Chapter 44
Alterations of Musculoskeletal Function
Christy L. Crowther-Radulewicz and Kathryn L. McCance
Musculoskeletal Injuries
Skeletal Trauma
Fractures
A fracture is a break in the continuity of a bone. A break occurs when force is applied that exceeds the tensile or compressive strength of the bone. The highest incidence of fractures occurs in young males (between ages 15 and 24 years) and in adults 65 years of age and older, and varies for individual bones according to age, gender, and possibly race. In a study of more than 158,000 people, fractures were most prevalent among black males younger than age 65, whereas fractures in whites were highest in those age 65 and older.1 Fractures of healthy bones, particularly the tibia, clavicle, and lower humerus, tend to occur in young persons and are often the result of trauma. Fractures of the hands and feet are usually caused by accidents in the workplace. The incidence of fractures of the upper femur, upper humerus, vertebrae, and pelvis is highest in older or older adults and is often associated with osteoporosis (see p. 1550). In 1990, an estimated 1.66 million hip fractures occurred worldwide; that number is expected to increase to 6.3 million by the year 2050.2
Classification
Fractures can be classified as complete or incomplete and open or closed (Figure 44-1). In a complete fracture the bone is broken all the way through, whereas in an incomplete fracture the bone is damaged but still in one piece. Complete or incomplete fractures also can be classified as open (formerly referred to as compound) if the skin is broken or as closed (formerly called simple) if it is not. A fracture in which a bone breaks into more than two fragments is termed a comminuted fracture. Fractures are classified also according to the direction of the fracture line. A linear fracture runs parallel to the long axis of the bone. An oblique fracture is a slanted fracture of the shaft of the bone. A spiral fracture encircles the bone, and a transverse fracture occurs straight across the bone.
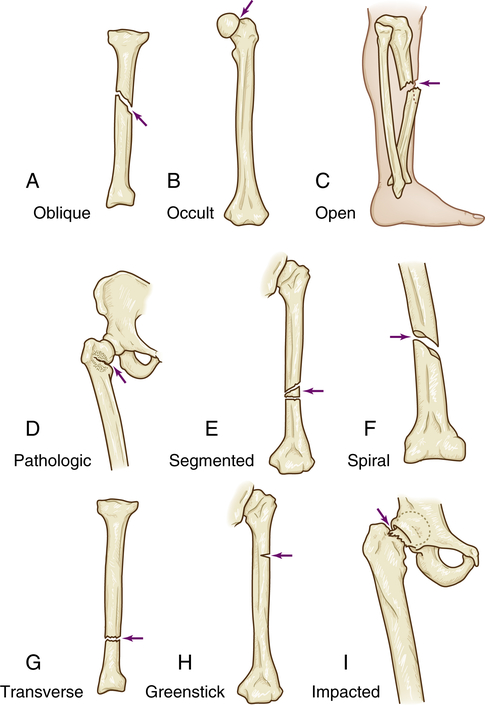
A, Oblique: fracture at oblique angle across both cortices. Cause: direct or indirect energy, with angulation and some compression. B, Occult: fracture that is hidden or not readily discernible. Cause: minor force or energy. C, Open: skin broken over fracture; possible soft tissue trauma. Cause: moderate to severe energy that is continuous and exceeds tissue tolerances. D, Pathologic: transverse, oblique, or spiral fracture of bone weakened by tumor pressure or presence. Cause: minor energy or force, which may be direct or indirect. E, Segmented: fracture with two or more pieces or segments. Cause: direct or indirect moderate to severe force. F, Spiral: fracture that curves around cortices and may become displaced by twist. Cause: direct or indirect twisting energy or force with distal part held or unable to move. G, Transverse: horizontal break through bone. Cause: direct or indirect energy toward bone. H, Greenstick: break in only one cortex of bone. Cause: minor direct or indirect energy. I, Impacted: fracture with one end wedged into opposite end of inside fractured fragment. Cause: compressive axial energy or force directly to distal fragment. (Redrawn from Mourad L: Musculoskeletal system. In Thompson JM et al, editors: Mosby’s clinical nursing, ed 7, St Louis, 2002, Mosby.)
Incomplete fractures tend to occur in the more flexible, growing bones of children. The three main types of incomplete fractures are greenstick, buckle or torus, and bowing. A greenstick fracture perforates one cortex and splinters the spongy bone and is relatively unstable.3 The name is derived from the damage sustained by a young tree branch (a green stick) when it is bent sharply. The outer surface is disrupted, but the inner surface remains intact. Greenstick fractures typically occur in the metaphysis or diaphysis of the tibia, radius, and ulna. In a buckle or torus fracture, the cortex buckles but does not break; this is a relatively stable fracture. Bowing fractures usually occur when longitudinal force is applied to bone. This type of fracture is common in children and usually involves the paired radius-ulna or fibula-tibia. A complete diaphyseal fracture occurs in one of the bones of the pair, which disperses the stress sufficiently to prevent a complete fracture of the second bone, which bows. A bowing fracture resists correction (reduction) because the force necessary to reduce it must be equal to the force that bowed it. Treatment of bowing fractures is difficult also because the bowed bone interferes with reduction of the fractured bone. A fracture that results from a low-level trauma (one that would not normally cause a fracture) is called a fragility fracture, which is often seen in osteoporosis. Types of fractures are summarized in Table 44-1.
TABLE 44-1
| TYPE | DEFINITION |
| Typical Complete Fractures | |
| Closed fracture | The skin overlying the bone is intact |
| Open fracture | Communicating wound between bone and skin |
| Comminuted fracture | Multiple bone fragments |
| Linear fracture | Fracture line parallel to long axis of bone |
| Oblique fracture | Fracture line at an angle to long axis of bone |
| Spiral fracture | Fracture line encircling bone (as a spiral staircase) |
| Transverse fracture | Fracture line perpendicular to long axis of bone |
| Impacted | Fracture fragments are pushed into each other |
| Pathologic | Fracture occurs at a point in the bone weakened by disease (e.g., bones with tumors or osteoporosis) |
| Avulsion | A fragment of bone connected to a ligament or tendon breaks off from the main bone |
| Compression | Fracture is wedged or squeezed together on one side of bone |
| Displaced | Fracture with one, both, or all fragments out of normal alignment |
| Extracapsular | Fragment is close to the joint but remains outside the joint capsule |
| Intracapsular | Fragment extends into or is within the joint capsule |
| Fragility | Fracture caused by low-level trauma |
| Typical Incomplete Fractures | |
| Greenstick fracture | Break on one cortex of bone with splintering of inner bone surface (commonly occurs in children and older adults) |
| Torus fracture | Buckling of cortex |
| Bowing fracture | Bending of the bone |
| Stress fracture | Microfracture |
| Transchondral fracture | Separation of cartilaginous joint surface (articular cartilage) from main shaft of bone |
A transchondral fracture consists of fragmentation and separation of a portion of the articular cartilage that covers the end of a bone at a joint. (Joint structures are defined in Chapter 43.) The fragments may consist of cartilage alone or cartilage and bone. Typical sites of transchondral fracture are the distal femur, the ankle, the kneecap, the elbow, and the wrist. Transchondral fractures are most prevalent in adolescents.
Pathophysiology
When a bone is broken the periosteum and blood vessels in the cortex, marrow, and surrounding soft tissues are disrupted. Bleeding occurs from the damaged ends of the bone and from the neighboring soft tissue. A clot (hematoma) forms within the medullary canal, between the fractured ends of the bone, and beneath the periosteum. Bone tissue immediately adjacent to the fracture dies. This necrotic tissue (along with any debris in the fracture area) stimulates an intense inflammatory response characterized by vasodilation, exudation of plasma and leukocytes, and infiltration by inflammatory leukocytes and mast cells. Cytokines, including transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), prostaglandins, and other factors that promote healing are released. Within 48 hours after the injury, vascular tissue invades the fracture area from surrounding soft tissue and the marrow cavity, and blood flow to the entire bone is increased. Bone-forming cells in the periosteum, endosteum, and marrow are activated to produce subperiosteal procallus along the outer surface of the shaft and over the broken ends of the bone (Figure 44-2). Healing generally occurs in three overlapping phases. The initial inflammatory phase of healing lasts 3 to 4 days. During the next few days, the repair phase begins and capillary ingrowth, together with mononuclear cells and fibroblasts, begins the transformation of a hematoma into granulation tissue. Osteoblasts within the procallus synthesize collagen and matrix, which becomes mineralized to form callus. As the repair process continues, remodeling occurs, during which unnecessary callus is resorbed and trabeculae are formed along lines of stress; at the end of this stage, bone can withstand normal stresses. The last phase, remodeling, lasts for months to years. Except for the liver, bone is unique among all body tissues in that after a fracture it will heal with normal, not scar, tissue.
Evaluation and Treatment
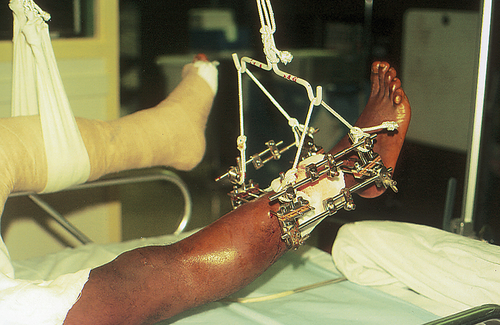
External fixation can be used to reduce and immobilize significantly displaced open fractures. Pins are placed in the bone proximal and distal to the break and then stabilized by an external frame of clamps and rods (Figure 44-3).
Splints and casts are used to immobilize and hold a reduction in place. Improper reduction or immobilization of a fractured bone may result in nonunion, delayed union, or malunion. Nonunion is failure of the bone ends to grow together (Figure 44-4). The gap between the broken ends of the bone fills with dense fibrous and fibrocartilaginous tissue instead of new bone. Occasionally the fibrous tissue contains a fluid-filled space that resembles a joint and is termed a false joint, or pseudarthrosis. Delayed union is union that does not occur until approximately 8 to 9 months after a fracture. Malunion is the healing of a bone in a nonanatomic position. Treatment of delayed union and nonunion includes use of various modalities designed to stimulate new bone formation. Physical modalities, such as implantable or external electric current devices, electromagnetic field generations, and low-density ultrasound, have been effective in stimulating bone formation.4 Stem cells and gene therapy also show promise in promoting formation of new bone.5,6 Large defects in bone can be filled with bone graft or synthetic materials, such as calcium phosphate cement.
Dislocation and Subluxation
Dislocation and subluxation are most common in persons younger than 20 years and are generally associated with fractures. Dislocation and subluxation, however, may result from congenital or acquired disorders that cause (1) muscular imbalance, as occurs with congenital hip dislocation or neurologic disorders; (2) incongruities in the articulating surfaces of the bones, such as with rheumatoid arthritis (see p. 1568); or (3) joint instability.
Most often dislocated or subluxated are the joints of the shoulder, elbow, wrist, finger, hip, and knee (Figure 44-5). The shoulder’s glenohumeral joint is a relatively unstable joint because the articular surface of the glenoid cavity is only one third as large as the surface of the humeral head. As a result, the glenohumeral joint is often injured. Physical trauma to the shoulder can cause anterior, posterior, superior, or inferior dislocation. Anterior dislocation is the most common and is usually the result of an indirect force that places the shoulder in extreme external rotation. Posterior dislocations usually occur as a result of trauma. A superior dislocation is rare and usually the result of an extreme forward and upward force on an adducted arm. Inferior displacement is often seen in persons with neurologic injuries of the brachial plexus and is believed to be caused by stretching of the supporting muscles or by joint effusion.
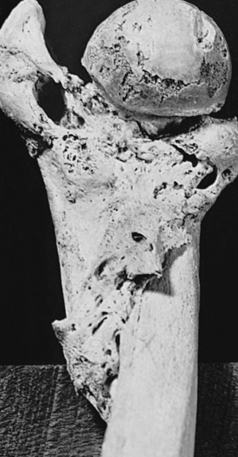
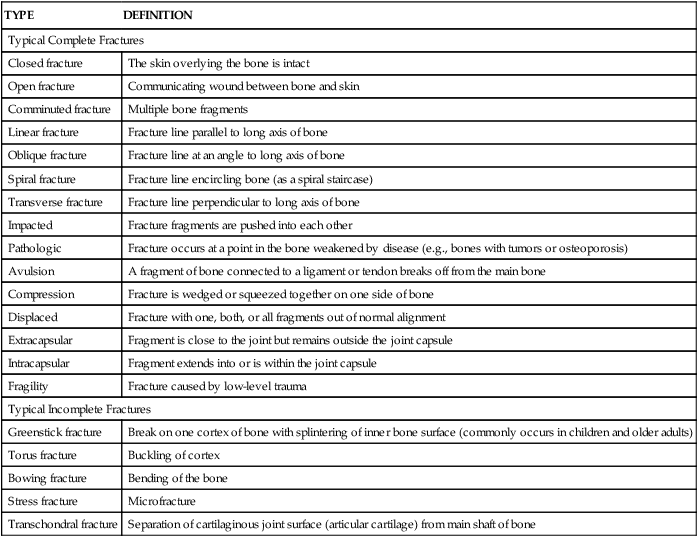
An x-ray showing a displaced fracture of the base of the first metacarpal, also known as a Bennett fracture.
Support Structure Trauma
Sprains and Strains of Tendons and Ligaments
Pathophysiology
When a tendon or ligament is torn, an extensive cascade of inflammatory processes begins. An inflammatory exudate develops between the torn ends. Later, granulation tissue containing macrophages, fibroblasts, and capillary buds grows inward from the surrounding soft tissue and cartilage to begin the repair process. Within 3 to 4 days after the injury, collagen formation begins. At first, collagen formation is random and disorganized. As the collagen fibers interweave and connect with pre-existing tendon fibers, they become organized parallel to the lines of stress. Eventually vascular fibrous tissue fuses the new and surrounding tissues into a single mass. As reorganization takes place, the healing tendon or ligament separates from the surrounding soft tissue. Usually a healing tendon or ligament lacks sufficient strength to withstand strong pull for 4 to 5 weeks after the injury. If strong muscle pull does occur during this time, the tendon or ligament ends may separate again, which causes the tendon or ligament to heal in a lengthened shape with an excessive amount of scar tissue that renders the tendon or ligament functionless. Scar remodeling may take months to years before it is complete.7
Tendinopathy and Bursitis
Trauma and repetitive stress can cause painful degradation of collagen fibers (tendinosis), inflammation of tendons (tendinitis), or inflammation in bursal sacs (bursitis). The term tendinopathy includes tendinitis, tendinosis, and paratendinitis. Studies have shown that vascular ingrowth in tendinopathy (neovascularization) is accompanied with nerve ingrowth, facilitating pain transmission in Achilles and patellar tendinopathies.8 Other causes of tendinopathy include crystal deposits, postural misalignment, and hypermobility in a joint. Table 44-2 summarizes classes of tendinopathies.
TABLE 44-2
HISTOPATHOLOGIC CLASSIFICATION OF TENDON DISORDERS
| PATHOLOGIC DIAGNOSIS | MACROSCOPIC PATHOLOGY | HISTOPATHOLOGIC FINDINGS |
| Tendinosis | Intratendinous degeneration (commonly due to aging, microtrauma, muscular compromise) | Collagen disorientation, disorganization, and fiber separation by an increase in mucoid ground substance, increased preponderance of cells and vascular spaces with or without neovascularization and focal necrosis or calcification |
| Tendinitis | Symptomatic degeneration of the tendon with vascular disruption and inflammatory repair response | Degenerative changes as noted above with superimposed evidence of tear, including fibroblastic and myofibroblastic proliferation, hemorrhage, and organizing granulation tissue |
| Paratenonitis | “Inflammation” of the outer layer of the tendon (paratenon) alone, whether or not the paratenon is lined by synovium | Mucoid degeneration if the areolar tissue is seen; a scattered mild mononuclear infiltrate with or without focal fibrin deposition and fibrinous exudate |
| Paratenonitis with tendinosis | Paratenonitis associated with intratendinous degeneration | Degenerative changes as noted in tendinosis with mucoid degeneration with or without fibrous and scattered inflammatory cells in the paratenon alveolar tissue |
From Maffulli N, Wong J, Almekinders LC: Clin Sports Med 22(4):675–692, 2003.
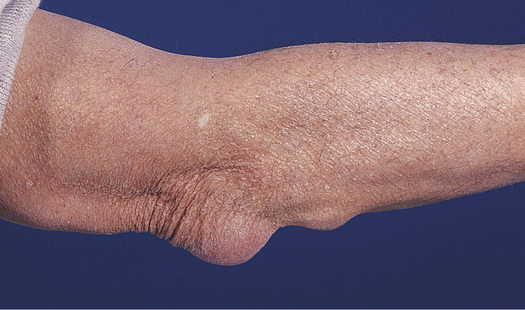
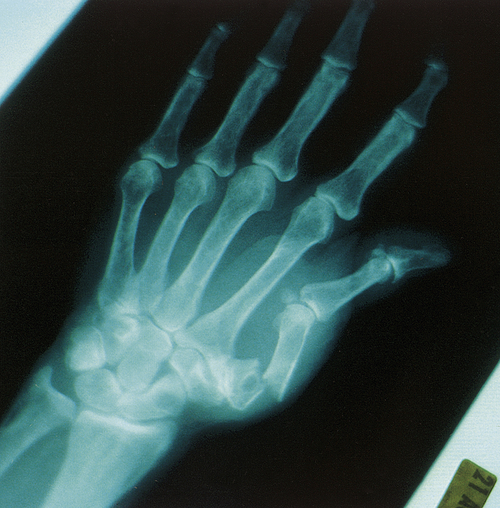
Epicondylitis is inflammation of a tendon where it attaches to a bone (at its origin). Most tendon pathology, however, is caused by tissue degeneration rather than inflammation.9 Epicondylar areas of the humerus, radius, or ulna and the area around the knee are most often involved. Lateral epicondylopathy, commonly called tennis elbow, is the result of tissue degeneration or irritation of the extensor carpi radialis brevis tendon at its origin. Medial epicondylopathy, referred to as golfer’s elbow, is a degenerative process of the pronator teres, flexor carpi radialis, and palmaris longus tendons at the medial humeral condyle (Figure 44-6). Epicondylopathy is also related to smoking, obesity, and work activities that involve forceful or repetitive cyclic flexion and extension of the elbow, or cyclic pronation, supination, extension, and flexion of the wrist that generates loads to the elbow and forearm region.
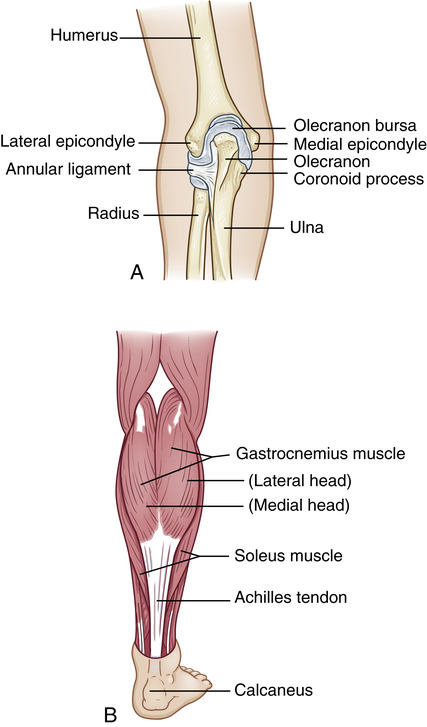
A, Medial or lateral epicondyles of humerus, site of epicondylitis. B, Achilles tendon, site of commonly occurring tendinitis.
Pathophysiology
The typical bursitis is an inflammation that is reactive to overuse or excessive pressure. The inflamed bursal sac becomes engorged, and the inflammation can spread to adjacent tissues (Figure 44-7). The inflammation may decrease with rest, heat, and aspiration of the fluid. (Inflammation is discussed in Chapter 7.)
Clinical Manifestations
Tendinopathy may be asymptomatic, but generally there is localized pain that worsens with active more than passive motion. With symptomatic tendinopathy, the pain is localized over the involved tendon and movement in the affected joint is limited. In bursitis, onset of pain may be gradual or sudden, but movement of the joint itself is normal. Shoulder bursitis impairs arm abduction because of pain and swelling of the bursa. Bursitis in the knee produces pain when climbing stairs, and crossing the legs is painful in bursitis of the hip. Lying on the side of an inflamed trochanteric bursa is also very painful. Table 44-3 summarizes common sites of bursitis. Signs of infectious bursitis may include the presence of a puncture site, prior corticosteroid injection, severe inflammation, or an adjacent source of infection.
TABLE 44-3
COMMON SITES AND CAUSES OF BURSITIS
| SITE | COMMON CAUSES |
| Shoulder (subacromial) | Repetitive overhead activities |
| Elbow (olecranon) | Rheumatoid arthritis (RA), gout, tuberculosis, leaning on elbow |
| Hip (greater trochanter) | Acute trauma, chronic stress |
| Ischial (weaver’s bottom) | Overuse (runner, ballet dancers), lumbosacral disease, RA, osteoarthritis (OA) |
| Knee | |
| Prepatellar (housemaid’s knee) | Trauma, frequent kneeling, infection |
| Pes anserine (medial knee) | Obesity, long-distance runner, OA, type 2 diabetes |
| Heel (calcaneal) | Poorly fitting footwear, Achilles tendinitis |
Muscle Strains
Mild injury such as muscle strain is usually seen after traumatic or sports injuries. Muscle strain is a general term for local muscle damage. It is often the result of sudden, forced motion causing the muscle to become stretched beyond normal capacity. Strains often involve the tendon as well. Muscles are ruptured more often than tendons in young people; the opposite is true in older adults. Muscle strain may be chronic when the muscle is repeatedly stretched beyond its usual capacity. There is evidence of tissue disruption with subsequent signs of muscle regeneration and connective tissue repair when a biopsy is performed. Hemorrhage into the surrounding tissue and signs of inflammation also may be present. Knife and gunshot wounds also cause traumatic rupture. Regardless of the cause of trauma, muscle cells usually can regenerate. Regeneration may take up to 6 weeks, and the affected muscle should be protected during this time. Types of muscle strain, together with their manifestations and treatment, are summarized in Table 44-4.
TABLE 44-4
| TYPE | MANIFESTATIONS | TREATMENT |
| First degree (e.g., bench press in untrained athlete) | Muscle overstretched, painful | Ice should be applied 5 or 6 times in the first 24-48 hours; complete rest for up to 2 weeks, followed by weightbearing 3 times per week and range of motion daily |
| Second degree (e.g., any muscle strain with bruising and pain) | Muscle intact with some tearing pain, mild bruising; fascia is intact | Treatment similar to that for first-degree strains, with added mild analgesia; cryokinetics (a treatment system of alternating applications of cold with progressive exercise) |
| Third degree (e.g., traumatic injury) | Caused by tearing of fascia; muscle rupture palpable, bleeding present | Surgery to approximate ruptured edges; immobilization and rest for 6 weeks, followed by an individualized rehabilitation regimen of strengthening exercises |
A late complication of localized muscle injury is abnormal bone formation in soft tissue, often called myositis ossificans or heterotopic ossification (HO). Its exact pathophysiology remains unknown, but the basic problem seems to be the inability of mesenchymal cells to differentiate into osteoblastic stem cells and the improper development of fibroblasts into bone-forming cells. Though uncommon, HO is associated with burns, joint surgery, and trauma to the musculoskeletal system or central nervous system. HO may involve muscle or tendons, ligaments, or bones near a muscle.10 Examples include “rider’s bone,” in which the adductor muscle of the thigh of equestrians becomes calcified, as well as in football players after muscle injury to thigh muscles; and “drill bone,” in which the same complication is seen in the deltoid and pectoral muscles of fencers and infantry soldiers.
Rhabdomyolysis
Pathophysiology
Rhabdomyolysis is sometimes incorrectly used interchangeably with crush injury (a description of injuries resulting from crushing of a body part), compartment syndrome (the consequences of increased intracompartmental pressures of a muscle), or crush syndrome (the pathophysiologic events caused by rhabdomyolysis, primarily involving the kidneys and coagulation syndrome).11 Although relatively rare, rhabdomyolysis has many causes (Box 44-1) and can result in serious complications, including hyperkalemia (because of the release of intracellular potassium into the circulation), metabolic acidosis (from liberation of intracellular phosphorus and sulfate), acute renal failure (myoglobin precipitates in the tubules, obstructing flow through the nephron and producing injury), and even disseminated intravascular coagulation (DIC) (likely caused by activation of the clotting cascade by sarcolemma damage and release of intracellular components from the damaged muscles). Even the weight of a limp extremity can generate enough pressure to produce muscle ischemia (Figure 44-8).