Chapter 38
Alterations of Renal and Urinary Tract Function
Alexa K. Doig and Sue E. Huether
Urinary Tract Obstruction
Urinary tract obstruction is an interference with the flow of urine at any site along the urinary tract (Figure 38-1). An obstruction may be anatomic or functional. It impedes flow proximal to the obstruction, dilates structures distal to the obstruction, increases risk for infection, and compromises renal function. Anatomic changes in the urinary system caused by obstruction are referred to as obstructive uropathy. The severity of an obstructive uropathy is determined by: (1) the location of the obstructive lesion, (2) the involvement of one or both upper urinary tracts (ureters and renal pelvis), (3) the completeness of the obstruction, (4) the duration of the obstruction, and (5) the nature of the obstructive lesion.1,2 Obstructions may be relieved or partially alleviated by correction of the obstruction, although permanent impairments occur if a complete or partial obstruction persists over weeks to months or longer.
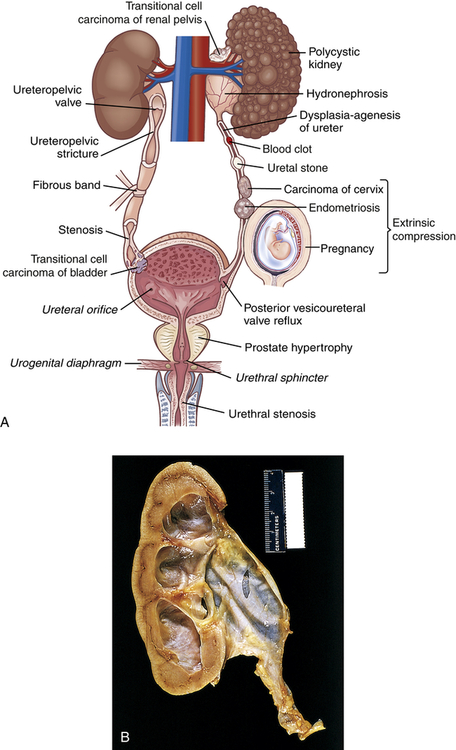
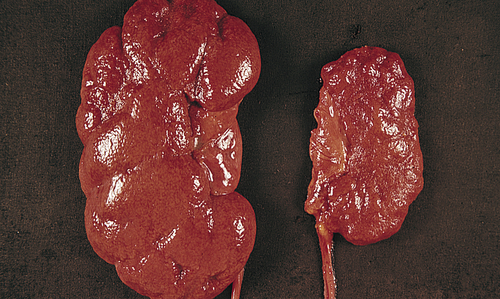
A, Causes of urinary tract obstruction. Terms in italics are normal structures. B, Hydronephrosis, marked dilation of renal pelvis and calyces with thinning of parenchyma.
Upper Urinary Tract Obstruction
Obstruction of the upper urinary tract causes dilation of the ureter, renal pelvis, calyces, and renal parenchyma proximal to the site of urinary blockage. Dilation of the ureter is referred to as hydroureter (accumulation of urine in the ureter), and dilation of the renal pelvis and calyces proximal to a blockage leads to hydronephrosis (enlargement of the renal pelvis and calyces) or ureterohydronephrosis (dilation of both the ureter and the pelvicaliceal system) (Figure 38-2). Dilation of the upper urinary tract is an early response to obstruction and reflects smooth muscle hypertrophy and accumulation of urine above the level of blockage (urinary stasis/retention). The increased pressure is transmitted to the glomerulus, which decreases filtration. Unless the obstruction is relieved, this dilation leads to enlargement with tubulointerstitial fibrosis and apoptosis affecting the distal nephron and renal function. Tubulointerstitial fibrosis is the deposition of excessive amounts of extracellular matrix (collagen and other proteins). Deposition of extracellular matrix is a normal process of organ repair and maintenance, and the deposition of extracellular matrix is balanced by its breakdown under the influence of metalloproteinases. Multiple cytokines and growth factors have been implicated in the process of tubulointerstitial fibrosis and irreversible loss of kidney function, including transforming growth factor-beta-1 (TGF-β1), angiotensin II, aldosterone, and various tumor necrosis factors. Apoptosis is a normal process that the body uses to replace damaged or senescent cells with new ones (see Chapter 1), but the imbalance in growth factors provoked by obstruction leads to excess cellular destruction and death, ultimately resulting in loss of functioning nephrons and kidney damage.
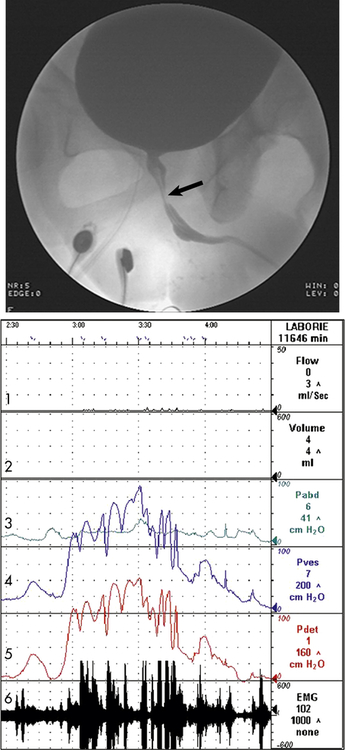
The arrow indicates narrowing of the striated sphincter consistent with electromyographic activity (Line 6) noted on the urodynamic tracing. Note the characteristic poor flow pattern (Line 1) with elevated voiding pressures (Lines 4 and 5) indicating obstruction. Line 1 = Urine flow rate; Line 2 = urine volume; Line 3 = abdominal pressure (Pabd); Line 4 = intravesicular (inside bladder) pressure (Pves); Line 5 = detrusor muscle pressure (Pdet); Line 6 = bladder electromyelogram (EMG).
Tubulointerstitial fibrosis and apoptosis result in detectable damage to the distal renal tubules within approximately 7 days. By 14 days, obstruction has adversely affected both distal and proximal aspects of the nephron. Within 28 days the glomeruli of the kidney have been damaged and the renal cortex and medulla are reduced in size (thinned). Distal tubular damage occurs initially and decreases the kidney’s ability to concentrate urine, causing an increase in urine volume despite a decrease in glomerular filtration rate (GFR). The affected kidney is unable to conserve sodium, bicarbonate, and water or to excrete hydrogen or potassium, leading to metabolic acidosis and dehydration. The magnitude of this damage, and the kidney’s ability to recover normal homeostatic function, is affected by the severity and duration of the obstruction. With complete obstruction, damage to the renal tubules and compression of the renal vasculature occurs in a matter of hours, and irreversible damage occurs within 3 to 4 weeks. Nevertheless, even in the face of a complete obstruction, the human kidney may recover at least partial homeostatic function provided the blockage is removed within 56 to 69 days.3,3a This recovery requires approximately 4 months. Partial obstruction, in the absence of renal infection, leads to subtler but ultimately permanent impairments including loss of the kidney’s ability to concentrate urine, reabsorb bicarbonate, excrete ammonia, or regulate metabolic acid-base balance if the obstruction is not relieved. Complete bilateral obstruction causes anuria because the retrograde increase in tubular hydrostatic pressure completely opposes glomerular filtration.
The body is able to partially counteract the negative consequences of unilateral obstruction by a process called compensatory hypertrophy and hyperfunction.4 The compensatory response is the result of two growth processes: obligatory growth occurs under the influence of somatomedins, and compensatory growth occurs under the influence of a hormone or hormones that have not yet been identified. These processes cause the contralateral (unobstructed) kidney to increase the size of individual glomeruli and tubules but not the total number of functioning nephrons. The ability of the body to engage in compensatory hypertrophy and hyperfunction diminishes with age, and the process is reversible when relief of obstruction results in recovery of function by the obstructed kidney. Unilateral obstruction may remain silent for a long time.
Relief of bilateral, partial urinary tract obstruction or complete obstruction of one kidney is usually followed by a brief period of diuresis (commonly called postobstructive diuresis).5 It is a physiologic response and is typically mild, representing a restoration of fluid and electrolyte imbalance caused by the obstructive uropathy. Alterations in tubular transport and water reabsorption and volume expansion contribute to the diuresis. Occasionally relief of obstruction will cause rapid excretion of large volumes of water, sodium, or other electrolytes, resulting in a urine output of 10 L/day or more (minimal normal daily urine output is approximately 720 ml/day). Rapid postobstructive diuresis causes dehydration and fluid and electrolyte imbalances if not promptly corrected. Risk factors for severe postobstructive diuresis include bilateral obstruction, impairment of one or both kidneys’ ability to concentrate urine or reabsorb sodium (nephrogenic diabetes insipidus), hypertension, edema and weight gain, congestive heart failure, and uremic encephalopathy.
Kidney Stones
Calculi, or urinary stones (urolithiasis), are masses of crystals, protein, or other substances that are a common cause of urinary tract obstruction in adults. They can be located in the kidneys, ureters, and urinary bladder. The prevalence of stones in the United States is approximately 6% in women and 15% in men, and is more common in whites.6 The recurrence rate is approximately 30% to 50% within 5 years.7 The risk of urinary calculi formation is influenced by a number of factors, including age, gender, race, geographic location, seasonal factors, fluid intake, diet, occupation, genetic predisposition, and other conditions including urinary tract infection, hypertension, atherosclerosis, metabolic syndrome, obesity, and diabetes.8 Most persons develop their first stone before age 50 years. Geographic location influences the risk of stone formation because of indirect factors, including average temperature, humidity, and rain fall, and their influence on fluid and dietary patterns. Persons who regularly consume an adequate volume of water and those who are physically active are at reduced risk when compared with people who are inactive or consume lower volumes of fluid. Most kidney stones are unilateral and are a risk factor for chronic kidney disease and an increased risk for myocardial infarction.9,10
Pathophysiology
Renal calculus formation is complex and related to: (1) supersaturation of one or more salts in the urine, (2) precipitation of the salts from a liquid to a solid state (crystals), (3) growth through crystallization or agglomeration (sometimes called aggregation), and (4) the presence or absence of stone inhibitors.11 Supersaturation is the presence of a higher concentration of a salt within a fluid (in this case, the urine) than the volume is able to dissolve to maintain equilibrium. Human urine contains many positively and negatively charged ions capable of precipitating from solution and forming a variety of salts. The salts form crystals that are retained and grow into stones. Crystallization is the process by which crystals grow from a small nidus or nucleus to larger stones in the presence of supersaturated urine. Although supersaturation is essential for free stone formation, the urine need not remain continuously supersaturated for a calculus to grow once its nidus has precipitated from solution. Intermittent periods of supersaturation after the ingestion of a meal or during times of dehydration are sufficient for stone growth in many individuals. In addition, the apical papillae have interstitial sites where hydroxyapatite deposits (Randall plaque) become exposed and serve as sites for calcium oxalate stone formation (but not calcium phosphate stone formation).12 Matrix is an organic material (i.e., mucoprotein) in which the components of a kidney stone are embedded.11
The size of a stone determines the likelihood that it will pass through the urinary tract and be excreted through micturition.13 Stones smaller than 5 mm have about a 50% chance of spontaneous passage, whereas stones that are 1 cm have almost no chance of spontaneous passage. Nevertheless, the person with ureteral dilation from the previous passage of a stone may be able to excrete larger stones when compared with the person experiencing an initial obstructing calculus.
Calcium stones (urolithiasis) account for 70% to 80% of all stones requiring treatment. Calcium oxalate accounts for about 80% of these stones and calcium phosphate about 15%.14 Both genetic and environmental factors may increase susceptibility. Most affected individuals have idiopathic calcium oxalate urolithiasis (ICOU), a condition whose exact etiology has not yet been defined. Stones can form freely in supersaturated urine or detach from interstitial sites of formation.15 Hypercalciuria, hyperoxaluria, hyperuricosuria, hypocitraturia, mild renal tubular acidosis, crystal growth inhibitor deficiencies, and alkaline urine are associated with calcium stone formation.16,17 Hypercalciuria and hyperoxaluria are usually attributable to intestinal hyperabsorption and less commonly to a defect in renal calcium reabsorption. Hyperparathyroidism and bone demineralization associated with prolonged immobilization are also known to cause hypercalciuria.
Struvite stones primarily contain magnesium-ammonium-phosphate as well as varying levels of matrix. Matrix forms in an alkaline urine and during infection with a urease-producing bacterial pathogen, such as a Proteus, Klebsiella, or Pseudomonas. Struvite calculi may grow quite large and branch into a staghorn configuration (staghorn calculus) that approximates the pelvicaliceal collecting system. Women are at greater risk for struvite stones because they have an increased incidence of urinary tract infection (see p. 1349).
Clinical Manifestations
Renal colic, described as moderate to severe pain often originating in the posterior hypochondrium (flank) and radiating to the groin, usually indicates obstruction of the renal pelvis or proximal ureter. Colic that radiates to the lateral flank or lower abdomen typically indicates obstruction in the midureter, and bothersome lower urinary tract symptoms (urgency, frequent voiding, urge incontinence) indicate obstruction of the lower ureter or ureterovesical junction. The pain can be severe and incapacitating and may be accompanied by nausea and vomiting. Gross (visible blood in the urine) or microscopic hematuria (three or more red blood cells per high power microscopic field) may be present.18
Evaluation and Treatment
The evaluation and diagnosis of urinary calculi are based on presenting symptoms and history combined with a focused physical assessment, imaging studies, and possibly a functional study of renal pelvic and ureteral pressures.16 The history queries dietary habits; the age of the first stone episode; stone analysis; and presence of complicating factors, including recurrent urinary tract infection, hyperparathyroidism, or recent gastrointestinal or genitourinary surgery. Urinalysis (including pH) is obtained and a 24-hour urine is completed to identify calcium oxalate, citrate, and other significant constituents. In addition, every effort is made to retrieve and analyze calculi that are passed spontaneously or retrieved through aggressive intervention. Additional tests are obtained in selected individuals, such as those with suspected hyperparathyroidism or cystine or uric acid stones, in order to diagnose and manage underlying metabolic disorders. Imaging of kidney stones includes plain abdominal radiography, ultrasound, intravenous pyelogram, computed tomography, and magnetic resonance imaging.19
The goals of treatment are to manage acute pain, promote stone passage, reduce the size of stones already formed, and prevent new stone formation. The components of treatment include: (1) administering parenteral and/or oral analgesics for acute pain, (2) providing medical therapy to promote stone passage (alpha antagonists or calcium channel blockers), (3) reducing the concentration of stone-forming substances by increasing urine flow rate with high fluid intake, (4) decreasing the amount of stone-forming substances in the urine by decreasing dietary intake or endogenous production or by altering urine pH,20 and (5) removing stones using percutaneous nephrolithotomy, ureteroscopy, or ultrasonic or laser lithotripsy to fragment stones for excretion in the urine.21,22 Obstructing kidney stones with a suspected proximal urinary tract infection are urologic emergencies requiring emergent decompression and antibiotics.23
Lower Urinary Tract Obstruction
Obstructive disorders of the lower urinary tract (LUT) are primarily related to storage of urine in the bladder or emptying of urine through the bladder outlet. The causes of the obstruction include neurogenic and anatomic alterations or, in some instances, a combination of both. Incontinence is a common symptom. Types of incontinence are reviewed in Table 38-1.
TABLE 38-1
| TYPE | DESCRIPTION |
| Urge incontinence (most common in older adults) | Involuntary loss of urine associated with an abrupt and strong desire to void (urgency). Often associated with involuntary contractions of the detrusor. When associated with a neurologic disorder this is called detrusor hyperreflexia. When no neurologic disorder exists this is called detrusor instability. May be associated with decreased bladder wall compliance. |
| Stress incontinence (most common in women younger than 60 years and men who have had prostate surgery) | Involuntary loss of urine during coughing, sneezing, laughing, or other physical activity associated with increased abdominal pressure. |
| Overflow incontinence | Involuntary loss of urine with overdistention of the bladder. Associated with neurologic lesions below S1, polyneuropathies, and urethral obstruction (i.e., an enlarged prostate). |
| Mixed incontinence (most common in older women) | A combination of stress and urge incontinence. |
| Functional incontinence | Involuntary loss of urine caused by dementia or immobility. |
Data from Agency for Healthcare Research and Quality: Overview: urinary incontinence in adults, clinical practice guideline update, Rockville, MD, 1996, Author. Available at www.ahrq.gov/clinic/uiovervw.htm.
Neurogenic Bladder
Neurogenic bladder is a general term for bladder dysfunction caused by neurologic disorders. The types of dysfunction are related to the sites in the nervous system that control sensory and motor bladder function (Figure 38-3). Lesions that develop in upper motor neurons of the brain and spinal cord result in dyssynergia (loss of coordinated neuromuscular contraction) and overactive or hyperreflexive bladder function. Lesions in the sacral area of the spinal cord or peripheral nerves result in underactive, hypotonic, or atonic (flaccid) bladder function, often with loss of bladder sensation.
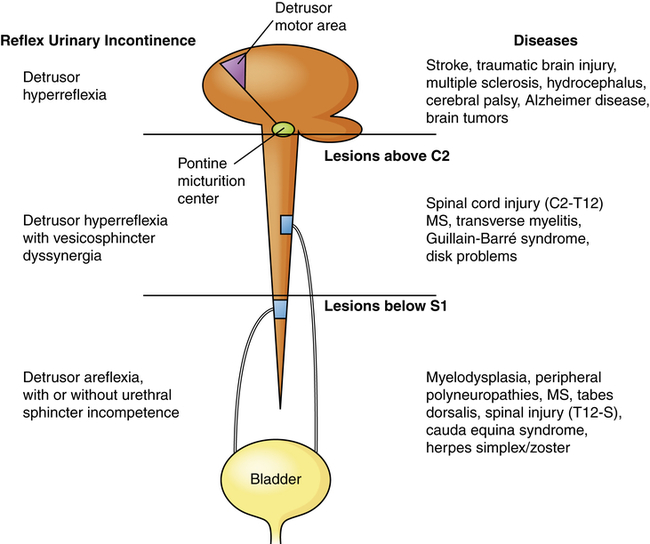
Neurologic lesions that occur below the pontine micturition center but above the sacral micturition center (between C2 and S1) are also upper motor neuron lesions and result in detrusor hyperreflexia with vesicosphincter (detrusor sphincter) dyssynergia. There is loss of pontine coordination of detrusor muscle contraction and external sphincter relaxation, so both the bladder and the sphincter are contracting at the same time, causing a functional obstruction of the bladder outlet.24 Spinal cord injury, multiple sclerosis, Guillain-Barré syndrome, and intervertebral disk problems are causes of this disorder. There is diminished bladder relaxation during storage with small urine volumes and high intravesicular (inside the bladder) pressures. This results in an overactive bladder syndrome with symptoms of urgency, frequency, urge incontinence, and increased risk for urethral turbulence and urinary tract infection.
Overactive Bladder Syndrome
Overactive bladder syndrome (OAB) is a chronic syndrome of detrusor overactivity in the absence of infection. It is estimated that 29.8 million adults over the age of 40 have bothersome OAB symptoms.25 OAB is characterized by urgency with involuntary detrusor contractions during the bladder filling phase. The contraction may be spontaneous or provoked and associated with hyperexcitable nerves or involuntary reflexes.26 There is coordination between the contracting bladder and the external sphincter, but the detrusor is too weak to empty the bladder, resulting in urinary retention with overflow or stress incontinence. Overactive bladder is defined by the International Continence Society as a symptom syndrome of urgency, with or without urge incontinence and usually associated with frequency and nocturia.27 Overactive bladder syndrome affects millions of men, women, and children; adults are often reluctant to discuss this syndrome with their healthcare provider. Sexual dysfunction and bowel problems often accompany OAB. Diagnosis is usually made by evaluation of symptoms. Urodynamic evaluation confirms the diagnosis. Treatment options include lifestyle modifications, behavioral therapy, pharmacotherapy (e.g., antimuscarinics), neuromodulation, botulinum toxin therapy, and surgical interventions. When left untreated, OAB is costly, impairs health and quality of life, causes depression, and leads to social isolation.28,29 OAB is a risk for urinary tract infection and a risk for falls in older adults.30
Obstructions to Urine Flow
A urethral stricture is a narrowing of its lumen. It occurs when infection, injury, or surgical manipulation produces a scar that reduces the caliber of the urethra. The vast majority of urethral strictures occur in men; they are rare in women.31,32 The severity of obstruction is influenced by its location within the urethra, its length, and the minimum caliber of urethral lumen within the stricture. Specifically, proximal urethral strictures cause more severe obstruction than do strictures of the distal urethra, longer strictures tend to be more obstructive, and the magnitude of blockage is in reverse proportion to the urethral caliber.33
Prostate enlargement is caused by acute inflammation, benign prostatic hyperplasia, or prostate cancer (see Chapter 25). Each of these disorders can cause encroachment on the urethra with obstruction to urine flow and the symptoms summarized previously.
Severe pelvic organ prolapse (see Chapter 24) in a woman causes bladder outlet obstruction when, most commonly, a cystocele (the downward protrusion of the bladder into the vagina) descends below the level of the urethral outlet.34 Cystoceles (see Figure 24-11) that reach or protrude beyond the vaginal introitus create the greatest risk for obstruction, particularly if the bladder neck (in females the bladder neck and proximal urethra constitute the internal sphincter of the bladder) has been surgically repaired without simultaneous repair of the cystocele.35 In men the bladder may rarely herniate into the scrotum, causing a similar type of obstruction.
Evaluation and Treatment
Various diagnostic tests assist with evaluation. A cystometric test uses a catheter and manometer to evaluate bladder urine volume and pressure in relation to involuntary bladder contraction (the leak point pressure) and the urge to void. The postvoid residual urine is measured by catheterization within 5 to 15 minutes of urination or through a bladder ultrasound machine that measures bladder height and width to provide an approximation of urine within the vesicle. This measurement may be combined with uroflowmetry, a graphic representation of the force of the urinary stream expressed as milliliters voided per second. Each of these measurements assesses the lower urinary tract’s efficiency in evacuating urine through micturition but neither differentiates poor detrusor contraction strength from obstruction as a cause of urinary retention. Instead, multichannel urodynamic testing is used to identify obstruction, quantify its severity, and measure detrusor contraction strength (see Figure 38-2). Video-urodynamic recordings can also demonstrate overactive bladder and detrusor sphincter dyssynergia. An evaluation of renal function, including functional imaging studies and measurement of serum creatinine level, is completed particularly when obstruction is severe and associated with elevated residuals or urinary tract infection. Electromyography measures electrical activity in the bladder neck using surface or needle electrodes.
Because the bladder neck consists of circular smooth muscle with adrenergic innervation, detrusor sphincter dyssynergia may be managed by α-adrenergic blocking (antimuscarinic) medications or botulinum toxin.36 Obstruction that is not adequately managed by pharmacotherapy may require bladder neck incision. Detrusor sphincter dyssynergia may be managed by intermittent catheterization in combination with higher dose antimuscarinic drugs to prevent overactive detrusor contractions and associated dyssynergia while ensuring regular, complete bladder evacuation through catheterization. Alternatively, men with dyssynergia may be managed by condom catheter containment, supplemented by an α-adrenergic blocking drug or transurethral sphincterotomy (surgical incision of the striated sphincter) in order to relieve obstruction. Low bladder wall compliance may be managed by antimuscarinic drugs and intermittent catheterization; however, more severe cases may require augmentation enterocystoplasty (enlargement of the low compliant bladder wall using a detubularized piece of small bowel), urinary diversion, or long-term indwelling catheterization.37 Prostate enlargement is managed by treating the underlying cause of the prostate enlargement with medication or surgery. Acute prostatitis is initially managed by broad-spectrum antibiotics until the results of a urine culture are obtained. Urinary retention may require transient placement of a suprapubic catheter. The management of benign prostatic hyperplasia and treatment options for prostate cancer are presented in Chapter 25.
A pessary (rubber or silicone device designed to compensate for vaginal wall prolapse) may be inserted to mechanically reverse severe pelvic organ (bladder, uterus, or rectum) prolapse. Depending on the device, the woman may be able to remove, cleanse, and replace the pessary, or it may be changed during a clinic visit. Intravaginal hormone replacement therapy and regular follow-up visits are critical to the long-term success of a pessary.38 Alternatively, pelvic organ prolapse may be repaired surgically; the procedure may be combined with a urethral suspension to correct stress urinary incontinence or rectocele repair.39
Tumors
Renal Tumors
Renal tumors account for about 65,150 (3.9%) new cancer cases and 13,680 (2.3%) deaths in 2013.40 There are a number of different types of kidney tumors. Renal adenomas (benign tumors) are uncommon but are increasing in number. The tumors are solid and encapsulated and are usually located near the cortex of the kidney. Because they can become malignant, they are usually surgically removed. Renal transitional cell carcinoma (RTCC) is rare and primarily arises in the renal parenchyma and renal pelvis. Renal cell carcinoma (RCC) is the most common renal neoplasm (85% to 90% of all renal neoplasms) (Figure 38-4). Renal cell carcinoma usually occurs in men (two times more often than in women) between 50 and 60 years of age and the incidence is increasing. Blacks have a higher incidence and mortality. Risk factors include cigarette smoking, obesity, hypertension, and advanced stage chronic renal failure (CRF).41,42 Five-year survival for all stages is about 50% and about 10% for stage IV cancer (metastatic disease).43
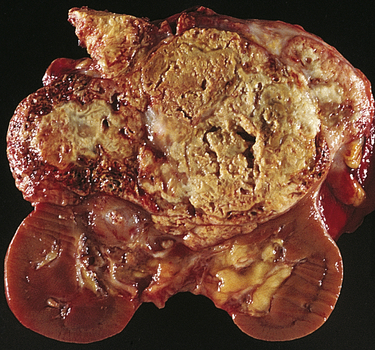
Renal cell carcinomas usually are spheroidal masses composed of yellow tissue mottled with hemorrhage, necrosis, and fibrosis. (From Damjanov I, Linder J, editors: Anderson’s pathology, ed 10, St Louis, 1996, Mosby.)
Pathogenesis
Renal cell carcinomas are adenocarcinomas and the etiology is unknown. They are classified according to cell type and extent of metastasis. Clear cell tumors, the most common, are dominated by mutations of the von Hippel-Lindau (VHL) gene located on chromosome 3p in 90% of cases. They present a better prognosis than papillary (10%), chromophobe (5%), oncocytoma (3% to 4%), collecting duct tumors (1%), or unclassified (rare) tumors.44 Clear cell and papillary tumors arise from proximal tubular epithelium. Chromophobe, oncocytoma, and collecting duct tumors are believed to arise from the distal nephron epithelium. Confinement within the renal capsule, together with treatment, is associated with a better survival rate. The tumors usually occur unilaterally (see Figure 38-4). About 25% to 30% of individuals with RCC present with metastasis (stage IV).45,46 The most common sites of distant metastasis are the lung, lymph nodes, liver, bone, thyroid, and central nervous system.47,48
Evaluation and Treatment
Diagnosis is based on the clinical symptoms, plain x-ray films of the abdomen, intravenous pyelography, renal angiography, and computed tomography (CT). (Staging of renal cell carcinoma is presented in Table 38-2.) Staging systems using molecular tumor markers (measurable biologic molecules released from a tumor or the host tissues that distinguish a malignancy) are rapidly advancing.49 Treatment for localized disease is surgical removal of the affected kidney (radical nephrectomy) or partial nephron sparing nephrectomy for smaller tumors. Surgery is combined with the use of chemotherapeutic agents, although RCC is resistant to conventional chemotherapy.50 Use of traditional cytokine therapy, specifically high-dose interleukin-2, is limited by significant toxicity (hypotension requiring vasopressor support, oliguria, pulmonary congestion, arrhythmias, and neurologic toxicity).51 Agents targeting the vascular endothelial growth factor pathway and the mammalian target of rapamycin (mTOR) have shown efficacy in randomized clinical trials for treatment of metastatic disease.52,53 There is ongoing research to identify molecular tumor markers that predict individual response to targeted molecular therapies.54 Radiation therapy may be used for palliation, and new techniques using stereotactic radiofrequency ablation, cryoablation, and laparoscopy are promising. Tumor obstruction is relieved by placement of ureteral catheters or nephrostomy tubes or by completion of urinary diversion procedures. Survival is related to tumor grade, tumor cell type, and extent of metastasis.
TABLE 38-2
STAGING OF RENAL CELL CARCINOMA (TNM SYSTEM)
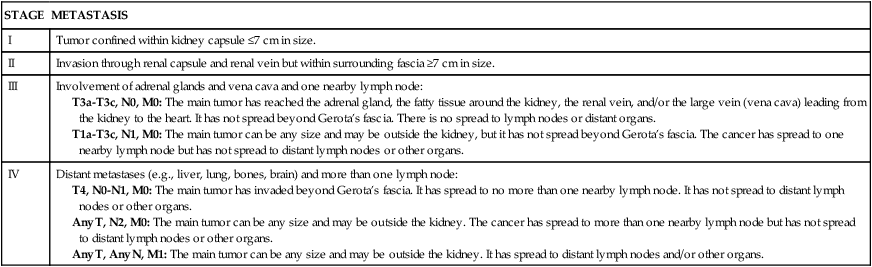
T, Tumor; N, node; M, metastasis.
Adapted from American Cancer Society: Kidney cancer (adult)—renal cell carcinoma, 2013. Available at www.cancer.org/cancer/kidneycancer/detailedguide/kidney-cancer-adult-staging. Accessed April 2013.
Bladder Tumors
Bladder tumors represent about 4.5% of all malignant tumors and are the fourth most common malignancy in men. Approximately 72,570 people developed bladder cancer with 15,210 deaths (2.5% of all cancer deaths) in 2013.40 The development of bladder cancer is most common in men older than 60 years. Urothelial (transitional cell) carcinoma is the most common bladder malignancy, appearing on the inner lining of the bladder.
Pathogenesis
The risk of primary bladder cancer is greater among people who smoke or are exposed to any of the following: metabolites of aniline dyes or other aromatic amines or chemicals, high levels of arsenic in drinking water, or heavy consumption of phenacetin.55 Oncogenes of the ras gene family and tumor-suppressor genes including TP53 mutations and inactivation of retinoblastoma gene (pRb) are implicated in bladder cancer. Loss of heterozygosity at chromosome 9 has been found in all stages of urothelial cell carcinoma.56 The tumor is usually composed of uroepithelial cells (cells lining the bladder, ureters, urethra, and renal pelvis), most have a papillary growth pattern (a tuftlike lesion attached to a stalk), and they rarely progress to invasive disease (Figure 38-5). Nonpapillary tumors (10% to 30% of bladder tumors) are not as common as papillary tumors, but they tend to be more invasive and have a poorer prognosis. Carcinoma in situ can present with papillary tumors.57 Worldwide, renal squamous cell carcinoma is the most prevalent and certain forms present at a higher stage and with a poorer prognosis.58 Metastasis is usually to lymph nodes, liver, bones, lungs, and adrenal glands. Staging for bladder carcinoma is presented in Table 38-3. Secondary bladder cancer develops by invasion of cancer from bordering organs, such as cervical carcinoma in women or prostatic carcinoma in men.
TABLE 38-3
STAGING OF BLADDER CARCINOMA (TNM SYSTEM)
| STAGE | DESCRIPTION | |
| Primary Tumor | ||
| T0 | No primary tumor identified | |
| Ta | Noninvasive papillary carcinoma—not in bladder muscle | |
| Tis | Carcinoma in situ (CIS) | |
| T1 | Tumor invades connective tissue | |
| T2 | Tumor invades detrusor muscle | |
| T3 | Invasion of fatty tissue around bladder | |
| T4 | Tumor has invaded adjacent structures | |
| Region of Lymph Nodes | ||
| N0 | No lymph node involvement | |
| N1 to N3 | Lymph node metastasis to pelvic or adjacent region | |
| Distant Metastasis | ||
| M0 | No metastasis | |
| M1 | Distant metastasis | |
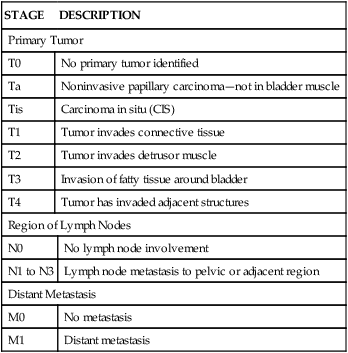
T, Tumor; N, node; M, metastasis.
Adapted from American Cancer Society: Bladder cancer, 2013. Available at www.cancer.org/cancer/bladdercancer/detailedguide/bladder-cancer-staging. Accessed April 2013.
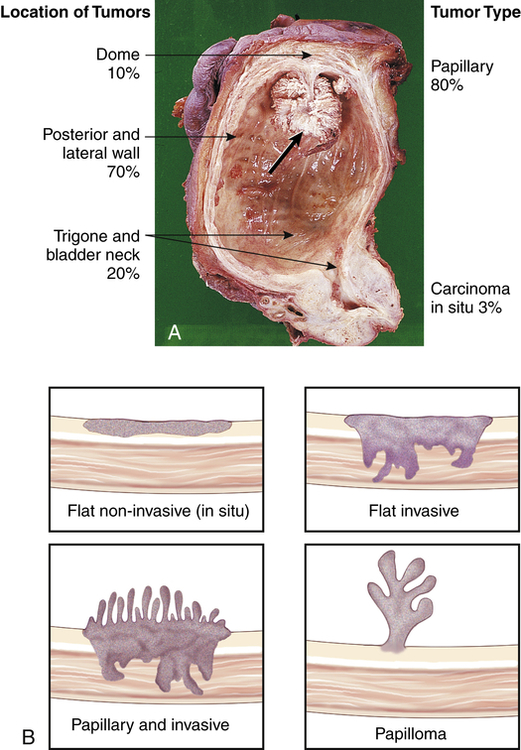
A, Papillary transitional cell carcinoma arising in the dome of the bladder as a cauliflower-like lesion (black arrow); location and frequency of bladder tumor types noted. B, Bladder cancer with morphologic patterns of most common tumors. (A from Stevens A, Lowe J, Scott I, editors: Core pathology, ed 3, London, 2009, Mosby; B from Kissane JM, editor: Anderson’s pathology, ed 9, St Louis, 1990, Mosby.)
Clinical Manifestations
Gross painless microscopic hematuria is the archetypal clinical manifestation of bladder cancer. Episodes of hematuria tend to recur, and they are often accompanied by bothersome lower urinary tract symptoms including daytime voiding frequency, nocturia, urgency, and urge urinary incontinence. Flank pain may occur if tumor growth obstructs one or both ureterovesical junctions. Bothersome lower urinary tract symptoms are particularly intense in individuals with carcinoma in situ. Metastasis is the cause of death from bladder cancer, usually from high-grade muscle-invasive tumors.59
Evaluation and Treatment
Urinalysis for evidence of hematuria in the absence of infection provides a useful screening tool for high-risk patients. Urine cytology (pathologic analysis of sloughed cells within the urine) is completed in individuals with evidence of hematuria from unknown causes; cystoscopy or fluorescence cystoscopy with tissue biopsy can confirm the diagnosis. Biologic markers for the diagnosis and prognosis of bladder cancer are under investigation (e.g., cadherins, cyclooxygenase-2 [COX-2] protein, karyopherin-α2 [KPNA2], tyrosine kinase HER2 [RTOG-0524], and epidermal growth factor receptor [EGFR)]).60,61
Newer magnetic resonance techniques are helpful for local staging and whole-body computed tomography detects metastasis.62 Transurethral resection or laser ablation, combined with intravesical chemotherapy or immunotherapy, is effective for superficial tumors. Radical cystectomy with urinary diversion and adjuvant chemotherapy is required for locally invasive tumors, and radiation therapy may be used to support palliative or adjuvant treatment for muscle-invasive tumors.63–65
Urinary Tract Infection
Causes of Urinary Tract Infection
A urinary tract infection (UTI) is an inflammation of the urinary epithelium usually caused by bacteria from gut flora. A UTI can occur anywhere along the urinary tract including the urethra, bladder, ureter, or kidney. At risk are premature newborns; prepubertal children; sexually active and pregnant women; women treated with antibiotics that disrupt vaginal flora; spermicide users; estrogen-deficient postmenopausal women; individuals with indwelling catheters; and persons with diabetes mellitus, neurogenic bladder, or urinary tract obstruction (see What’s New? Catheter-Associated Urinary Tract Infections). UTIs are commonly classified by their location or complicating factors: cystitis (bladder inflammation), pyelonephritis (inflammation of upper urinary tract), uncomplicated UTI (occur in a normally functioning urinary system), and complicated UTI (occur with defects in the urinary system
or in individuals with health problems that compromise host defenses or response to treatment).
Host Defense Mechanisms and Urinary Tract Infection
Host defense mechanisms maintain a sterile posterior urethra and bladder in a healthy individual. Even if bacteria manage to enter the bladder, these defense mechanisms prevent it from clinging to the walls of the bladder or ascending to the upper urinary tracts.66 Several factors normally combine to protect against UTI. Most bacteria are washed out of the urethra during micturition. The low pH and high osmolality of urea, the presence of Tamm-Horsfall protein, and the presence of secretions from the uroepithelium provide a bactericidal effect. The ureterovesical junction closes during bladder contraction, preventing reflux of urine to the ureters and kidneys. Periurethral mucus-secreting glands surround the distal two thirds of the female urethra. Mucus from these glands traps bacteria before it can ascend from the proximal urethra to the bladder. In men, the length of the male urethra and secretions from the prostate and accessory periurethral glands combine to form a protective barrier against infection. In addition, the urethral sphincter mechanism acts as a mechanical barrier to bacterial ascent from the distal urethra.
Bacteria that successfully ascend the urethra face detection and destruction by components of the body’s immune system provided they come into contact with the bladder wall. Protective uroepithelial immune responses include Toll-like receptor (e.g., TLR4) recognition of pathogen-associated molecular patterns on the bacteria, neutrophil and macrophage recruitment and phagocytosis, and the presence of antimicrobial proteins (defensins, cathelicidin, and Tamm-Horsfall protein). Susceptibility to infection also is influence by genetic variation in the host immune response to bacterial virulence and to the virulence of the pathogen.67 Unfortunately, time is required for the immune system to respond to the potential threat, and this period may provide adequate time for bacteria or other pathogens to reproduce several times.
The efficiency of the bladder’s defenses is also influenced by the person’s Lewis blood group.68 This taxonomy is based on recognition of inherited antigens associated with the ABO blood factors. Individuals with certain Lewis blood groups are more prone to UTIs because they secrete fewer antigens capable of resisting bacterial adherence by pili formation.
Most people are able to rapidly rid the urinary tract of invading bacteria, but some show evidence of bacteria in the urine that does not provoke an infection. This condition, called asymptomatic bacteriuria, does not harm urinary function or require intervention except in pregnant women.69 A UTI occurs when a pathogen circumvents or overwhelms the host’s defense mechanisms and rapidly reproduces.
Virulence of Uropathogens
Virulence is a pathogen’s ability to evade or overwhelm the host defense mechanisms and cause disease in a host (see Chapter 10). Several factors contribute to bacterial virulence within the urinary tract, including the ability of uropathic bacteria to adhere (attach) to the uroepithelium. Uropathic strains of Escherichia coli have type-1 pili. Type-1 pili have adhesins that allow them to bind to mucosal cellular receptors and enter uroepithelial cells and resist flushing during normal micturition. Once inside the cell, they can persist quiescently for long periods and cause recurrent infection or multiply rapidly, safe from host defenses and protected from antibiotic therapy.70 Additionally, uropathic strains have pyelonephritis-associated fimbriae (P. fimbriae) that bind to the uroepithelial P-blood group antigen (present in most of the human population) and readily ascend the urinary tract. Strains of E. coli also produce siderophores for acquiring nutrient iron, are resistant to bactericidal effects of complement, and express toxins including cytotoxin necrotizing factor-1 and hemolysins. Certain bacterial species also enhance their virulence by acting together to form a biofilm that enhances colonization and resists efficiency of innate host defense mechanisms and antimicrobial therapy, particularly in catheter-associated UTI.71,72
Types of Urinary Tract Infection
Acute Cystitis
Acute cystitis is an inflammation of the bladder and is the most common site of UTI. Cystitis is more common in women because of the shorter urethra and the closeness of the urethra to the vagina and anus (increasing the possibility of bacterial contamination).73
Pathophysiology
Two factors account for the presence of a UTI: the efficiency of defense mechanisms within the host (individual) and the virulence of the pathogen (bacterium, fungus, or parasite). The most common infecting microorganisms are uropathic strains of E. coli (80% to 85%) and the second most common is Staphylococcus saprophyticus (10%). Less common microorganisms include Klebsiella, Proteus, Pseudomonas, fungi, viruses, parasites, or tubercular bacilli. Bacterial contamination of the normally sterile urine usually occurs by retrograde movement of gram-negative bacilli into the urethra and bladder and then to the ureter and kidney. Some women may be genetically susceptible to certain strains of E. coli attachment and may harbor pathogenic strains in their vaginal flora.73 In rare instances bloodstream infections (sepsis) can spread to the bladder tissue.
Fungal infections are comparatively uncommon. The most common pathogen is Candida, but multiple fungal species may colonize the urinary tract or urinary catheters and produce symptomatic UTIs, particularly in those who are immunosuppressed.74
Schistosomiasis haematobium is the most common cause of parasitic invasion of the urinary tract on a global basis; it infects more than 200 million people.75 Although rare among people living in the United States, the parasite dwells in waters of the various rivers of fresh water bodies in Africa, South America, and Pacific Rim countries. It usually enters the human by swimming up the urethra while the host swims or is partly submerged in an infected body of water. The parasite burrows into the walls of the urinary tract, causing inflammation and scarring of the urinary tract, and an increased risk for urothelial malignancies or renal failure.76
Clinical Manifestations
Many individuals with bacteriuria are asymptomatic. Clinical manifestations of cystitis are related to the host inflammatory response and usually include frequency, urgency, dysuria (painful urination), and suprapubic and low back pain. Hematuria, cloudy and foul-smelling urine, and flank pain are more serious symptoms. Approximately 10% of individuals with bacteriuria have no symptoms and have low TLR4 levels,77 and 30% of individuals with symptoms are abacteriuric. Older adults with cystitis may be asymptomatic or demonstrate confusion or vague abdominal discomfort. Older adults with recurrent UTI and other concurrent illness have a higher risk of morbidity and mortality.78
Evaluation and Treatment
Infections are diagnosed by urine culture of specific microorganisms with counts of 10,000/ml or more from freshly voided urine. Dipstick urinalysis and microscopy are adequate to diagnose an uncomplicated UTI, but urine culture is critical for complicated infections.79 Risk factors, such as a urinary tract obstruction, which are associated with a complicated UTI, should be identified and treated. Increased fluid intake and analgesics can relieve symptoms. Evidence of bacteria from urine culture and antibiotic sensitivity warrants treatment with a microorganism-specific antibiotic to eradicate the underlying pathogen. A single large dose of antibiotic or a 3-day course may be effective when symptoms are of short duration and there are no complications. A treatment period of 3 to 7 days is most common depending on antimicrobial selection; older adults with obstructive disorders may require 7 to 14 days of treatment. Frequent, recurrent, acute uncomplicated UTI requires low-dose antimicrobial therapy from 6 months to 2 years or prophylactic treatment, depending on frequency and individual responses.80 Follow-up urine cultures should be obtained 1 week after initiation of treatment and at monthly intervals for 3 months. Clinical symptoms are frequently relieved, but bacteriuria may still be present, particularly with the development of antimicrobial-resistant bacterial strains. Repeat cultures should be obtained every 3 to 4 months until 1 year after treatment for evaluation of recurrent infection. Urosepsis and septic shock are medical emergencies that usually demand parenteral, broad-spectrum antibiotic therapy and may require hospitalization. A UTI caused by Schistosomiasis is treated with praziquantel, and vaccines are under development.81
Painful Bladder Syndrome/Interstitial Cystitis
Painful bladder syndrome/interstitial cystitis (PBS/IC) is a condition that includes nonbacterial infectious cystitis (viral, mycobacterial, chlamydial, fungal) and noninfectious cystitis (radiation, chemical, autoimmune, hypersensitivity).82 It occurs most commonly in women ages 20 to 40 years who have symptoms of cystitis, such as frequency, urgency, dysuria, and nocturia, for more than 6 weeks duration but with negative urine cultures and no other known etiology. Nonbacterial infectious cystitis is most common among those who are immunocompromised. Noninfectious cystitis is associated with radiation or chemotherapy treatment for pelvic and urogenital cancers.
The cause of PBS/IC is unknown, but an autoimmune reaction may be responsible for the inflammatory response, which includes mast cell activation, altered epithelial permeability, neuroinflammation, and increased sensory nerve sensitivity.83 The inflammation is associated with a derangement of the glycosaminoglycan layer of the bladder mucosa that makes it more susceptible to penetration by bacteria and noxious urinary solutes. Inflammation and fibrosis of the bladder wall are accompanied by the presence of hemorrhagic ulcers (Hunner ulcers) and bladder volume may decrease as a result of fibrosis, particularly in older individuals.84 More recently, the identification of antiproliferative factor (APF), a protein expressed by the bladder uroepithelium in those with IC, is important. APF appears to block the normal growth of cells that line the inside wall of the bladder and indirectly increases bladder sensation.85 Characteristic symptoms of IC include bladder fullness, frequency (including nocturia), small urine volume, and chronic pelvic pain with symptoms lasting longer than 9 months. Chronic pain and sleep deprivation can lead to depression. Diagnosis of IC requires the exclusion of other diagnoses, and extensive evaluations are completed.86 No single treatment is effective. Oral and intravesical therapies, sacral nerve stimulation, and onabotulinumtoxinA are used for symptom relief. Surgery is used in refractory cases.87 More research is needed to understand the pathogenesis and treatment of this disease.
Acute Pyelonephritis
Pyelonephritis is an infection of one or both upper urinary tracts (ureter, renal pelvis, and kidney interstitium). Common causes are summarized in Table 38-4. Urinary obstruction and reflux of urine from the bladder (vesicoureteral reflux) are the most common underlying risk factors. Most cases occur in young adult women.
TABLE 38-4
COMMON CAUSES OF PYELONEPHRITIS
| PREDISPOSING FACTORS | PATHOLOGIC MECHANISMS |
| Kidney stones | Obstruction and stasis of urine contributing to bacteriuria and hydronephrosis; irritation of epithelial lining with entrapment of bacteria |
| Vesicoureteral reflux | Chronic reflux of urine up the ureter and into kidney during micturition, contributing to bacterial infection |
| Pregnancy | Dilation and relaxation of ureter with hydroureter and hydronephrosis; partly caused by obstruction from enlarged uterus and partly from ureteral relaxation caused by higher progesterone levels |
| Neurogenic bladder | Neurologic impairment interfering with normal bladder and urethral sphincter contraction with residual urine and ascending infection |
| Instrumentation | Introduction of organisms into urethra and bladder by catheters and endoscopes introduced into the urinary tract for diagnostic purposes |
| Female sexual trauma | Movement of organisms from the urethra into the bladder with infection and retrograde spread to kidney |
Pathophysiology
Microorganisms usually associated with acute pyelonephritis include E. coli, Proteus, or Pseudomonas. The latter two microorganisms are more commonly associated with infections after urethral instrumentation or urinary tract surgery. These microorganisms also split urea into ammonia, making alkaline urine that increases the risk of stone formation. The infection is probably spread by ascending uropathic microorganisms along the ureters, but dissemination also may occur by way of the bloodstream. The inflammatory process is usually focal and irregular, primarily affecting the pelvis, calyces, and medulla. The infection causes medullary infiltration of white blood cells with renal inflammation, renal edema, and purulent urine. In severe infections, localized abscesses may form in the medulla and extend to the cortex. Primarily affected are the renal tubules; the glomeruli usually are spared. Necrosis of renal papillae can develop. After the acute phase, healing occurs with deposition of scar tissue, fibrosis, and atrophy of affected tubules (Figure 38-6). Acute pyelonephritis rarely causes renal failure.88
Evaluation and Treatment
Differentiating symptoms of cystitis from those of pyelonephritis by clinical assessment alone is difficult. The specific diagnosis is established by urine culture, urinalysis, and clinical signs and symptoms. White blood cell casts indicate pyelonephritis, but they are not always present in the urine. Complicated pyelonephritis requires blood cultures and urinary tract imaging.89
Uncomplicated acute pyelonephritis responds well to 2 to 3 weeks of microorganism-specific antibiotic therapy. Follow-up urine cultures are obtained at 1 and 4 weeks after treatment if symptoms recur. Antibiotic-resistant microorganisms or reinfection may occur in cases of urinary tract obstruction or reflux.90 Intravenous pyelography and voiding cystourethrography identify surgically correctable lesions.
Chronic Pyelonephritis
Pathophysiology
Chronic urinary tract obstruction prevents elimination of bacteria and starts a process of progressive inflammation, alterations of the renal pelvis and calyces, destruction of the tubules, atrophy or dilation and diffuse scarring, and, finally, impaired urine-concentrating ability, leading to chronic kidney failure. The lesions of chronic pyelonephritis are sometimes termed chronic interstitial nephritis because the inflammation and fibrosis are located in the interstitial spaces between the tubules (see Figure 38-6).
Glomerular Disorders
Glomerulonephritis
Glomerulonephritis is an inflammation of the glomerulus caused by primary glomerular injury, including immunologic responses, ischemia, free radicals, drugs, toxins, vascular disorders, and infection. Secondary glomerular injury is a consequence of systemic diseases, including diabetes mellitus, systemic lupus erythematosus, and, less commonly, congestive heart failure and HIV-related kidney disease. Glomerular disease is a significant cause of chronic kidney disease and end-stage renal failure worldwide.91,92
Acute Glomerulonephritis
Pathophysiology
Immune mechanisms and inflammation are a major cause of injury for both primary and secondary types of acute glomerulonephritis (Figure 38-7). Immune injury includes: (1) deposition of circulating antigen-antibody immune complexes on the glomerulus (type III hypersensitivity reaction); (2) antibodies reacting in situ against planted antigens within the glomerulus (type III hypersensitivity); (3) action of antibodies directed against the glomerular capillary wall (antiglomerular basement membrane antibodies), the least common and most severe form of immune injury (type II hypersensitivity); and (4) cell-mediated immune injury (type IV hypersensitivity) (Table 38-5). In nearly all types of acute glomerulonephritis, the epithelial or podocyte layer of the glomerular capillary membrane is disturbed with loss of negative charges and changes in membrane permeability; the mesangial matrix may be expanded or the basement membrane thickened (see Figure 38-7). Different causes of injury may result in more than one type of glomerular lesion; thus lesions are not necessarily disease specific (Table 38-6). Many types of acute glomerulonephritis occur, most often in children or young adults, including acute postinfectious glomerulonephritis, Henoch-Schönlein purpura nephritis, and minimal change nephropathy (lipoid nephrosis) associated with nephrotic syndrome. Details of these diseases are presented in Chapter 39. The types, causes, and histopathology of acute glomerulonephritis are summarized in Table 38-7. The mechanisms of acute glomerulonephritis are presented next and include IgA nephropathy, membranous glomerulonephritis, crescentic or rapidly progressive glomerulonephritis, mesangial proliferative glomerulonephritis, and membranous proliferative glomerulonephritis.
TABLE 38-5
IMMUNOLOGIC PATHOGENESIS OF GLOMERULONEPHRITIS
| GLOMERULAR INJURY | MECHANISM |
| Soluble immune-complex glomerulonephritis (90%) | Formation of antibodies stimulated by the presence of endogenous or exogenous antigens results in circulating soluble antigen-antibody complexes, which are deposited in glomerular capillaries, or the in situ formation of immune complexes to planted antigens or to structural components within the glomerulus; glomerular injury occurring with complement deposition and activation and release of immunologic substances that lyse cells and increase membrane permeability; immune deposits with a microscopic appearance that fluoresce in a granular pattern when stained with fluorescein and viewed under ultraviolet light; severity of glomerular injury related to the number of complexes formed; a type III hypersensitivity reaction |
| Antiglomerular basement membrane glomerulonephritis (5%) | Antibodies are formed and act directly against the glomerular basement membrane; immune response that causes crescent formation and a linear pattern of immunofluorescence; generally associated with rapidly progressive renal failure such as Goodpasture syndrome (type II hypersensitivity reaction) |
| Alternative complement pathway | A relatively obscure mechanism associated with low levels of complement and membranoproliferative glomerulonephritis |
| Cell-mediated immunity | A delayed hypersensitivity response that damages the glomerulus; actual cellular mechanism not clearly understood but may involve cytokine secretion, activation of effector cells such as macrophages or by inducing autoantibodies or immune complexes; cytotoxic CD8+ T-cell responses and failure of regulatory T cells may represent two additional types of antirenal hypersensitivity∗ |
∗Data from Kurts C et al: Semin Immunopathol 29(4):317–335, 2007.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree