30
Antidepressant Agents
CASE STUDY
A 47-year-old woman presents to her primary care physician with a chief complaint of fatigue. She indicates that she was promoted to senior manager in her company approximately 11 months earlier. Although her promotion was welcome and came with a sizable raise in pay, it resulted in her having to move away from an office and group of colleagues she very much enjoyed. In addition, her level of responsibility increased dramatically. The patient reports that for the last 7 weeks, she has been waking up at 3 AM every night and been unable to go back to sleep. She dreads the day and the stresses of the workplace. As a consequence, she is not eating as well as she might and has dropped 7% of her body weight in the last 3 months. She also reports being so stressed that she breaks down crying in the office occasionally and has been calling in sick frequently. When she comes home, she finds she is less motivated to attend to chores around the house and has no motivation, interest, or energy to pursue recreational activities that she once enjoyed such as hiking. She describes herself as “chronically miserable and worried all the time.” Her medical history is notable for chronic neck pain from a motor vehicle accident for which she is being treated with tramadol and meperidine. In addition, she is on hydrochlorothiazide and propranolol for hypertension. The patient has a history of one depressive episode after a divorce that was treated successfully with fluoxetine. Medical workup including complete blood cell count, thyroid function tests, and a chemistry panel reveals no abnormalities. She is started on fluoxetine for a presumed major depressive episode and referred for cognitive behavioral psychotherapy. What CYP450 and pharmacodynamic interactions might be associated with fluoxetine use in this patient? Which class of antidepressants would be contraindicated in this patient?
The diagnosis of depression still rests primarily on the clinical interview. Major depressive disorder (MDD) is characterized by depressed mood most of the time for at least 2 weeks or loss of interest or pleasure in most activities, or both. In addition, depression is characterized by disturbances in sleep and appetite as well as deficits in cognition and energy. Thoughts of guilt, worthlessness, and suicide are common. Coronary artery disease, diabetes, and stroke appear to be more common in depressed patients, and depression may considerably worsen the prognosis for patients with a variety of comorbid medical conditions.
According to the Centers for Disease Control, antidepressants are consistently among the three most commonly prescribed classes of medications in the USA. The wisdom of such widespread use of antidepressants is debated. However, it is clear that American physicians have been increasingly inclined to use antidepressants to treat a host of conditions and that patients have been increasingly receptive to their use.
The primary indication for antidepressant agents is the treatment of MDD. Major depression, with a lifetime prevalence of around 17% in the USA and a point prevalence of 5%, is associated with substantial morbidity and mortality. MDD represents one of the most common causes of disability in the developed world. In addition, major depression is commonly associated with a variety of medical conditions—from chronic pain to coronary artery disease. When depression coexists with other medical conditions, the patient’s disease burden increases, and the quality of life—and often the prognosis for effective treatment—decreases significantly.
Some of the growth in antidepressant use may be related to the broad application of these agents for conditions other than major depression. For example, antidepressants have received FDA approvals for the treatment of panic disorder, generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD). In addition, antidepressants are commonly used to treat pain disorders such as neuropathic pain and the pain associated with fibromyalgia. Some antidepressants are used for treating premenstrual dysphoric disorder (PMDD), mitigating the vasomotor symptoms of menopause, and treating stress urinary incontinence. Thus, antidepressants have a broad spectrum of use in medical practice. However, their primary use remains the treatment for MDD.
Pathophysiology of Major Depression
There has been a marked shift in the last decade in our understanding of the pathophysiology of major depression. In addition to the older idea that a deficit in function or amount of monoamines (the monoamine hypothesis) is central to the biology of depression, there is evidence that neurotrophic and endocrine factors play a major role (the neurotrophic hypothesis). Histologic studies, structural and functional brain imaging research, genetic findings, and steroid research all suggest a complex pathophysiology for MDD with important implications for drug treatment.
Neurotrophic Hypothesis
There is substantial evidence that nerve growth factors such as brain-derived neurotrophic factor (BDNF) are critical in the regulation of neural plasticity, resilience, and neurogenesis. The evidence suggests that depression is associated with the loss of neurotrophic support and that effective antidepressant therapies increase neurogenesis and synaptic connectivity in cortical areas such as the hippocampus. BDNF is thought to exert its influence on neuronal survival and growth effects by activating the tyrosine kinase receptor B in both neurons and glia (Figure 30–1).
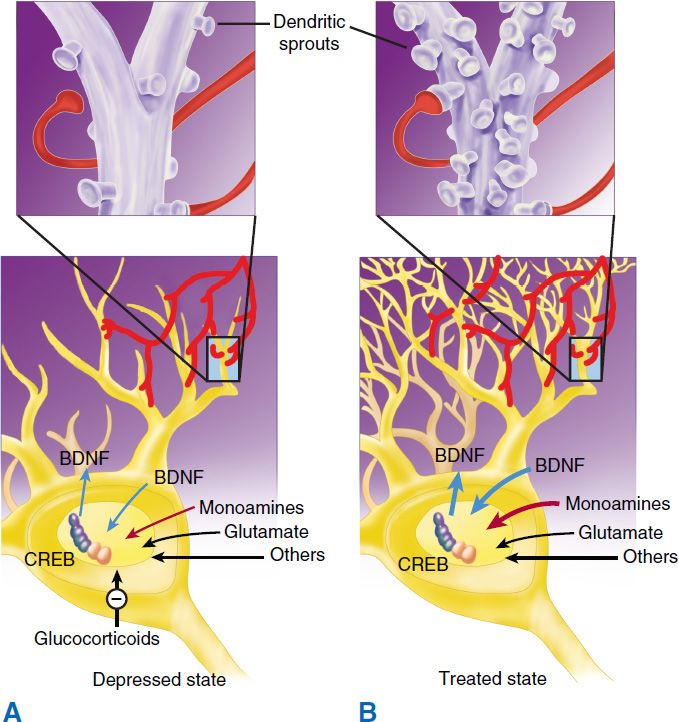
FIGURE 30–1 The neurotrophic hypothesis of major depression. Changes in trophic factors (especially brain-derived neurotrophic factor, BDNF) and hormones appear to play a major role in the development of major depression (A). Successful treatment results in changes in these factors (B). CREB, cAMP response element-binding (protein). BDNF, brain-derived neurotrophic factor. (Reproduced, with permission, from Nestler EJ: Neurobiology of depression. Neuron 2002;34[1]:13–25. Copyright Elsevier.)
Several lines of evidence support the neurotrophic hypothesis. Animal and human studies indicate that stress and pain are associated with a drop in BDNF levels and that this loss of neurotrophic support contributes to atrophic structural changes in the hippocampus and perhaps other areas such as the medial frontal cortex and anterior cingulate. The hippocampus is known to be important both in contextual memory and regulation of the hypothalamic-pituitary-adrenal (HPA) axis. Likewise, the anterior cingulate plays a role in the integration of emotional stimuli and attention functions, whereas the medial orbital frontal cortex is also thought to play a role in memory, learning, and emotion.
Over 30 structural imaging studies suggest that major depression is associated with a 5–10% loss of volume in the hippocampus, although some studies have not replicated this finding. Depression and chronic stress states have also been associated with a substantial loss of volume in the anterior cingulate and medial orbital frontal cortex. Loss of volume in structures such as the hippocampus also appears to increase as a function of the duration of illness and the amount of time that the depression remains untreated.
Another source of evidence supporting the neurotrophic hypothesis of depression comes from studies of the direct effects of BDNF on emotional regulation. Direct infusion of BDNF into the midbrain, hippocampus, and lateral ventricles of rodents has an antidepressant-like effect in animal models. Moreover, all known classes of antidepressants are associated with an increase in BDNF levels in animal models with chronic (but not acute) administration. This increase in BDNF levels is consistently associated with increased neurogenesis in the hippocampus in these animal models. Other interventions thought to be effective in the treatment of major depression, including electroconvulsive therapy, also appear to robustly stimulate BDNF levels and hippocampus neurogenesis in animal models.
Human studies seem to support the animal data on the role of neurotrophic factors in stress states. Depression appears to be associated with a drop in BDNF levels in the cerebrospinal fluid and serum as well as with a decrease in tyrosine kinase receptor B activity. Conversely, administration of antidepressants increases BDNF levels in clinical trials and may be associated with an increase in hippocampus volume in some patients.
Much evidence supports the neurotrophic hypothesis of depression, but not all evidence is consistent with this concept. Animal studies in BDNF knockout mice have not always suggested an increase in depressive or anxious behaviors that would be expected with a deficiency of BDNF. In addition, some animal studies have found an increase in BDNF levels after some types of social stress and an increase rather than a decrease in depressive behaviors with lateral ventricle injections of BDNF.
A proposed explanation for the discrepant findings on the role of neurotrophic factors in depression is that there are polymorphisms for BDNF that may yield very different effects. Mutations in the BDNF gene have been found to be associated with altered anxiety and depressive behavior in both animal and human studies.
Thus, the neurotrophic hypothesis continues to be intensely investigated and has yielded new insights and potential targets in the treatment of MDD.
Monoamines & Other Neurotransmitters
The monoamine hypothesis of depression (Figure 30–2) suggests that depression is related to a deficiency in the amount or function of cortical and limbic serotonin (5-HT), norepinephrine (NE), and dopamine (DA).
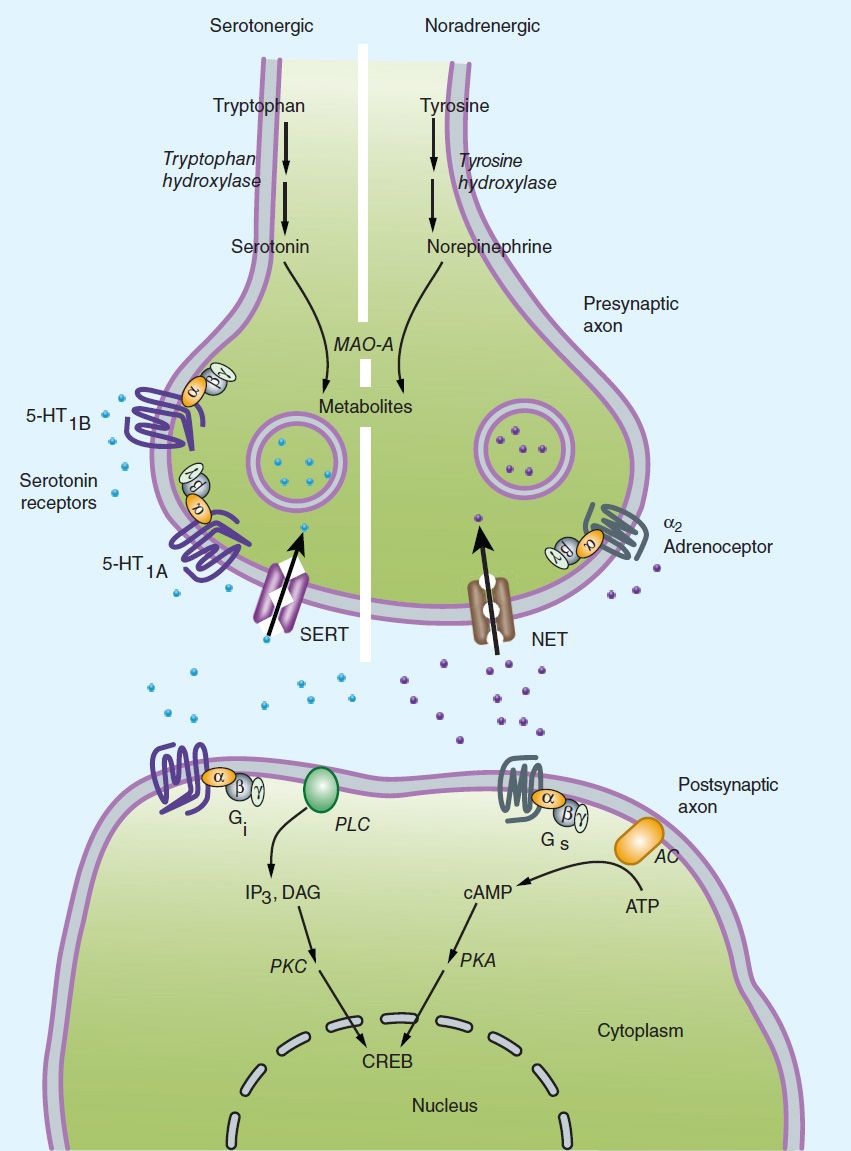
FIGURE 30–2 The amine hypothesis of major depression. Depression appears to be associated with changes in serotonin or norepinephrine signaling in the brain (or both) with significant downstream effects. Most antidepressants cause changes in amine signaling. AC, adenylyl cyclase; 5-HT, serotonin; CREB, cAMP response element-binding (protein); DAG, diacyl glycerol; IP3, inositol trisphosphate; MAO, monoamine oxidase; NET, norepinephrine transporter; PKC, protein kinase C; PLC, phospholipase C; SERT, serotonin transporter. (Adapted from Belmaker R, Agam G: Major depressive disorder. N Engl J Med 2008;358:59.)
