Chapter 47
Alterations of the Integument in Children
Noreen Heer Nicol and Sue E. Huether
Acne Vulgaris
Acne vulgaris is the most common skin disease and affects 85% of the population between the ages of 12 and 25 years. Onset is occurring at a younger age and may be associated with earlier onset of puberty in the United States. Genetic influences may determine an individual’s susceptibility and severity of disease. Severe acne tends to have a genetic predisposition.1 The incidence of acne is the same in both genders, although severe disease affects males more often.
Acne develops at distinctive pilosebaceous units, known as sebaceous follicles. Located primarily on the face and upper parts of the chest and back, these follicles have many large sebaceous glands, a small vellus hair (very short, nonpigmented, and very thin hair), and a dilated follicular canal that is visible as a pore on the skin surface. Acne lesions may be inflammatory (pustules, papules, nodules) or noninflammatory. In noninflammatory acne the comedones are open (blackheads) and closed (whiteheads), with the accumulated material causing distention of the follicle and thinning of follicular canal walls. Inflammatory acne develops in closed comedones when the follicular wall ruptures, expelling sebum into the surrounding dermis and initiating inflammation. Pustules form when the inflammation is close to the surface; papules and cystic nodules can develop when the inflammation is deeper, causing mild to severe scarring (Figure 47-1). Many types of lesions may exist in the same individual.
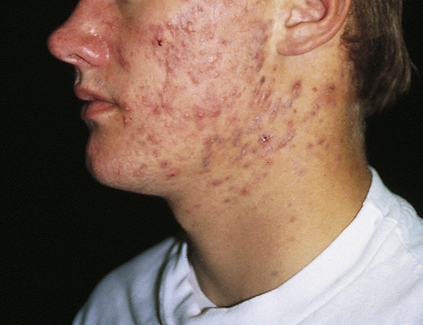
Multiple pustules (erythematous papules and pustules) are present, and several have become confluent. Note areas of scarring. (Courtesy Department of Dermatology, School of Medicine, University of Utah.)
The exact cause of acne is unknown and various pathophysiologic factors contribute to the development of acne. The principal factors are follicular hyperkeratinization, excessive sebum production, follicular proliferation of Propionibacterium acnes, and inflammation and rupture of a follicle from accumulated debris and bacteria within the follicle. Androgens (dihydrotestosterone and testosterone) synthesized in increasing amounts during puberty boost the size and productivity of the sebaceous glands and promote P. acnes. Acne also is associated with polycystic ovarian syndrome, congenital adrenal hyperplasia, and various endocrine tumors as a result of higher circulating levels of androgens. The P. acnes anaerobic bacteria produce extracellular porphyrins and proinflammatory molecules, including chemotactic factors and lipolytic and proteolytic enzymes. The hydrolytic action of the enzymes converts triglycerides into free fatty acids that stimulate inflammation and edema and result in breakdown of the follicle wall. Chemotactic substances also may be released that involve mediation of inflammation by attraction of polymorphonuclear leukocytes.2
Treatment of acne should address the causative factors and be individualized according to severity. The combination of a topical retinoid, benzoyl peroxide, and antimicrobial agents is preferred. Retinoids are anticomedogenic and comedolytic and have some anti-inflammatory effects. Benzoyl peroxide is antimicrobial with some keratolytic effects. Topical antibiotics (e.g., erythromycin and clindamycin) have anti-inflammatory and antimicrobial effects. Combined oral contraceptives may be effective for acne in women.3 Use of systemic therapies, including oral antibiotics, corticosteroids, and isotretinoin, is encouraged in more severe cases but may be limited by side effects. Dietary based guidelines need further investigation.4 Acne surgery, including comedo extraction, intralesional steroids, and cryosurgery, may be useful in selected individuals. Severe scarring may be treated with dermabrasion, lasers, and resurfacing techniques. Special consideration must be given to treatment for those with darker skin because they have greater risk for hyperpigmentation and keloidal scarring.5 Psychosocial morbidity is significant, particularly in adolescents.5–7
Dermatitis
Atopic Dermatitis
Atopic dermatitis (AD), also known as atopic eczema, occurs with a prevalence rate up to 20% in children. AD is increasing throughout the world.8 Onset is usually from 2 to 6 months of age, and 85% of cases occur within the first 5 years of life; 75% to 80% of individuals with AD have a personal or family history of asthma, allergic rhinitis (hay fever), or food allergy. The cause of this chronic relapsing form of pruritic eczema involves an interplay of genetic predisposition, altered skin barrier function associated with filaggrin gene missense mutations (proteins that bind keratin in the epidermis), reduced ceramide (a stratum corneum lipid) levels, altered innate immunity, and altered immune responses to allergens, irritants, and microbes.9
There is debate as to whether the pathophysiology favors an “inside-out” explanation with immunologic dysregulation leading to epidermal barrier abnormality or an “outside-in” explanation with primary barrier dysfunction as the cause of the immunologic perturbations.10 Regardless of the debate, both are involved in the disease. Positive skin tests to a variety of common food and inhalant allergens are seen in approximately 80% of individuals. Children with atopic dermatitis are more likely to have asthma.11
In AD, memory T cells in the blood express cutaneous lymphocyte antigen (CLA), which leads to the homing of lymphocytes to the skin. Inflammation is associated with activation of Th2 and Th1 cells with release of numerous cytokines, chemokines, interferon-gamma (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Activation of mast cells, eosinophils, and macrophages and expression of IgE contribute to the inflammation.12
Alterations in filaggrin protein lead to a defect of the epidermal barrier that causes transepidermal water loss and allows easy penetration of pathogens and allergens through the skin and a systemic hyperactive immune response. Filaggrin gene mutations also are associated with increased risk for asthma in AD and ichthyosis vulgaris (dry, scaly skin). In AD keratinocytes are deficient in their ability to express Toll-like antimicrobial peptides (see Chapter 7), and may predispose such individuals to skin colonization and infection with Staphylococcus aureus, viruses, and fungi.13
AD has a long-term course with frequent exacerbations, severe pruritus, and characteristic eczematoid appearance with redness, edema, and scaling (Figure 47-2). The skin becomes increasingly dry, sensitive, itchy, and easily irritated because the barrier function is impaired. Microscopic epidermal cracks that allow water to be expelled and irritants, allergens, and microbes to enter further inflammation, drying, and cracking. Itching is the hallmark of atopic dermatitis, and rubbing and scratching to relieve the itch are responsible for many of the clinical skin changes of AD. Histamine level is elevated in AD lesions but is not considered a major pruritogen because blockage of H1 and H2 receptors alone is ineffective at relieving itch. However, H4 receptor blockers are being evaluated.14 Pruritus is a complex process with immune and neurologic pathways.15,16
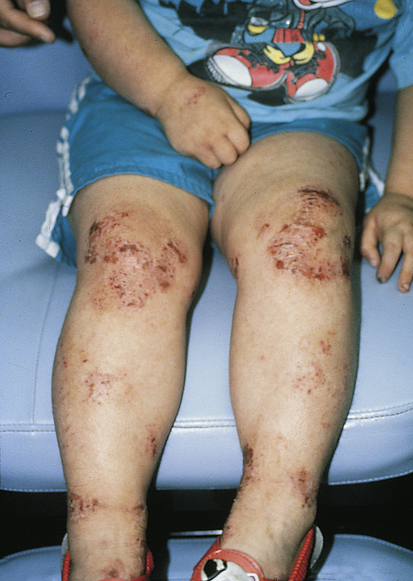
Characteristic lesions with crusting from irritation and scratching over knees and around ankles. (Courtesy Department of Dermatology, School of Medicine, University of Utah.)
There are no specific laboratory features of AD that can be used for diagnostic purposes, and diagnosis is based on clinical history and presentation of symptoms. Management of AD requires a systematic, multidimensional, individualized approach that considers the individual’s skin reaction pattern and acuity of the rash.17 Treatment guidelines establish the foundation of AD management, addressing the skin barrier defect with regular use of emollients and skin hydration along with the identification and avoidance of specific and nonspecific trigger factors. Various topical agents are mainstays of treatment. Topical corticosteroids and calcineurin inhibitors continue to dominate therapy and, to date, their safety record seems good (despite the Black Box warning issued in the United States). In severe cases that cannot be controlled, guidelines suggest systemic treatment options including systemic corticosteroids, cyclosporine A, ultraviolet light therapy, and wet wrap therapy being added to the topical treatment regimen. Systemic corticosteroids are known to be effective in short-term treatment of AD, but evidence is limited to support their use because of long-term side effects and rebound flaring.18–20 Research is in progress to develop targeted molecular therapy that is safe and cost-effective.
Diaper Dermatitis
Diaper dermatitis is a form of irritant contact dermatitis initiated by a combination of factors that include prolonged exposure to and irritation by urine and feces, maceration by wet diapers, complications from use of airtight plastic diaper covers, and possibly increased association with intercurrent illnesses and early introduction of cereals. Disposable diaper designs have decreased diaper dermatitis in infants.21 Diaper dermatitis is frequently secondarily infected with Candida albicans.
The lesions vary from mild erythema to erythematous papular lesions. Candidal (monilial) diaper dermatitis is usually very erythematous, with sharp margination and pustulovesicular satellite lesions (Figure 47-3).
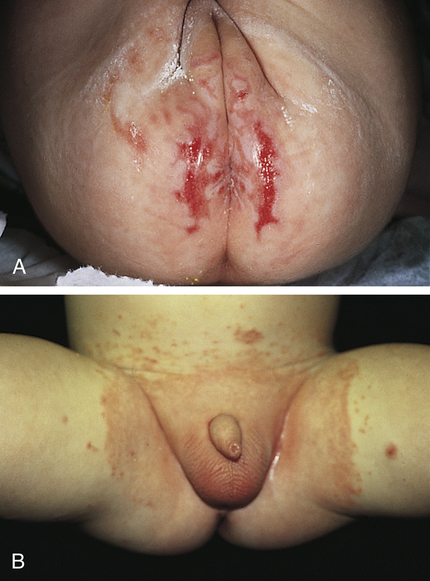
A, Diaper dermatitis with erosions. B, Diaper dermatitis with Candida albicans secondary infection. (Courtesy Department of Dermatology, School of Medicine, University of Utah.)
Treatment includes changing the diaper frequently to keep the area clean and dry or frequently exposing the perineal area to air, using super-absorbent diapers, and applying topical protection with a product containing petrolatum or zinc oxide, or both. Topical antifungal medication is used to treat C. albicans when present.22
Infections of the Skin
Bacterial Infections
Impetigo Contagiosum
Nonbullous impetigo is a contagious, acute, superficial, vesiculopustular form of impetigo caused by Staphylococcus aureus or group A Streptococcus pyogenes (alone or in combination with S. aureus). The microorganisms are disseminated by direct physical contact from other infected individuals or through insect bites. The lesions begin as small vesicles with a honey-colored serum. Yellow to white-brown crusts form as the vesicles rupture (Figure 47-4). The lesions must be differentiated from herpes simplex lesions (see Figure 46-17, p. 1636). Untreated lesions may last for weeks and extend to cover a large area. In contrast to bullous impetigo, regional lymphadenitis is common.
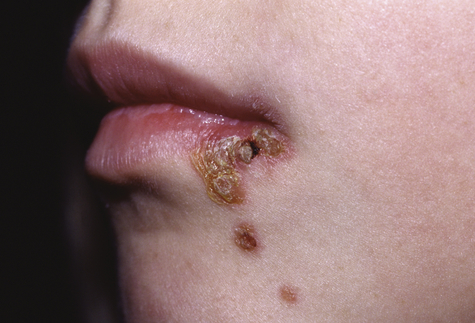
Multiple crusted and oozing lesions of impetigo. (From Kliegman RM et al: Nelson textbook of pediatrics, ed 19, Philadelphia, 2011, Saunders.)
Bullous impetigo is a rarer variant of impetigo caused by S. aureus.23 The pathogen is often carried in the anterior nares, perineal region, or fingernails and is transmitted by contact with the individual or contaminated equipment.24 The staphylococci produce bacterial toxins called exfoliative toxins (ETs) that cause a disruption in desmosomal adhesion molecules with blister formation.25 The blisters enlarge or coalesce to form superficial bullae. There may be a few localized lesions or many lesions scattered over the skin. As the bullae rupture, a thin, flat, honey-colored crust appears.
The crust is the hallmark of impetigo. A moist, inflamed serum-weeping base is revealed when the crust is removed. The lesions are often located on the face around the nose and mouth, but the hands and other exposed areas are also involved. Regional lymphadenitis is uncommon. Treatment of choice for both types of impetigo is topical mupirocin and topical fusidic acid or oral antibiotics. Antibiotic therapy should be determined by bacterial culture and drug sensitivity because the prevalence of community-acquired methicillin-resistant S. aureus–associated impetigo is increasing. For extensive or complicated impetigo, systemic antibiotics may be warranted but beta-lactam antibiotics should be avoided if methicillin-resistant S. aureus (MRSA) is suspected.23,26 Prompt treatment avoids complications, such as glomerulonephritis, necrotizing fasciitis, and septic shock syndrome. Handwashing and isolation of the infected child’s washcloth, towels, drinking glass, and linen are important practices to control this highly contagious disease.
Staphylococcal Scalded-Skin Syndrome
Staphylococcal scalded-skin syndrome (SSSS) is the most serious staphylococcal infection that affects the skin and usually is seen in infants and children younger than 5 years.27 SSSS is caused by virulent group II staphylococci, which produce an exfoliative toxin that attacks desmoglein and adhesion molecules and causes a separation of the skin just below the granular layer of the epidermis.28 The toxins are usually produced at body sites other than the skin and arrive at the epidermis through the circulatory system. Staphylococci typically are not found in the skin lesions themselves. Adults have circulating antistaphylococcal antibodies and are better able to metabolize and excrete the toxin. Newborns and premature infants are at the highest risk because of immature immunity (absence of prior exposure to the toxin).
The clinical symptoms begin with fever, malaise, rhinorrhea, and irritability followed by generalized erythema with exquisite tenderness of the skin. There may be an associated impetigo, but the infection often begins in the throat or chest. The erythema spreads from the face and trunk to cover the entire body except the palms, soles, and mucous membranes. Within 48 hours, blisters and bullae may develop, giving the child the appearance of being scalded (Figure 47-5). The pain is severe. Fluid loss from ruptured blisters and water evaporation from denuded areas may cause dehydration and loss of body heat. Perioral and nasolabial crusting and fissures develop. In severe cases the skin of the entire body may slough. When secondary infection can be prevented, healing of the involved skin occurs in 10 to 14 days, usually without scarring.
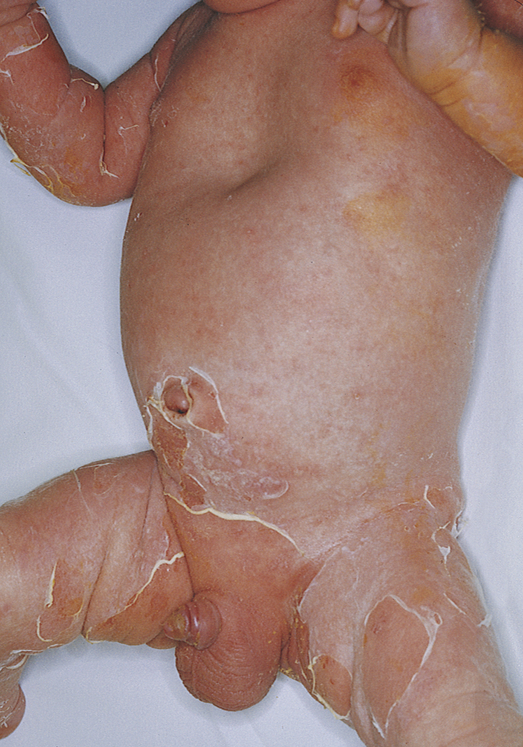
The skin lesions, showing desquamation and wrinkling of the skin margins, appeared 1 day after drainage of a staphylococcal abscess. (From Habif TP: Clinical dermatology: a color guide to diagnosis and therapy, ed 5, St Louis, 2010, Mosby.)
Before medical intervention is begun, culture, histology, or exfoliative cytology must be done to differentiate SSSS from toxic epidermal necrolysis (TEN) (see p. 1664). When the infection is confirmed, treatment with oral or intravenous antibiotics is begun. Topical antibiotics are ineffective. The skin should be treated the same as that with a severe burn—with meticulous aseptic technique. Skin substitutes may be used for adjuvant therapy.29 Special care is required in serious cases and when the lips and eyelids are involved.27
Fungal Infections
Fungal disorders are known as mycoses and, when caused by dermatophytes (fungi that thrive on keratin), the mycoses are termed tinea (dermatophytosis or ringworm). The different types of tinea are classified according to their location on the body. Tinea pedis (a chronic, superficial fungal infection of the skin of the foot) occurs in children but is rare. Scaling disorders of the toes and feet in prepubertal children are usually eczema. Tinea capitis (infection of the scalp) and tinea corporis (infection of the body) are much more common in children than in adults. Dermatophytes of the genus Epidermophyton are the major cause of superficial fungal infections in children.30 These dermatophytes invade the stratum corneum and not the remainder of the epidermis or dermis. The inflammatory response is thought, in part, to be secondary to the toxins released by the dermatophyte. It is important to confirm by culture the exact microorganism that is causing the fungal infection before commencing therapy.
Tinea Capitis
Tinea capitis, a fungal infection of the scalp, is the most common fungal infection of childhood. It rarely affects infants and is seen in children younger than age 12. Primary microorganisms responsible for the disease are Trichophyton tonsurans and Microsporum canis. T. tonsurans is transmitted by human-to-human contact. Areas of crowding are the most prevalent environments for this microorganism, which frequently affects inner-city children. T. tonsurans is often the predominant dermatophyte found in North America; many of these infections are not symptomatic.31 The prevalence of asymptomatic carriers among household contacts of a child with active T. tonsurans disease is high. M. canis is found on cats, dogs, and certain rodents. Humans appear to be a terminal host for M. canis, and children who handle such animals are possible hosts. Human-to-human transmission does not occur with M. canis.
When symptoms are present, the clinical presentations vary, depending on the microorganism. Often the lesions are circular and manifest by broken hairs 1 to 3 mm above the scalp, leaving a partial alopecia 1 to 5 cm in diameter32 (Figure 47-6). Slight erythema and scaling with raised borders can be observed.
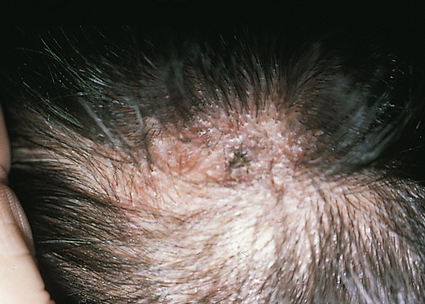
Diagnosis is best confirmed by performing Wood light examination, potassium hydroxide (KOH) examination, and fungal culture, in that order. T. tonsurans does not fluoresce with Wood light examination. Oral griseofulvin is the treatment of choice because topical fungicides do not penetrate to the hair bulb. Terbinafine, itraconazole, and fluconazole are effective alternatives.31 Adjunct therapy includes 2% ketoconazole and 1% selenium sulfide shampoos. Treatment of household contacts with a sporicidal shampoo should be considered and co-sleeping and comb-sharing must be discouraged.
Tinea Corporis
Tinea corporis (ringworm) is a common superficial dermatophyte infection in children. The microorganisms most commonly responsible for this disease are M. canis and Trichophyton mentagrophytes. As in tinea capitis, contact with kittens and puppies is a common source of the disorder. Tinea corporis preferentially affects the nonhairy parts of the face, trunk, and limbs. Lesions are often erythematous, round or oval scaling patches that spread peripherally with clearing in the center, creating the ring appearance, which is why this disease is commonly referred to as ringworm. The lesions are distributed asymmetrically, and multiple lesions (when present) overlap. Transmission occurs by direct contact with an infected lesion and through indirect contact with personal items used by the infected person. Potassium hydroxide examination of the scale from the border of the lesions confirms the diagnosis for most lesions. Most lesions respond well to applications of appropriate topical antifungal medications.30
Thrush
Thrush is the term used to describe the presence of Candida albicans in the mucous membranes of the mouth of infants and less commonly in adults, and both may be immune compromised. C. albicans penetrates the epidermal barrier more easily than other microorganisms because of its keratolytic proteases and other enzymes. C. albicans attracts neutrophils to skin sites of invasion but evades neutrophil killing.33 Thrush is characterized by the formation of white plaques or spots in the mouth that lead to shallow ulcers. The tongue may have a dense, white covering. The underlying mucous membrane is red and tender and may bleed when the plaques are removed. The disease is often accompanied by fever and gastrointestinal irritation. The infection commonly spreads to the groin, buttocks, and other parts of the body. Treatment may be difficult and may include oral antifungal washes, such as nystatin oral suspension. Gentian violet can also be effective but is messy.34 Simultaneous treatment of a Candida nipple infection or vaginitis in the mother is helpful in reducing the C. albicans surface colonization of the infant. Feeding bottles and nipples should be sterilized to prevent reinfection. The diaper area should be kept clean and dry.
Viral Infections
Molluscum Contagiosum
Molluscum contagiosum is a common highly contagious poxvirus infection of the skin and occasionally conjunctiva that affects school-aged children and sexually active young adults. The disease can be more severe or prolonged in individuals with atopic dermatitis and a variety of immunodeficient states, including acquired immunodeficiency syndrome (AIDS). It is transmitted by skin-to-skin contact (e.g., contact sports), autoinoculation, and fomites, such as clothing, wash devices, and towels. The poxvirus induces epidermal cell proliferation and blocks immune responses that would control the virus. The epidermis grows down into the dermis to form saccules containing clusters of virus. The characteristic molluscum body is composed of mature, immature, and incomplete viruses and cellular debris.35
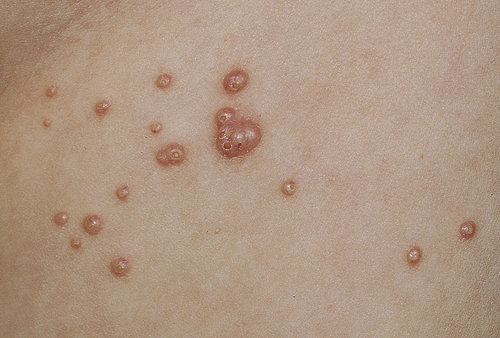
The lesions of molluscum are discrete, slightly umbilicated, dome-shaped papules 1 to 5 mm in diameter that appear anywhere on the skin or conjunctiva. The skin distribution in children is mainly on the trunk, face, and extremities (Figure 47-7). The pubic, genital, and perineal areas are favored in adults. Usually no inflammation surrounds molluscum lesions unless they are traumatized or secondary infection occurs. Scarring may occur with healing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree