korean and Malaysian populations). The risk of carbamazepine-induced SJS/TEN is 25–220 times greater in these populations than in users with non-Asian ancestry. There have been reports of SJS/TEN induced by other AEDs in patients with the HLA-B*1502 allele, such as lamotrigine and phenytoin, but the number of cases is not high enough to demonstrate a clear risk association. The HLA-B*1502 association is only seen with carbamazepine-induced SJS/TEN, not other carbamazepine-induced cutaneous reactions (e.g., DRESS).
Presentation
There are no defining features of AED-induced SJS/TEN compared to all-cause SJS/TEN. Briefly, the presentation of SJS/TEN begins with a flu-like prodrome lasting for several days. Onset is usually 1–3 weeks after drug initiation. The cutaneous manifestations are sudden onset in a symmetric generalized distribution, predominantly on the face and trunk (Fig. 28.2). Lesions are ill-defined erythematous or purpuric macules, sometimes with central duskiness. Lesions coalesce to form large patches, which progress to necrotic, sloughing epidermis, often detaching in sheets, particularly at sites of friction. The dermoepidermal separation also manifests as flaccid bullae. Mucous membranes are prominently involved early in the course of the disease; involvement of at least two mucosal sites is included in the diagnostic criteria.
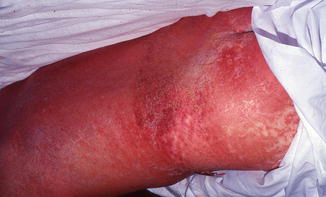

Fig. 28.2
Erythematous drug eruption in a patient with TEN. Note positive Nikolsky’s sign developing on the inner edge of the thigh with erosive epidermal peeling
There are significant systemic manifestations of TEN due to extensive sloughing of both internal and external mucocutaneous membranes. These include, but are not limited to, renal, pulmonary, cardiovascular, and gastrointestinal dysfunction. Depending on the severity, SJS/TEN may progress to involve anywhere from <10 to 100 % of the body surface area. Progression lasts for 4–5 days but may be longer if the half-life of the offending drug is long. The leading cause of death in TEN is sepsis leading to multi-organ failure. Those who survive the acute phase of TEN experience significant morbidity, including ocular dysfunction, dyspigmentation and scarring, alopecia, xerostomia, genitourinary dysfunction, and onychodystrophy.
Histopathology
Dermoepidermal separation
Full-thickness epidermal necrosis
Sparse CD4+ lymphocytic infiltrate in dermis
CD8+ lymphocytic infiltrate in epidermis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