Evidence to support the monoamine hypothesis comes from several sources. It has been known for many years that reserpine treatment, which is known to deplete monoamines, is associated with depression in a subset of patients. Similarly, depressed patients who respond to serotonergic antidepressants such as fluoxetine often rapidly suffer relapse when given diets free of tryptophan, a precursor of serotonin synthesis. Patients who respond to noradrenergic antidepressants such as desipramine are less likely to relapse on a tryptophan-free diet. Moreover, depleting catecholamines in depressed patients who have previously responded to noradrenergic agents likewise tends to be associated with relapse. Administration of an inhibitor of norepinephrine synthesis is also associated with a rapid return of depressive symptoms in patients who respond to noradrenergic but not necessarily in patients who had responded to serotonergic antidepressants.
Another line of evidence supporting the monoamine hypothesis comes from genetic studies. A functional polymorphism exists for the promoter region of the serotonin transporter gene, which regulates how much of the transporter protein is available. Subjects who are homozygous for the s (short) allele may be more vulnerable to developing major depression and suicidal behavior in response to stress. In addition, homozygotes for the s allele may also be less likely to respond to and tolerate serotonergic antidepressants. Conversely, subjects with the l (long) allele tend to be more resistant to stress and may be more likely to respond to serotonergic antidepressants.
Studies of depressed patients have sometimes shown an alteration in monoamine function. For example, some studies have found evidence of alteration in serotonin receptor numbers (5-HT1A and 5-HT2C) or norepinephrine (α2) receptors in depressed and suicidal patients, but these findings have not been consistent. A reduction in the primary serotonin metabolite 5-hydroxyindoleacetic acid in the cerebrospinal fluid is associated with violent and impulsive behavior, including violent suicide attempts. However, this finding is not specific to major depression and is associated more generally with violent and impulsive behavior.
Finally, perhaps the most convincing line of evidence supporting the monoamine hypothesis is the fact that (at the time of this writing) all available antidepressants appear to have significant effects on the monoamine system. All classes of antidepressants appear to enhance the synaptic availability of 5-HT, norepinephrine, or dopamine. Attempts to develop antidepressants that work on other neurotransmitter systems have not been effective to date.
The monoamine hypothesis, like the neurotrophic hypothesis, is at best incomplete. Many studies have not found an alteration in function or levels of monoamines in depressed patients. In addition, some candidate antidepressant agents under study do not act directly on the monoamine system.
In addition to the monoamines, the excitatory neurotransmitter glutamate appears to be important in the pathophysiology of depression. A number of studies of depressed patients have found elevated glutamate content in the cerebrospinal fluid of depressed patients and decreased glutamine/glutamate ratios in their plasma. In addition, postmortem studies have revealed significant increases in the frontal and dorsolateral prefrontal cortex of depressed patients. Likewise, structural neuroimaging studies have consistently found volumetric changes in the brain areas of depressed patients in which glutamate neurons and their connections are most abundant, including the amygdala and hippocampus.
Antidepressants are known to impact glutamate neurotransmission in a variety of ways. For example, chronic antidepressant use is associated with reducing glutamatergic transmission, including the presynaptic release of glutamate in the hippocampus and cortical areas. Similarly, the chronic administration of antidepressants significantly reduces depolarization-evoked release of glutamate in animal models. Stress is known to enhance the release of glutamate in rodents, and antidepressants inhibit stress-induced presynaptic release of glutamate in these models.
Given the effect of antidepressants on the glutamate system, there has been a growing interest in the development of pharmaceutical agents that might modulate the glutamate system. Ketamine is a potent, high-affinity, noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist that has long been used in anesthesia and is a common drug of abuse in some parts of the world. A number of preclinical and clinical studies have demonstrated rapid antidepressant effects of ketamine. Multiple studies have suggested that a single dose of intravenous ketamine at subanesthetic doses produces rapid relief of depression, even in treatment-resistant patients, that may persist for 1 week or longer. Unfortunately, ketamine is associated with cognitive, dissociative, and psychotomimetic properties that make it impractical as a long-term treatment for depression. Still, a number of other NMDA receptor antagonists, partial antagonists, and metabotropic glutamate receptor modulators (see Chapter 29) are under investigation as potential antidepressants.
Neuroendocrine Factors in the Pathophysiology of Depression
Depression is known to be associated with a number of hormonal abnormalities. Among the most replicated of these findings are abnormalities in the HPA axis in patients with MDD. Moreover, MDD is associated with elevated cortisol levels (Figure 30–1), nonsuppression of adrenocorticotropic hormone (ACTH) release in the dexamethasone suppression test, and chronically elevated levels of corticotropin-releasing hormone. The significance of these HPA abnormalities is unclear, but they are thought to indicate a dysregulation of the stress hormone axis. More severe types of depression, such as psychotic depression, tend to be associated with HPA abnormalities more commonly than milder forms of major depression. It is well known that both exogenous glucocorticoids and endogenous elevation of cortisol are associated with mood symptoms and cognitive deficits similar to those seen in MDD.
Thyroid dysregulation has also been reported in depressed patients. Up to 25% of depressed patients are reported to have abnormal thyroid function. These abnormalities include a blunting of response of thyrotropin to thyrotropin-releasing hormone, and elevations in circulating thyroxine during depressed states. Clinical hypothyroidism often presents with depressive symptoms, which resolve with thyroid hormone supplementation. Thyroid hormones are also commonly used in conjunction with standard antidepressants to augment therapeutic effects of the latter.
Finally, sex steroids are also implicated in the pathophysiology of depression. Estrogen deficiency states, which occur in the postpartum and postmenopausal periods, are thought to play a role in the etiology of depression in some women. Likewise, severe testosterone deficiency in men is sometimes associated with depressive symptoms. Hormone replacement therapy in hypogonadal men and women may be associated with an improvement in mood and depressive symptoms.
Integration of Hypotheses Regarding the Pathophysiology of Depression
The several pathophysiologic hypotheses just described are not mutually exclusive. It is evident that the monoamine, neuroendocrine, and neurotrophic systems are interrelated in important ways. For example, HPA and steroid abnormalities may contribute to suppression of transcription of the BDNF gene. Glucocorticoid receptors are found in high density in the hippocampus. Binding of these hippocampal glucocorticoid receptors by cortisol during chronic stress states such as major depression may decrease BDNF synthesis and may result in volume loss in stress-sensitive regions such as the hippocampus. The chronic activation of monoamine receptors by antidepressants appears to have the opposite effect of stress and results in an increase in BDNF transcription. In addition, activation of monoamine receptors appears to down-regulate the HPA axis and may normalize HPA function.
One of the weaknesses of the monoamine hypothesis is the fact that amine levels increase immediately with antidepressant use, but maximum beneficial effects of most antidepressants are not seen for many weeks. The time required to synthesize neurotrophic factors has been proposed as an explanation for this delay of antidepressant effects. Appreciable protein synthesis of products such as BDNF typically takes 2 weeks or longer and coincides with the clinical course of antidepressant treatment.
 BASIC PHARMACOLOGY OF ANTIDEPRESSANTS
BASIC PHARMACOLOGY OF ANTIDEPRESSANTS
Chemistry & Subgroups
The currently available antidepressants make up a remarkable variety of chemical types. These differences and the differences in their molecular targets provide the basis for distinguishing several subgroups.
A. Selective Serotonin Reuptake Inhibitors
The selective serotonin reuptake inhibitors (SSRIs) represent a chemically diverse class of agents that have as their primary action the inhibition of the serotonin transporter (SERT; Figure 30–3). Fluoxetine was introduced in the United States in 1988 and quickly became one of the most commonly prescribed medications in medical practice. The development of fluoxetine emerged out of the search for chemicals that had high affinity for monoamine receptors but lacked the affinity for histamine, acetylcholine, and α adrenoceptors that is seen with the tricyclic antidepressants (TCAs). There are currently six available SSRIs, and they are the most common antidepressants in clinical use. In addition to their use in major depression, SSRIs have indications in GAD, PTSD, OCD, panic disorder, PMDD, and bulimia. Fluoxetine, sertraline, and citalopram exist as isomers and are formulated in the racemic forms, whereas paroxetine and fluvoxamine are not optically active. Escitalopram is the (S) enantiomer of citalopram. As with all antidepressants, SSRIs are highly lipophilic. The popularity of SSRIs stems largely from their ease of use, safety in overdose, relative tolerability, cost (all are available as generic products), and broad spectrum of uses.
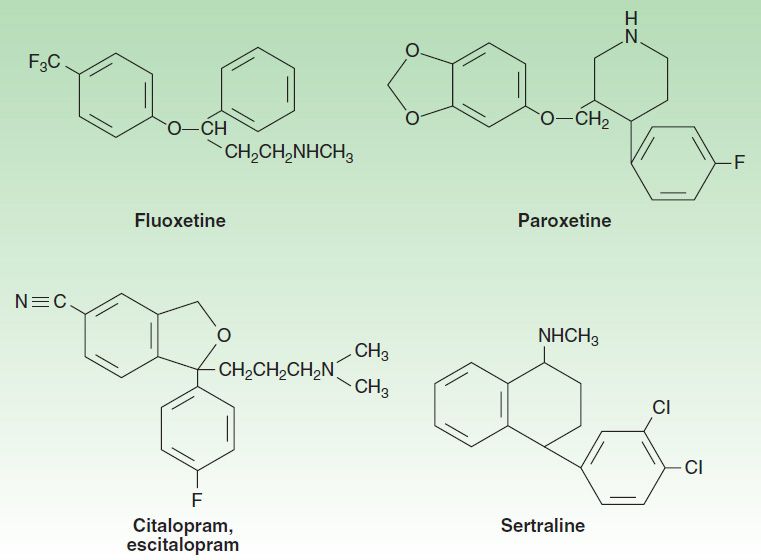
FIGURE 30–3 Structures of several selective serotonin reuptake inhibitors (SSRIs).
B. Serotonin-Norepinephrine Reuptake Inhibitors
Two classes of antidepressants act as combined serotonin and norepinephrine reuptake inhibitors: selective serotonin-norepinephrine reuptake inhibitors (SNRIs) and TCAs.
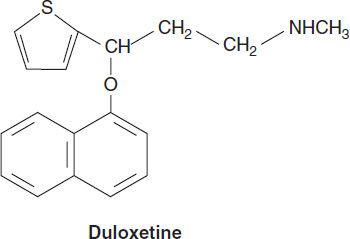
1. Selective serotonin-norepinephrine reuptake inhibitors—The SNRIs include venlafaxine, its metabolite desvenlafaxine, duloxetine, and levomilnacipran. Levomilnacipran is the active enantiomer of a racemic SNRI, milnacipran. Milnacipran has been approved for the treatment of fibromyalgia in the USA and has been used in the treatment of depression in Europe for many years. In addition to their use in major depression, SNRIs have applications in the treatment of pain disorders including neuropathies and fibromyalgia. SNRIs are also used in the treatment of generalized anxiety, stress urinary incontinence, and vasomotor symptoms of menopause.
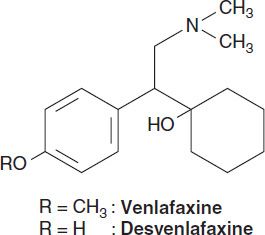
SNRIs are chemically unrelated to each other. Venlafaxine was discovered in the process of evaluating chemicals that inhibit binding of imipramine. Venlafaxine’s in vivo effects are similar to those of imipramine but with a more favorable adverse-effect profile. All SNRIs bind the serotonin (SERT) and norepinephrine (NET) transporters, as do the TCAs. However, unlike the TCAs, the SNRIs do not have much affinity for other receptors. Venlafaxine and desvenlafaxine are bicyclic compounds, whereas duloxetine is a three-ring structure unrelated to the TCAs. Milnacipran contains a cyclopropane ring and is provided as a racemic mixture.
2. Tricyclic antidepressants—The TCAs were the dominant class of antidepressants until the introduction of SSRIs in the 1980s and 1990s. Nine TCAs are available in the USA, and they all have an iminodibenzyl (tricyclic) core (Figure 30–4). The chemical differences between the TCAs are relatively subtle. For example, the prototype TCA imipramine and its metabolite, desipramine, differ by only a methyl group in the propylamine side chain. However, this minor difference results in a substantial change in their pharmacologic profiles. Imipramine is highly anticholinergic and is a relatively strong serotonin as well as norepinephrine reuptake inhibitor. In contrast, desipramine is much less anticholinergic and is a more potent and somewhat more selective norepinephrine reuptake inhibitor than is imipramine.
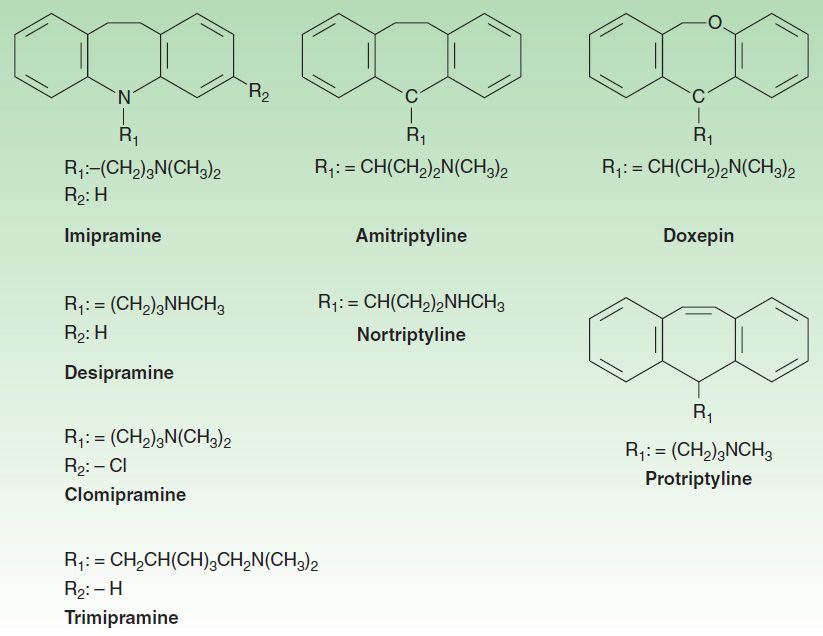
FIGURE 30–4 Structures of some tricyclic antidepressants (TCAs).
At the present time, the TCAs are used primarily in depression that is unresponsive to more commonly used antidepressants such as the SSRIs or SNRIs. Their loss of popularity stems in large part from relatively poorer tolerability compared with newer agents, difficulty of use, and lethality in overdose. Other uses for TCAs include the treatment of pain conditions, enuresis, and insomnia.
C. 5-HT2 Receptor Modulators
Two antidepressants are thought to act primarily as antagonists at the 5-HT2 receptor: trazodone and nefazodone. Trazodone’s structure includes a triazolo moiety that is thought to impart antidepressant effects. Its primary metabolite, m-chlorphenylpiperazine (m-cpp), is a potent 5-HT2 antagonist. Trazodone was among the most commonly prescribed antidepressants until it was supplanted by the SSRIs in the late 1980s. The most common use of trazodone in current practice is as an unlabeled hypnotic, since it is highly sedating and not associated with tolerance or dependence.
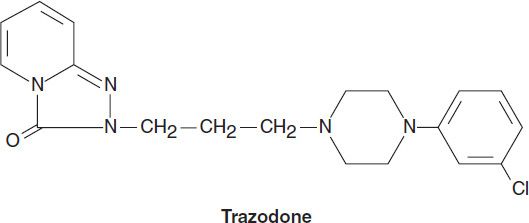
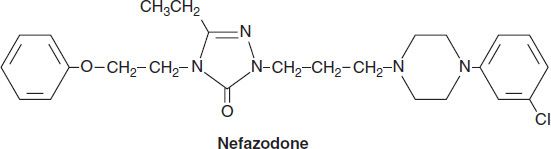
Nefazodone is chemically related to trazodone. Its primary metabolites, hydroxynefazodone and m-cpp are both inhibitors of the 5-HT2 receptor. Nefazodone received an FDA black box warning in 2001 implicating it in hepatotoxicity, including lethal cases of hepatic failure. Though still available generically, nefazodone is no longer commonly prescribed. The primary indications for both nefazodone and trazodone are major depression, although both have also been used in the treatment of anxiety disorders.
Vortioxetine is a newer agent that acts as an antagonist of the 5-HT3, 5-HT7, and 5-HT1D receptors, a partial agonist of the 5-HT1B receptor, and an agonist of the 5HT1A receptor. It also inhibits the serotonin transporter but its actions are not primarily related to SERT inhibition and it is therefore not classified as an SSRI. Vortioxetine has demonstrated efficacy on major depression in a number of controlled clinical studies. In addition, there is some preliminary evidence that the drug also may improve some aspects of cognition in depressed patients.
D. Tetracyclic and Unicyclic Antidepressants
A number of antidepressants do not fit neatly into the other classes. Among these are bupropion, mirtazapine, amoxapine, vilazodone, and maprotiline (Figure 30–5). Bupropion has a unicyclic aminoketone structure. Its unique structure results in a different side-effect profile than most antidepressants (described below). Bupropion somewhat resembles amphetamine in chemical structure and, like the stimulant, has central nervous system (CNS) activating properties.
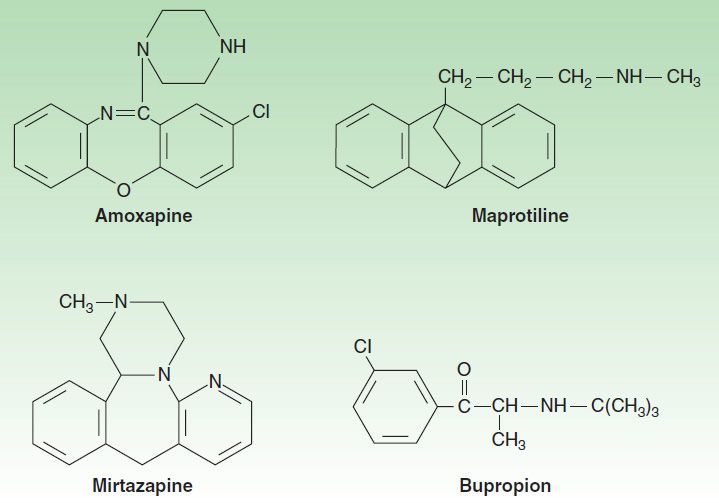
FIGURE 30–5 Structures of the tetracyclics, amoxapine, maprotiline, and mirtazapine and the unicyclic, bupropion.
Mirtazapine was introduced in 1994 and, like bupropion, is one of the few antidepressants not commonly associated with sexual effects. It has a tetracyclic chemical structure and belongs to the piperazino-azepine group of compounds.
Mirtazapine, amoxapine, and maprotiline have tetracyclic structures. Amoxapine is the N-methylated metabolite of loxapine, an older antipsychotic drug. Amoxapine and maprotiline share structural similarities and side effects comparable to the TCAs. As a result, these tetracyclics are not commonly prescribed in current practice. Their primary use is in MDD that is unresponsive to other agents. Vilazodone has a multi-ring structure that allows it to bind potently to the serotonin transporter but minimally to the dopamine and norepinephrine transporter.
E. Monoamine Oxidase Inhibitors
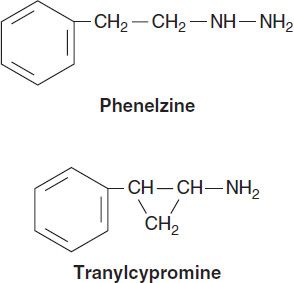
Arguably the first modern class of antidepressants, monoamine oxidase inhibitors (MAOIs) were introduced in the 1950s but are now rarely used in clinical practice because of toxicity and potentially lethal food and drug interactions. Their primary use now is in the treatment of depression unresponsive to other antidepressants. However, MAOIs have also been used historically to treat anxiety states, including social anxiety and panic disorder. In addition, selegiline is used in the treatment of Parkinson’s disease (see Chapter 28).
Current MAOIs include the hydrazine derivatives phenelzine and isocarboxazid and the non-hydrazines tranylcypromine, selegiline, and moclobemide (the latter is not available in the USA). The hydrazines and tranylcypromine bind irreversibly and nonselectively with MAO-A and -B, whereas other MAOIs may have more selective or reversible properties. Some of the MAOIs such as tranylcypromine resemble amphetamine in chemical structure, whereas other MAOIs such as selegiline have amphetamine-like metabolites. As a result, these MAOIs tend to have substantial CNS-stimulating effects.
Pharmacokinetics
The antidepressants share several pharmacokinetic features (Table 30–1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


