Chapter 25
Alterations of the Male Reproductive System
George Rodway and Kathryn L. McCance
Alterations of the reproductive system span a wide range of concerns, from delayed sexual development and suboptimal sexual performance to structural and functional abnormalities. Many common male reproductive disorders carry potentially serious physiologic or psychologic consequences. Sexual or reproductive dysfunction, such as impotence or infertility, can dramatically affect self-concept, relationships, and overall quality of life. Conversely, organic and psychosocial problems, such as alcoholism, depression, situational stressors, chronic illness, and medications, can affect sexual performance and fertility and may be risk factors for the development of some types of reproductive tract cancers. Prostate cancer is the second leading cause of cancer death in men and is the most frequently diagnosed cancer in men aside from skin cancer. Incidence rates for prostate cancer changed substantially between the mid-1980s and mid-1990s and have since fluctuated widely from year to year, in large part reflecting changes in prostate cancer screening with the prostate specific antigen (PSA) blood test. Since 2004, incidence rates have act actually decreased by 2.7% per year among men 65 years of age and older and have remained stable among men younger than 65 years.1 Diagnosis and treatment of male reproductive system disorders are, like female reproductive system disorders, complicated because of the stigma and symbolism associated with the reproductive organs and the emotion-laden beliefs and behaviors related to reproductive health. Treatment and diagnosis for related problems may be delayed because of embarrassment, guilt, fear, or denial.
Alterations of Sexual Maturation
Delayed Puberty
About 3% of children in North America experience delayed development of secondary sex characteristics.2 Normally, boys tend to mature later than girls, around 14 to 14.5 years of age. In boys the first sign is enlargement of the testes and thinning of the scrotal skin. Puberty is considered delayed if there are no clinical signs of puberty by age 14 in boys (2 standard deviations [SDs] above the mean age of pubertal onset). Boys especially tend to be embarrassed by sexual immaturity;3 therefore, early diagnosis and treatment are recommended, as well as reassurance for boys as well as girls.
In 95% of cases delayed puberty is a physiologic delay, that is, hormonal levels are normal and the hypothalamic-pituitary-gonadal (HPG) axis is intact, but maturation is happening slowly.4 This constitutional delay tends to be familial and is much more common in boys than in girls. Physiologic delay is difficult to distinguish from isolated gonadotropin deficiency and is usually diagnosed retrospectively once pubertal progression is complete.
Delayed puberty also may be related to consequences of any chronic condition that delays bone aging (i.e., lung disease, renal failure, cystic fibrosis) (Box 25-1).2 Many clinicians recommend intervention (i.e., exogenous sex steroid administration) in physiologic cases of delayed puberty to reduce the psychologic effects (e.g., self-esteem issues, embarrassment) often associated with delayed puberty.4
The other 5% of cases are caused by a disruption of the HPG axis of various etiologies (see Box 25-1).5 Human gonadal function is partially controlled by luteinizing hormone (LH) and follicle-stimulating hormone (FSH), the release of which is regulated by the pulsatile secretion of hypothalamic gonadotropin-releasing hormone (GnRH).4,5 Most recently, the G-protein–coupled receptor 54 (GPR54) has been identified as the gatekeeper gene for activation of the GnRH axis based on loss of function studies in mice and humans. GPR54 is required for the normal function of this axis, and data suggest that the ligand kisspeptin-1 may act as a neurohormonal regulator of the GnRH axis.6 The mechanisms of childhood inhibition of GnRH release and activation are poorly understood but appear to involve feedback inhibition by sex steroids and presumably other central nervous system (CNS) pathways.7 Given the myriad etiologies contributing to the occurrence of delayed puberty, a thorough evaluation should be conducted that includes physical examination and medical and family history. Such evaluation should specifically target known contributors to delayed puberty.4 Laboratory workup generally consists of x-ray studies for bone age, measurement of thyroid function, serum levels of prolactin and adrenal and gonadal steroids, radioimmunoassay of plasma gonadotropins, and screening for systemic disorders. Adolescents with high gonadotropin levels require a karyotype, to rule out genetic causes, and those with low levels need skull imaging (lateral skull film, computed tomography [CT], or magnetic resonance imaging [MRI]) to rule out pituitary or other CNS infiltrate or tumor.2 Although several genes involved in the HPG maturation cascade have been characterized from familial or sporadic cases of primitive isolated hypogonadotropic hypogonadism, many genes regulating puberty onset remain undetermined. Treatment of delayed puberty depends on the cause; the goal of treatment is the development of secondary sex characteristics and fertility, when possible. Insufficient sex hormone secretion can be corrected by hormone replacement therapy, such as testosterone for boys.8 Idiopathic hypogonadotropic hypogonadism is treated with synthetic GnRH or sex hormone administration, or both, and may be lifelong.2,4,8
Precocious Puberty
Precocious puberty is a rare event in boys, affecting less than 1 in 50,000. Precocious puberty in boys has been redefined as sexual maturation before age 9.9 One study has noted observed mean ages of beginning male genital and pubic hair growth and early testicular volumes tending toward younger ages than earlier studies have suggested, although this seems to be dependent on race and ethnicity.10 For instance, black boys are showing significantly earlier mean ages for stages 2 to 4 genital development and stages 2 to 4 pubic hair than white and Hispanic boys. All cases of precocious puberty require thorough evaluation.
Precocious puberty may be partial, complete, or mixed (heterosexual) types (Box 25-2) and can be further categorized into central (GnRH-dependent) and peripheral (GnRH-independent) (Box 25-3). Central precocious puberty is GnRH-dependent and occurs when the HPG axis is working normally but prematurely. Besides the premature development of secondary sex characteristics, precocity causes premature closure of the epiphysis of long bones, which results in shorter stature. Central precocious puberty results from failure of central inhibition of the GnRH pulse generator (the gonadostat). The diagnosis of central precocious puberty is one of exclusion. Because a CNS lesion may be missed, children with presumed central precocious puberty require long-term surveillance. Peripheral puberty is GnRH-independent and develops when sex hormones are produced by some mechanism other than stimulation by the gonadotropins. Sex steroid–producing tumors (i.e., gonadal tumors), testotoxicosis, and exposure to exogenous sex steroids (i.e., hormonal contraceptives and environmental endocrine disruptors) are some of the causes (see Box 25-3).
The diagnosis and cause of premature development are often obvious. A thorough history and physical examination are done to determine the velocity of the process and to rule out life-threatening CNS or adrenal neoplasms. Family occurrence helps exclude tumors. Children with precocious puberty also have a tendency toward obesity.7,11
Treatment for all forms of precocious puberty includes identifying and removing the underlying cause (see Boxes 25-2 and 25-3) or administering appropriate hormones. In many cases precocious puberty can be reversed. Management goals include diagnosing and treating intracranial disease; arresting maturation until early teen years; maximizing eventual adult height; reducing emotional problems; and providing contraception, if necessary. The most common form, central precocious puberty, is usually treated with potent GnRH agonist analogs, which induce reversible, selective suppression of the HPG axis. Treatment does not seem to affect body composition or increase obesity in children with central precocious puberty. Because many of these children are obese and childhood obesity is predictive of morbidity in adolescence and adulthood, it is important for clinicians to include assessment and management of obesity as part of the treatment for central precocious puberty.
Mixed precocious puberty (e.g., feminization of a boy) causes the child to develop some secondary sex characteristics of the opposite sex. This condition is usually evident at birth and is rare in older children (Box 25-4).
Disorders of the Male Reproductive System
Disorders of the Urethra
Urethritis
Urethritis is an inflammatory process of the urethra without concurrent bladder infection that is usually, but not always, caused by a sexually transmitted microorganism. Biologic agents associated with infectious urethritis in males include Neisseria gonorrhoeae and Chlamydia trachomatis, Ueaplasma urealyticum, and other, less common, mycobacteria; parasites (e.g., Trichomonas vaginalis); and viruses (herpes simplex virus [HSV]).12,13 Infectious urethritis caused by N. gonorrhoeae often is called gonococcal urethritis (GU); infection caused by other microorganisms is called nongonococcal urethritis (NGU).14 (Sexually transmitted urethritis is described in Chapter 26.) Nonsexual origins of urethritis include inflammation or infection as a result of urologic procedures, insertion of foreign bodies into the urethra, anatomic abnormalities, or trauma.
Symptoms of urethritis include urethral tingling, itching, or burning sensation on urination (dysuria), frequency, and urgency. The individual may note a purulent or clear mucus-like discharge from the urethra. Nucleic acid detection amplification tests allow easy detection of N. gonorrhoeae and C. trachomatis in first-void urine.14 Treatment consists of appropriate antibiotic therapy for infectious urethritis and avoidance of future chemical or mechanical irritation.
Urethral Stricture
A urethral stricture is a fibrotic narrowing of the urethra caused by scarring. The scars may be congenital but are more likely to result from trauma or untreated or severe urethral infections, most often from long-term use of indwelling urinary catheters. It can present at any age and has a wide range of etiologic factors, including infection, trauma, and instrumentation. Large catheters and instruments cause internal trauma and ischemia, whereas external trauma, such as pelvic fracture, can partially or completely sever the urethra and cause severe and complex strictures.15 In addition, a report has concluded that stricture may occur decades after initial hypospadias surgery.16 Urethral carcinoma is a less common cause of urethral stricture. Prostatitis and infection secondary to urinary stasis are common complications. Severe and prolonged obstruction can result in hydronephrosis and renal failure. In addition, chronic, severe strictures may lead to urethral fistulas and periurethral abscesses.15,17
Disorders of the Penis
Phimosis and Paraphimosis
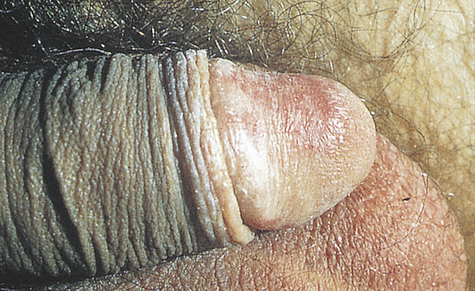
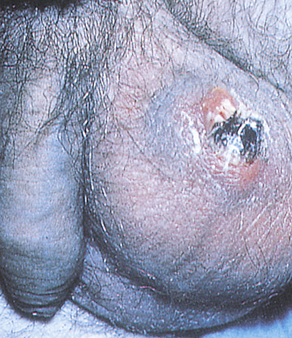
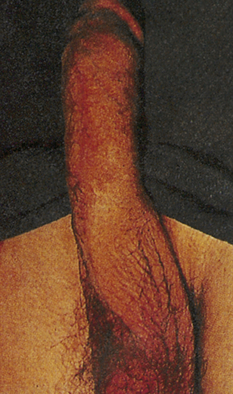
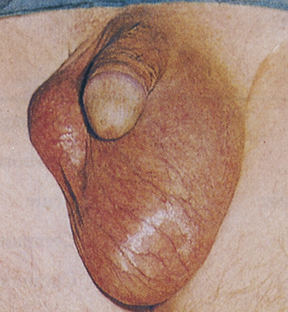
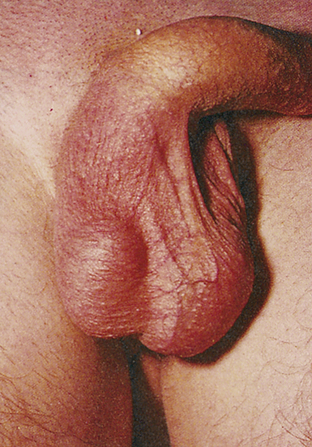
Phimosis and paraphimosis are disorders in which the foreskin (prepuce) is “too tight” to be moved easily over the glans penis. Phimosis is a condition in which the foreskin cannot be retracted back over the glans, whereas paraphimosis is the opposite: the foreskin is retracted and cannot be moved forward (reduced) to cover the glans (Figure 25-1). Both conditions can cause penile pathologic conditions.
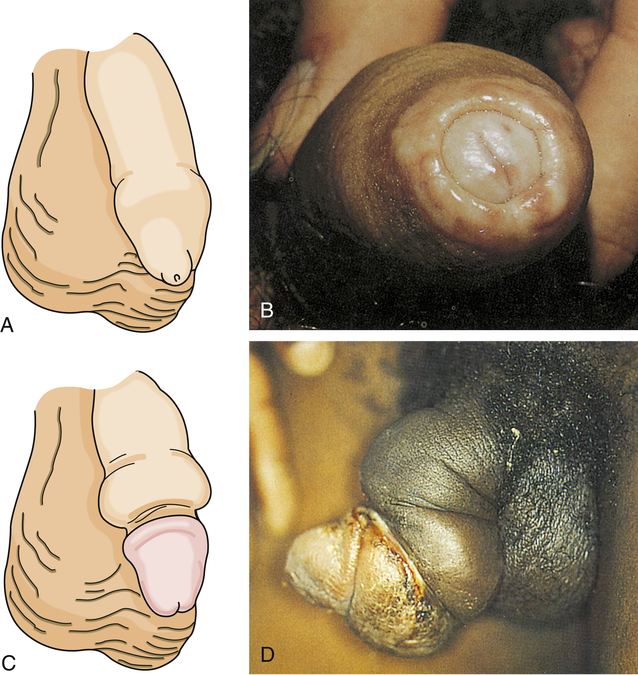
A, Phimosis: the foreskin has a narrow opening that is not large enough to permit retraction over the glans. B, Lesions on the prepuce secondary to infection cause swelling, and retraction of foreskin may be impossible. C, Paraphimosis: the foreskin is retracted over the glans but cannot be reduced to its normal position. Here it has formed a constricting band around the penis. D, Ulcer on the retracted prepuce with edema. (A, >C from Monahan FD, et al: Phipps’ medical-surgical nursing, ed 8, St Louis, 2007, Mosby; B from Taylor PK: Diagnostic picture tests in sexually transmitted diseases, London, 1995, Mosby-Wolfe; D from Morse SA, Holmes KK, Ballard RC: Atlas of sexually transmitted diseases and AIDS, ed 4, London, 2011, Saunders.)
The inability to retract the foreskin is normal in infancy and is caused by congenital adhesions. During the first 3 years of life these adhesions separate naturally with penile erections and are not an indication for circumcision. Although most cases occur in uncircumcised males, stenosis and resultant phimosis can occur in males with excessive skin remaining after circumcision.17 Phimosis can occur at any age and is caused most commonly by poor hygiene and chronic infection. Chronic balanoposthitis (inflammation of the glans and prepuce) predisposes older diabetic men to phimosis. It rarely occurs with normal foreskin.
Edema, erythema, and tenderness of the prepuce and purulent discharge are usually the reasons for seeking treatment; inability to retract the foreskin is a less common complaint. Circumcision, if needed, is performed after infection has been eradicated. Complications of phimosis include inflammation of the glans (balanitis) or prepuce (posthitis) and paraphimosis. There is a higher incidence of penile carcinoma in uncircumcised males, which is associated with morbidity and mortality, but chronic infection, most likely with HPV, is usually the underlying factor in such cases. Approximately 40% of invasive penile carcinomas are attributable to HPV.17,18
Peyronie Disease
Peyronie disease (bent nail syndrome) is a fibrotic condition of the tunica albuginea of the penis resulting in varying degrees of curvature and sexual dysfunction19 (Figure 25-2). Peyronie disease develops slowly and is characterized by tough, fibrous thickening of the fascia in the erectile tissue of the corpora cavernosa. A dense fibrous plaque is usually palpable on the dorsum of the penile shaft. The problem usually affects middle-age men and is associated with painful erection, painful intercourse (for both partners), and poor erection distal to the involved area. In some cases, impotence or unsatisfactory penetration occurs. There is no pain when the penis is flaccid.
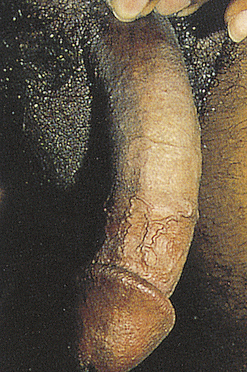
There is no definitive treatment for Peyronie disease. Spontaneous remissions occur in as many as 50% of cases. Treatment with pharmacologic therapies include colchicine, aminobenzoate potassium (Potaba), L-carnitine, and liposomal superoxide dismutase. Men suffering with Peyronie disease who have significant penile deformity precluding successful coitus can be appraised for surgical correction. Surgery is considered the gold standard and includes plication, incision and grafting, or penile-prosthesis-related procedures.17,19
Priapism
Priapism is an uncommon condition of prolonged penile erection. It is usually painful and is not associated with sexual arousal (Figure 25-3). Priapism is idiopathic in 60% of cases; the remaining 40% of cases are associated with spinal cord trauma, sickle cell disease, leukemia, pelvic tumors or infections, or penile trauma. Priapism also has been associated with cocaine use.20,21 Intracavernous injection therapy for impotence seems to be the most common cause. Prolonged sexual stimulation often is associated with initial development of the idiopathic type.17 The two corpora cavernosa within the erect penis are filled with blood and are tender to palpation; neither the corpus spongiosum nor the glans is engorged. The vascular congestion is thought to be associated with venous obstruction. If the erection remains over a period of days, edema and fibrosis develop, leading to erectile dysfunction (impotence).
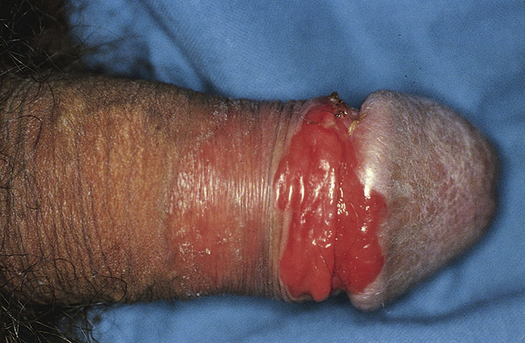
Balanitis
Balanitis is an inflammation of the glans penis (Figure 25-4) and usually occurs in conjunction with posthitis, an inflammation of the prepuce. It is associated with poor hygiene and phimosis. The accumulation under the foreskin of glandular secretions (smegma), sloughed epithelial cells, and Mycobacterium smegmatis can irritate the glans directly or lead to infection. Skin disorders (e.g., psoriasis, lichen planus, eczema) and candidiasis must be differentiated from inflammation resulting from poor hygienic practices. Balanitis is seen most commonly in men with poorly controlled diabetes mellitus and candidiasis. Antimicrobials are used to treat infection. Circumcision can prevent recurrences and can be considered after the inflammation has subsided.
Penile Cancer
In the United States, carcinoma of the penis is rare and affects about 1 in 100,000 men. Approximately 1570 cases and 310 deaths were estimated in the year 2012.1 Although rare in North America and Europe, where it accounts for about 0.2% of cancers and 0.1% of cancer deaths in men, penile cancer may account for up to 10% of cancers in African and South American men.
In the United States, about four out of five cases of the disease are diagnosed in men older than age 55 years.22 Major risk factors include infection with HPV (mainly serotypes 16 and 18), smoking, and psoriasis treated with a combination involving the drug psoralen and ultraviolet (UV) light. Men circumcised at birth have less than half the chance of getting penile cancer than those who were not. Penile cancer is more common in men with phimosis and those with acquired immunodeficiency syndrome (AIDS).22 About two thirds of men with penile cancer are diagnosed at more than 65 years of age.1
Before the development of penile cancer, signs of premalignant cancer or epidermal cancer in situ are present.23 These include thick white plaque (leukoplakia) that typically involves the meatus; red, inflamed areas of Paget disease; red, velvety, ulcerative lesions of erythroplasia of Queyrat that usually involve the glans; large, invasive, scaly growths of Buschke-Löwenstein tumor; red plaque with encrustations of Bowen disease; and in situ carcinoma that generally affects the penile shaft. Men with leukoplakia or erythroplasia of Queyrat may have concurrent invasive penile carcinoma.22,24 Pain and bleeding are late signs of penile cancer. Condylomata (genital warts) caused by HPV may be involved in the development of precancerous lesions (see Chapter 26 for a discussion of HPV). At times the penis might be the site of metastatic spread of solid tumors from the bladder, prostate, rectum, or kidney. Early squamous cell carcinoma and premalignant epidermal lesions are easily treated but are often ignored. Delays in seeking treatment are attributed to denial, embarrassment, failure to detect lesions under a phimotic foreskin, fear, guilt, and ignorance.
Penile cancer is mostly squamous cell carcinoma, which usually begins as a small, fat, ulcerative or papillary lesion on the glans or foreskin that grows to involve the entire penile shaft (Figure 25-5). Extensive lesions are associated with metastases and a poor prognosis.23 These lesions are not as painful as the amount of tissue involvement would seem to indicate. The regional femoral and iliac nodes are common metastatic sites. Rarely the urethra and bladder are involved. Weight loss, fatigue, and malaise accompany chronic suppurative lesions. Untreated, progressive disease causes death within 2 years.
The specific diagnosis is made by biopsy after examination to document the location, size, and fixation of the lesion. After a positive biopsy the extent of cancer spread is determined by imaging tests such as ultrasound, CT, or MRI. Fine-needle aspiration of lymph tissue confirms absence or presence of regional adenopathy.22 About 30% of penile cancers spread to lymph nodes before diagnosis. Distant metastases occur in less than 10% of cases and may involve lung, liver, bone, or brain.24 The following stages are used for penile cancer: stage 0 (carcinoma in situ), stage I, stage II, stage III, and stage IV.25
Penile carcinoma is primarily managed with surgery. Newer, innovative surgical techniques can preserve as much penile tissue as possible without compromising cancer control. For invasive penile carcinoma, complete excision leaving adequate tumor-free margins is the goal. A simple circumcision may be sufficient for localized lesions of the prepuce. If the primary site is the glans and distal shaft, removal of the penis may be necessary. Although conventional radical surgery continues to be an effective approach, the emasculating nature of the treatment has serious psychologic and sexual consequences. Recent studies have challenged the conventional belief that a 2-cm margin was required for adequate cancer control.23 Newer innovative surgical techniques can preserve as much penile tissue and functional integrity as possible without compromising cancer control. Inguinal lymph nodes also are removed if metastasis to these structures is known or suspected. Palliative treatment with radiation or chemotherapy may be used when the disease is inoperable and bulky inguinal metastases have occurred. Options for individuals with carcinoma in situ include local excision, radiation, laser surgery, cryosurgery, chemosurgery, or chemotherapy with topical (5%) 5-fluorouracil (5-FU). Differentiation, tumor stage, and age influence prognosis.23,26 The 5-year survival rate for stage I disease is greater than 80%; average 5-year survival rate for all stages is 50%.1,22
Disorders of the Scrotum, Testis, and Epididymis
Disorders of the Scrotum
Men may seek treatment for painful or painless scrotal masses. Masses may be serious (cancer or torsion) or benign (hydrocele or cyst) and may require immediate surgical intervention or allow for careful observation.27 Varicocele, hydrocele, and spermatocele are common intrascrotal disorders.28–30 A varicocele is an abnormal dilation of a vein within the spermatic cord and is classically described as a “bag of worms” (Figure 25-6). Most (95%) occur on the left side and may be painful or tender. Varicocele occurs in 10% of males and is seen most often after puberty. Sudden development of a varicocele in an older man is a late sign of renal tumor.31 Unilateral right-sided varicoceles are rare and result from compression or obstruction of the inferior vena cava by a tumor or thrombus. Color Doppler ultrasonography is used to confirm the diagnosis.27
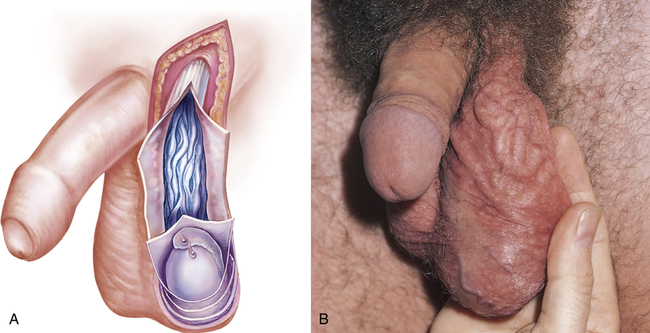
A, Dilation of veins within the spermatic cord. B, Varicocele on physical examination. (A from Seidel H et al: Mosby’s guide to physical examination, ed 7, St Louis, 2011, Mosby. B from Swartz MH: Textbook of physical diagnosis, ed 6, Philadelphia, 2010, Saunders.)
The cause of varicocele is incompetent or congenitally absent valves in the spermatic veins. The valves that normally prevent backflow are absent or do not close adequately, permitting blood to pool in the veins rather than flow into the venous system. Varicocele decreases blood flow through the testis. This interferes with spermatogenesis and is a cause of infertility.28,32 If infertility is a problem, treatment consists of ligation of the spermatic vein or occlusion of the vein by percutaneous methods, such as balloon catheter and sclerosing fluids.32 If varicocele is mild and fertility is not an issue, a scrotal support usually is sufficient to relieve symptoms of scrotal heaviness or “dragging.”
A hydrocele is a collection of fluid within the tunica vaginalis28,33 (Figure 25-7). It is the most common cause of scrotal swelling. Hydroceles occur in 6% of male newborns and are congenital malformations (patent processus vaginalis) that often resolve spontaneously in the first year of life. Surgical ligation is recommended if hydrocele persists after age 1 year.30 Hydroceles in adults may be caused by an imbalance between the secreting and absorptive capacities of scrotal tissues. Hydroceles range in size from slightly larger than the testes to the size of a grapefruit or larger and may be flaccid or tense. Compression of testicular blood supply may lead to atrophy.
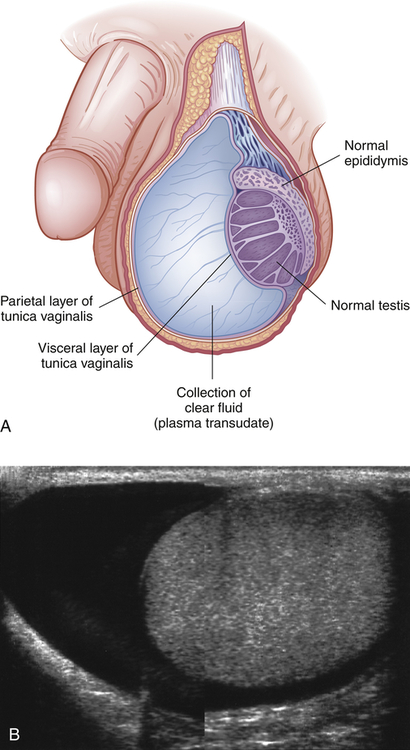
A, Accumulation of clear fluid between the visceral (inner) and parietal (outer) layers of the tunica vaginalis. B, The appearance of a hydrocele on ultrasound examination. (B from Adam A, et al: Grainger & Allison’s diagnostic radiology, ed 6, London, 2008, Churchill Livingstone.)
The exact mechanism of idiopathic hydrocele is unknown. Secondary hydrocele may result from trauma or infection of the testis or epididymis or from a testicular tumor. Rapid accumulation of fluid occurs after local injury, radiotherapy, or infection (epididymitis or orchitis), or it may accompany testicular neoplasm. Chronic hydroceles are more common and occur in men older than 40 because of an imbalance between fluid secretion and reabsorption in the tunica vaginalis. A painless, extratesticular mass that easily transilluminates is found on physical examination. Ultrasonography of a large hydrocele, which may conceal a testicular tumor, is recommended. Treatment is usually not required unless a large, bulky hydrocele causes considerable physical discomfort or undesirable cosmetic appearance.28 Treatment for uncomplicated hydrocele is aspiration of the fluid and injection of a sclerosing agent into the scrotal sac.33–35 The goal of treatment is to remove the hydrocele and prevent recurrence by sclerosing or excising the tunica vaginalis.
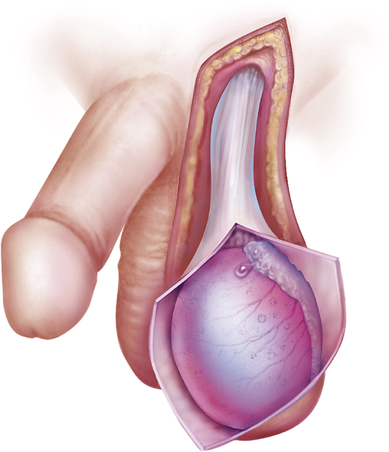
Spermatoceles (epididymal cysts) are benign cystic collections of fluid of the epididymis located between the head of the epididymis and the testis. Efferent ducts of the epididymis have potential for cystic dilation to form a spermatocele 33,35 (Figure 25-8). Spermatoceles are filled with milky fluid that contains sperm. Spermatocele is differentiated from a hydrocele in that aspiration of the hydrocele recovers a clear, yellow fluid, and unlike a hydrocele, a spermatocele does not cover the entire anterior surface of the testis.
Retention cyst of the head of the epididymis or of an aberrant tubule or tubules of the rete testis. The spermatocele lies outside the tunica vaginalis; therefore, on palpation it can be readily distinguished and separated from the testis. (From Lloyd-Davies RW, Gow JG, Davies DR: Color atlas of urology, ed 2, London, 1994, Mosby-Wolfe.)
Spermatoceles manifest as discrete, firm, freely mobile masses distinct from the testis that may be transilluminated. Epididymal cysts do not require treatment.33 A spermatic cord tumor may feel like a tense spermatocele but does not contain fluid and will not transilluminate.27 Spermatoceles that cause pain or discomfort are excised. Usually, however, spermatoceles are asymptomatic or produce mild discomfort that is relieved by scrotal support.35 Neither hydroceles nor spermatoceles are associated with infertility.
Cryptorchidism and Ectopy
Cryptorchidism is a condition of testicular maldescent, whereas an ectopic testis has strayed from the normal pathway of descent. Ectopy may be caused by an abnormal connection at the distal end of the gubernaculum testis that leads the gonad to an abnormal position, usually at the superficial inguinal site. In cryptorchidism the descent of one or both testes is arrested, with unilateral arrest occurring more often than bilateral arrest.35 The testes may remain in the abdomen, or testicular descent may be arrested in the inguinal canal or the puboscrotal junction. Cryptorchidism is a common congenital anomaly, with an incidence of approximately 3% in full-term infants. However, this rate increases significantly with low birth weight infants; for instance, the rate of cryptorchidism at 3 months has been found to be 7.7% for infants with birth weights less than 2000 g, 2.5% for birth weights of 2000 to 2500 g, and 1.41% for birth weights of 2500 g or more.35,36 The incidence of cryptorchidism in adults is 0.7% to 0.8%.32 Cryptorchidism is commonly associated with vasal or epididymal abnormalities. These congenital anomalies affect about one third to two thirds of newborns with cryptorchidism. Other structural anomalies include posterior urethral valves (less than 5%), upper tract abnormalities (less than 5%), and hypospadias. The presence of hypospadias as well as cryptorchidism raises the suspicion of mixed gonadal dysgenesis (intersex infant). It has been hypothesized that cryptorchidism may result from an absence or abnormality of the gubernaculum, a cordlike structure that extends from the lower pole of the testis to the scrotum; a congenital gonadal or dysgenetic defect that makes the testes insensitive to gonadotropins (a likely explanation for unilateral cryptorchidism); or lack of maternal gonadotropins (a likely explanation for bilateral cryptorchidism of prematurity).32 Mechanical possibilities include a short spermatic cord, fibrous bands or adhesions in the normal path of the testes, or a narrowed inguinal canal. Chromosomal studies do not support a genetic component. Physiologic cryptorchidism, also called retractile or migratory testis, is an involuntary retraction of the testes out of the scrotum that occurs with excitement, physical activity, or exposure to cold and is caused by the small mass of prepubertal testis and the strength of the cremaster muscle. This is a common phenomenon that is self-limiting (descent occurs at puberty).
Testicular cancer is also a well-established complication of cryptorchidism. In men with a history of unilateral cryptorchidism, neoplasms also develop more commonly in the contralateral testis. This finding suggests cryptorchidism affects the testes and is a process more significant than simply the position of the testis in childhood.35 The risk of testicular cancer is 35 to 50 times greater for men with cryptorchidism or a history of cryptorchidism than for the general male population. Because definite histologic change (decreased Leydig cells, loss of germ cells, and peritubular fibrosis) occurs in the cryptorchid testis by 1 year of age, surgical correction is recommended earlier.37 Treatment often begins with administration of GnRH or human chorionic gonadotropin (hCG), hormones that may initiate descent, making surgery unnecessary. GnRH is given as a nasal spray in Europe and may enhance germ-cell counts even when the testis does not descend.37 If hormonal therapy is not successful, the testis is located and moved surgically (orchiopexy) in young children or removed (orchiectomy) in adults and children older than 10 years.37 The testis that is properly placed in the scrotum provides adequate hormonal function and gives the scrotum a normal appearance. A successful operation does not ensure fertility if the testis is congenitally defective. Approximately 20% of males with unilateral undescended testis remain infertile even though orchiopexy is performed by age 1 year; most individuals with treated or untreated bilateral testicular maldescent have poor fertility. In addition, placement of the cryptorchid testis into the scrotal sac does not decrease the potential for malignancy; it does facilitate examination and tumor detection.
Torsion of the Testis
Torsion of the testis is rotation of a testis, which twists blood vessels in the spermatic cord. It causes an acute scrotum, which is testicular pain and swelling (Figure 25-9). Differentiation between testicular torsion and two other common causes of an acute scrotum is based on physical examination and history35 (Table 25-1). This event is most common among neonates and pubertal adolescents, but it can occur in males at any age.30 Onset may be spontaneous or follow physical exertion or trauma. Torsion twists the arteries and veins in the spermatic cord, reducing or stopping circulation to the testis. Vascular engorgement and ischemia develop, causing scrotal swelling and pain. These manifestations are not relieved by scrotal elevation (Prehn sign), rest, or scrotal support. On physical examination, men have a tender high-riding testis, a thickened spermatic cord, and an absent cremasteric reflex. Unlike epididymitis, the epididymis cannot be differentiated from the testis.35 Diagnostic testing includes urinalysis (to rule out infection) and color Doppler ultrasonography.32 Torsion of the testis is a surgical emergency. If the torsion cannot be reduced manually, surgery must be performed within 6 hours after the onset of symptoms to preserve normal testicular function. Surgery includes untwisting the spermatic cord and anchoring both testes in correct position within the scrotum to prevent recurrences. With successful manual detorsion, surgical fixation should be done within a few days.
TABLE 25-1
DIAGNOSIS OF SELECTED CONDITIONS RESPONSIBLE FOR THE ACUTE SCROTUM
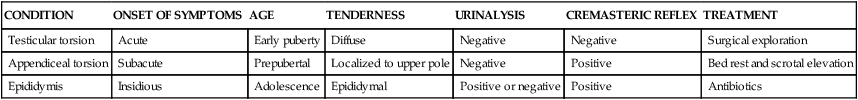
| CONDITION | ONSET OF SYMPTOMS | AGE | TENDERNESS | URINALYSIS | CREMASTERIC REFLEX | TREATMENT |
| Testicular torsion | Acute | Early puberty | Diffuse | Negative | Negative | Surgical exploration |
| Appendiceal torsion | Subacute | Prepubertal | Localized to upper pole | Negative | Positive | Bed rest and scrotal elevation |
| Epididymis | Insidious | Adolescence | Epididymal | Positive or negative | Positive | Antibiotics |
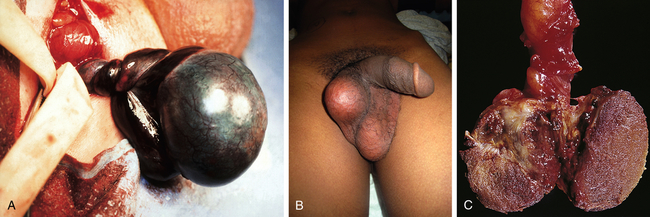
A, Left testicular torsion in an adolescent with acute scrotum; the testis is necrotic. B, Late phase torsion in an adolescent with severe testicular pain 1 month previously. Note the absence of inflammation and high position of testis in scrotum. C, The testes appear dark red and partially necrotic owing to hemorrhagic infarction. (A and B from Kliegman RM et al: Nelson’s textbook of pediatrics, ed 19, Philadelphia, 2011, Saunders. C from Damjanov I, Linder J, editors: Anderson’s pathology, ed 10, St Louis, 1996, Mosby.)
Orchitis
Orchitis is an acute infection of the testes (Figure 25-10) and is uncommon except as a complication of systemic infection or as an extension of an associated epididymitis38 (see p. 897). Infectious microorganisms may reach the testes through the blood or the lymphatics or, most commonly, by ascent through the urethra, vas deferens, and epididymis. Most cases of orchitis are actually cases of epididymo-orchitis. Occasionally, in middle-age men, a nonspecific, apparently noninfectious, inflammatory process (called granulomatous orchitis) occurs. It seems to be an autoimmune disease that triggers a granulomatous response to spermatozoa.
Inflammation of the testicle with enlargement or swelling. (From Seidel H et al: Mosby’s guide to physical examination, ed 7, St Louis, 2011, Mosby.)
Cancer of the Testis
Testicular cancer is a highly treatable, usually curable, cancer that most often develops in young and middle-aged men. For men with seminoma (all stages combined), the cure rate exceeds 90%. For those with low-stage seminoma or nonseminoma, the cure rate approaches 100%.39 Overall, testicular cancers are rare, yet they are the most common form of cancer in young men between ages 15 and 35. Approximately 8590 cases and 360 deaths were estimated for 2012.1 In the United States, the lifetime probability of developing testicular cancer is 0.2% for white men, an incidence that is four times higher than for blacks. Testicular tumors are slightly more common on the right side than on the left, a pattern that parallels the occurrence of cryptorchidism; about 1% to 2% of primary testicular cancers are bilateral (Figure 25-11), and 50% of these tumors arise from treated or untreated cryptorchid testes.
Pathogenesis
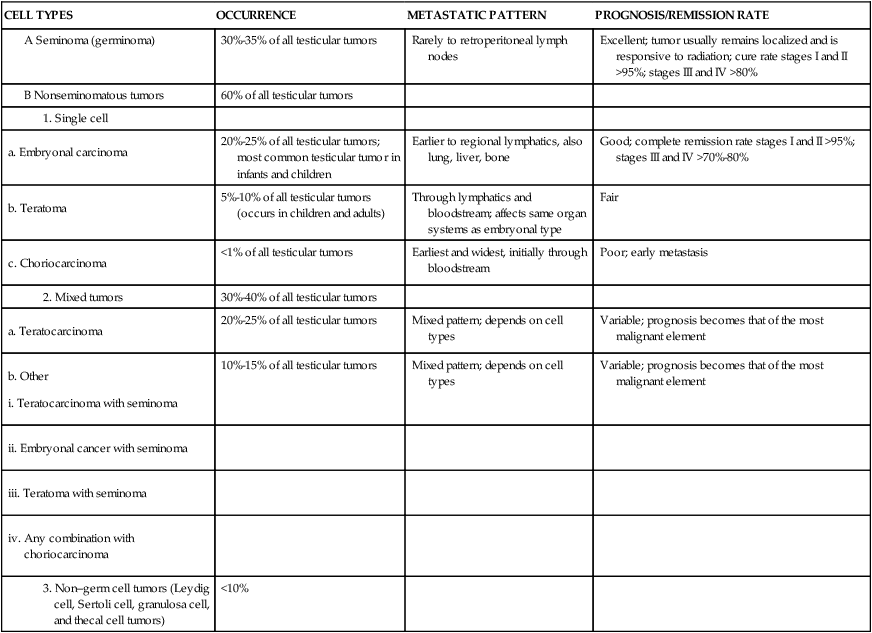
Ninety percent of testicular cancers are germ-cell tumors arising from the male gametes. Germ-cell tumors constitute 90% of testicular cancers and can be broadly classified into two types: seminomas and nonseminomas. Seminomas are the most common, are the least aggressive, and make up about 30% to 35% of testicular cancers. Nonseminomas include embryonal carcinomas, teratomas, and choriocarcinomas, the most aggressive but rare (less than 1%) form of testicular cancer. Testicular cancers can include a mix of types.40 In addition, testicular tumors can arise from specialized cells of the gonadal stroma. These tumors, which are named for their cellular origins, are Leydig cell, Sertoli cell, granulosa cell, and theca cell tumors and constitute less than 10% of all testicular cancers.41
The cause of testicular neoplasms is unknown. A genetic predisposition is suggested by the fact that the incidence is higher among brothers, identical twins, and other close male relatives. Genetic predisposition is supported further by statistics showing that the disease is relatively rare among black Africans, black Americans, Asians, and native New Zealanders. Familial testicular germ cell tumors may be associated with transgenerational inheritance of epigenetic events.42 Risk factors include history of cryptorchidism, abnormal testicular development, human immunodeficiency virus (HIV) and AIDS, Klinefelter syndrome, and history of testicular cancer.40
Clinical Manifestations
Painless testicular enlargement commonly is the first sign of testicular cancer. Enlargement is usually gradual and may be accompanied by a sensation of testicular heaviness or dull ache in the lower abdomen.40 Occasionally, acute pain occurs because of rapid growth, resulting in hemorrhage and necrosis. Ten percent of affected men have epididymitis, 10% have hydroceles,35 and 5% have gynecomastia or hydrocele. Incidence of gynecomastia increases considerably (30% to 45%) in men with Sertoli or Leydig tumors. Approximately 10% of individuals already have symptoms related to metastases at the time of initial diagnosis, which correlates with the typical delay of 3 to 6 months from initial recognition to definitive treatment. Lumbar pain may be present and usually is caused by retroperitoneal node metastasis. Signs of metastasis to the lungs include cough, dyspnea, and bloody sputum (hemoptysis). Supraclavicular node involvement may cause difficulty swallowing (dysphagia) and neck swelling. Alterations in vision or mental status, papilledema, and seizures may be experienced with metastasis to the CNS. Approximately 10% of affected individuals are asymptomatic; the tumor may be detected by the man’s sexual partner or incidentally following trauma.
Evaluation and Treatment
An incorrect diagnosis at the initial examination occurs in as many as 25% of men with testicular cancer. Epididymitis and epididymo-orchitis are the most common misdiagnoses; others include hydrocele and spermatocele. Evaluation begins with careful physical examination, including palpation of the scrotal contents with the individual in the erect and supine positions. The abdomen and lymph nodes are palpated to rule out metastases. Signs of testicular cancer include abnormal consistency, induration, nodularity, or irregularity of the testis. A firm, nontender testicular mass or diffuse enlargement is found in the majority of cases. Primary testicular cancer can be assessed rapidly and accurately by scrotal ultrasonography. Tumor markers are higher than normal in the presence of a tumor and may help detect a tumor that is too small to be palpated during physical examination or seen on imaging.35 Tumor type is identified after inguinal biopsy or orchiectomy. Scrotal incisions may cause dissemination of the tumor and increase the risk of local recurrence and therefore are avoided. Chest x-ray, lymphangiogram, intravenous pyelogram (IVP), abdominal ultrasound, and CT are used in clinical staging of disease. Treatment is based on type of tumor, stage of disease, general health, and age. Besides surgery, treatment involves radiation and chemotherapy singly or in combination. A number of factors influence the prognosis (Table 25-2). They include histology of the tumor, stage of the disease, and selection of appropriate treatment. Serum markers, such as alpha fetoprotein (AFP), β-hCG, and lactate dehydrogenase, are useful for detecting metastases and assessing responses to therapy. With appropriate treatment survival rates from testicular cancer are excellent, although some have persistent paresthesias, Raynaud phenomenon, or infertility. According to the National Cancer Institute, the overall 5-year survival rate from testicular cancer was 95.3% between 1999 and 2005. If the cancer was confined to the testis at the time of diagnosis, the survival rate was 99.2% and dropped only slightly to 95.9% with regional extension. For those with distant metastases, the survival rate was 71%.35,40 Orchiectomy does not affect sexual function, but infertility can result from chemotherapy or surgical removal of affected abdominal lymph nodes if nerves necessary for ejaculation are severed. After orchiectomy, testicular silicone implants may be used to restore “normal” scrotal appearance.
TABLE 25-2
TESTICULAR TUMORS OF GERM CELL ORIGIN
| CELL TYPES | OCCURRENCE | METASTATIC PATTERN | PROGNOSIS/REMISSION RATE |
| 30%-35% of all testicular tumors | Rarely to retroperitoneal lymph nodes | Excellent; tumor usually remains localized and is responsive to radiation; cure rate stages I and II >95%; stages III and IV >80% | |
| 60% of all testicular tumors | |||
| 20%-25% of all testicular tumors; most common testicular tumor in infants and children | Earlier to regional lymphatics, also lung, liver, bone | Good; complete remission rate stages I and II >95%; stages III and IV >70%-80% | |
| 5%-10% of all testicular tumors (occurs in children and adults) | Through lymphatics and bloodstream; affects same organ systems as embryonal type | Fair | |
| <1% of all testicular tumors | Earliest and widest, initially through bloodstream | Poor; early metastasis | |
| 30%-40% of all testicular tumors | |||
| 20%-25% of all testicular tumors | Mixed pattern; depends on cell types | Variable; prognosis becomes that of the most malignant element | |
| 10%-15% of all testicular tumors | Mixed pattern; depends on cell types | Variable; prognosis becomes that of the most malignant element | |
| <10% |
Data from American Cancer Society. In Cancer response system document #10029, New York, 1995, The Society; Cancer Net: Cancer facts: questions and answers about testicular cancer, National Cancer Institute, 2000. Available at www.cancernet.nci.nih.gov.
Epididymitis
Epididymitis, or inflammation of the epididymis, generally occurs in sexually active young males (younger than 35 years) and is rare before puberty (Figure 25-12). In young men the usual cause is a sexually transmitted microorganism, such as N. gonorrhoeae or C. trachomatis. Men who practice unprotected anal intercourse may acquire sexually transmitted epididymitis because of Escherichia coli, Haemophilus influenzae, tuberculosis (especially in regions where incidence of pulmonary tuberculosis is high), Cryptococcus, or Brucella.35 In men older than 35 years, enterobacteriaceae (intestinal bacteria) and Pseudomonas aeruginosa associated with urinary tract infections and prostatitis also may cause epididymitis. Besides an infectious etiology, epididymitis may result from a chemical inflammation caused by the reflux of sterile urine into the ejaculatory ducts.43 It is associated with urethral strictures, congenital posterior valves, and excessive physical straining in which increased abdominal pressure is transmitted to the bladder. Chemical epididymitis is usually self-limiting and does not require evaluation or intervention unless it persists.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree