Chapter 41
Alterations of Digestive Function
Alexa K. Doig and Sue E. Huether
Disorders of the Gastrointestinal Tract
Clinical Manifestations of Gastrointestinal Dysfunction
Anorexia
Vomiting
Vomiting is the forceful emptying of stomach and intestinal contents through the mouth. The vomiting center lies in the medulla oblongata and includes the reticular formation and tractus solitarius nucleus. Stimulation of the vomiting center occurs directly by irritants or indirectly. Indirect stimulation includes the cerebral cortex and thalamus (anxiety and pain); the vestibular system through the eighth cranial nerve (motion sickness); several types of intestinal, vagal, or sympathetic input, including the presence of ipecac or copper salts in the duodenum; side effects of many drugs; distention of the stomach or duodenum; or torsion or trauma affecting the ovaries, testes, uterus, bladder, or kidney. Serotonin (5-hydroxytryptamine [5-HT]) stimulates the vomiting center and appears to be released from enterochromaffin cells in the intestinal wall, which activate vagal afferents leading to the chemoreceptor trigger zone (CTZ).1 Activation of the CTZ, which lies in the area postrema between the medulla and floor of the fourth ventricle, leads to vomiting by triggering receptors for substances such as dopamine (D2), opioids, acetylcholine, substance P, serotonin (5-hydroxytryptamine type 3), and neurokinin-1. Serotonin and neurokinin-1 antagonists are effective antiemetics and have been used to treat nausea and vomiting associated with postoperative vomiting and cancer chemotherapy. Apomorphine, levodopa, and bromocriptine are dopamine D2 agonists that cause nausea and vomiting. Metoclopramide, domperidone, and haloperidol are D2 antagonists and are effective antiemetics.2
Projectile vomiting is spontaneous vomiting that is not preceded by nausea or retching. Projectile vomiting is caused by direct stimulation of the vomiting center by neurologic lesions (e.g., increased intracranial pressure, tumors, or aneurysms involving the brainstem [see Chapters 17 and 20]). The metabolic consequences of vomiting are fluid, electrolyte, and acid-base disturbances, including hyponatremia, hypokalemia, hypochloremia, and metabolic alkalosis (see Chapter 3).
Constipation
Constipation is difficult or infrequent defecation and is estimated to affect 2% to 28% of the population. Constipation must be individually defined because patterns of bowel evacuation differ greatly among individuals. Normal bowel habits range from two or three evacuations per day to one per week. Constipation is not significant until it causes health risks (e.g., severe abdominal distention or fecal impaction) or impairs quality of life.3
Pathophysiology
Secondary constipation can be caused by neurogenic disorders (e.g., stroke, Parkinson disease, spinal cord injury, multiple sclerosis) in which neurotransmitters are altered or neural pathways are diseased or degenerated, resulting in delayed colon transit time. Opiates, particularly codeine, antacids containing calcium carbonate or aluminum hydroxide; anticholinergics; iron; and bismuth tend to inhibit bowel motility. Endocrine or metabolic disorders associated with constipation include hypothyroidism, diabetes mellitus, hypokalemia, and hypercalcemia. Pelvic hiatal hernia (herniation of the bowel through the floor of the pelvis), diverticuli, irritable bowel syndrome–constipation predominant, and pregnancy also can be associated with constipation. Aging can result in constipation caused from decreased motility related to the degeneration of neurons in the myenteric plexus, decreased neurotransmitter function, use of medications, and comorbid medical conditions.4 Constipation as a notable change in bowel habits can be an indication of colorectal cancer.
Clinical Manifestations
Indicators of constipation include two of the following for at least 3 months: (1) straining with defecation at least 25% of the time, (2) lumpy or hard stools at least 25% of the time, (3) sensation of incomplete emptying at least 25% of the time, (4) manual maneuvers to facilitate stool evacuation for at least 25% of defecations, and (5) fewer than three bowel movements per week.5 Changes in bowel evacuation patterns, such as less frequent defecation, smaller stool volume, hard stools, difficulty passing stools (straining), or a feeling of bowel fullness and discomfort or blood in the stools, require investigation. Fecal impaction (hard, dry stool retained in the rectum) is associated with rectal bleeding, abdominal or cramping pain, nausea and vomiting, weight loss, and episodes of diarrhea. Straining to evacuate stool may cause engorgement of the hemorrhoidal veins and hemorrhoidal disease or thrombosis with rectal pain, bleeding, and itching. Passage of hard stools can cause painful anal fissures.
Evaluation and Treatment
The treatment for constipation is to manage the underlying cause or disease for each individual. Management usually consists of bowel retraining, in which the individual establishes a satisfactory bowel evacuation routine without becoming preoccupied with bowel movements. Moderate exercise, increased fluid and fiber intake, stool softeners, and laxative agents are useful for some individuals. Enemas can be used to establish bowel routine, but they should not be used habitually. Biofeedback training can be effective for dyssynergic defecation.6 Drugs used to treat constipation include the colonic secretagogues lubiprostone and plecanatide and the 5-HT4 agonist prucalopride. Methylnaltrexone is a peripherally acting μ-opioid receptor antagonist approved for opioid-induced constipation in terminally ill individuals.7
Diarrhea
Diarrhea is an increase in the frequency of defecation and the fluid content, volume, and weight of feces. Three or more loose or liquid stools per day or more frequently than is normal for the individual are considered abnormal.8 Many factors determine stool volume and consistency, including the water content of the colon and the presence of unabsorbed food, unabsorbable material, and intestinal secretions. Stool volume in the normal adult averages less than 200 g/day. Stool volume in children depends on age and size. An infant may pass up to 100 g/day. The adult intestine processes approximately 9 L of luminal contents per day; 2 L is ingested, and the remaining 7 L consists of intestinal secretions. Of this volume, 99% of the fluid is absorbed—90% (7 to 8 L) in the small intestine and 9% (1 to 2 L) in the colon. Normally, approximately 150 ml of water is excreted daily in the stool.
Pathophysiology
Diarrhea in which the volume of feces is increased is called large-volume diarrhea. Large-volume diarrhea generally is caused by excessive amounts of water or secretions, or both, in the intestines. Small-volume diarrhea, in which the volume of feces is not increased, usually results from excessive intestinal motility. The three major mechanisms of diarrhea are osmotic, secretory, and motility.9 (Specific mechanisms of diarrhea in children are described in Chapter 42.)
In osmotic diarrhea, a nonabsorbable substance in the intestine draws water into the lumen by osmosis. The excess water and the nonabsorbable substance cause large-volume diarrhea. Large oral doses of poorly absorbed ions, such as magnesium, sulfate, and phosphate, can increase intraluminal osmotic pressure. Excessive ingestion of synthetic, nonabsorbable sugars (e.g., sorbitol); introduction of full-strength tube feeding formulas; and dumping syndrome associated with gastric resection draw water into the intestinal lumen (see p. 1440). Osmotic diarrhea disappears when ingestion of the osmotic substance stops. Malabsorption related to lactase deficiency, pancreatic enzyme or bile salt deficiency, small intestine bacterial overgrowth, and celiac disease also cause diarrhea.
Secretory diarrhea is a form of large-volume diarrhea caused by excessive mucosal secretion of chloride- or bicarbonate-rich fluid or inhibition of net sodium absorption. Infectious causes include viruses (e.g., rotavirus), bacterial enterotoxins (e.g., Escherichia coli and Vibrio cholerae), or exotoxins from overgrowth of Clostridium difficile following antibiotic therapy. These infections cause secretion of transmitters from enteroendocrine cells (e.g., 5-HT) and activation of afferent neurons that stimulate submucosal secretomotor neurons and altered sodium and chloride transport resulting in decreased water absorption.10,11 Neoplasms (such as gastrinoma or thyroid carcinoma) produce hormones that stimulate intestinal secretion causing diarrhea.
Evaluation and Treatment
A thorough history is taken to document the onset, frequency, and volume of stools. Exposure to contaminated food or water is indicated if the individual has traveled in foreign countries or areas where drinking water might be contaminated. Iatrogenic diarrhea is suggested if the individual has undergone abdominal radiation therapy, intestinal resection, or treatment with selected drugs (e.g., antibiotics, diuretics, antihypertensives, laxatives). Physical examination helps the clinician to identify underlying systemic disease. Stool culture, examination of stool specimens for blood, abdominal roentgenograms, endoscopy, and intestinal biopsies provide more specific data.12
Treatment for diarrhea includes restoration of fluid and electrolyte balance, antimotility (e.g., loperamide [an opiate] or Lomotil [atropine]) and/or water-absorbent (e.g., attapulgite and polycarbophil) medications, and treatment of causal factors. Nutritional deficiencies need to be corrected in cases of chronic diarrhea or malabsorption. Natural bran and commercial preparations of psyllium are inexpensive and effective treatments for mild diarrhea. Probiotics can be useful for treating Clostridium difficile–associated diarrhea as an approach to restoring normal microflora in addition to antibiotic therapy.13 Fecal transplantation can be used for cases that are resistant to conventional therapies.14
Abdominal Pain
Abdominal pain is the presenting symptom of a number of gastrointestinal (GI) diseases and can be acute or chronic. The causal mechanisms are mechanical, inflammatory, or ischemic. (The physiology of pain is described in Chapter 16.) Abdominal pain may be generalized to the abdomen or localized to a particular abdominal quadrant. The pain is often described as sharp, dull, or colicky. Generally the abdominal organs are not sensitive to mechanical stimuli, such as cutting, tearing, or crushing. These organs are, however, sensitive to stretching and distention, which activate nerve endings in both hollow and solid structures. The onset of pain is associated with rapid distention; gradual distention causes little pain. Traction on the peritoneum caused by adhesions, distention of the common bile duct, or forceful peristalsis resulting from intestinal obstruction causes pain because of increased tension. Capsules that surround solid organs, such as the liver and gallbladder, contain pain fibers that are stimulated by stretching if these organs swell.
Visceral pain arises from a stimulus (distention, inflammation, ischemia) acting on an abdominal organ. Chronic low-grade inflammation can cause pain hypersensitivity with involvement of neurokinins, serotonin, and voltage-gated ion channels.15,16 Pain is usually felt near the midline in the epigastrium, midabdomen, or lower abdomen. The pain is poorly localized, is dull rather than sharp, and is difficult to describe. Visceral pain is diffuse and vague because nerve endings in abdominal organs are sparse and multisegmented. Pain arising from the stomach, for example, is experienced as a sensation of fullness, cramping, or gnawing in the midepigastric area. Referred pain is visceral pain felt at some distance from a diseased or an affected organ. Referred pain is usually well localized and is felt in the skin dermatomes or deeper tissues that share a central afferent pathway with the affected organ. Gallbladder pain is, for example, referred to the right shoulder or scapulae.
Gastrointestinal Bleeding
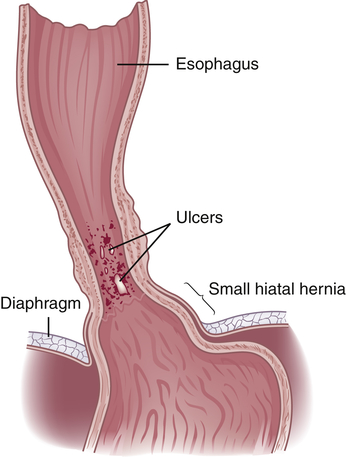
Upper gastrointestinal (GI) bleeding is bleeding in the esophagus, stomach, or duodenum and is characterized by frank, bright red bleeding in emesis or dark, grainy digested blood (“coffee grounds”) in stool. Upper GI bleeding is caused by esophageal or gastric varices, a Mallory-Weiss tear at the esophageal-gastric junction from severe retching, cancer, angiodysplasias, or peptic ulcers.17 Lower gastrointestinal (GI) bleeding, bleeding from the small intestine (jejunum or ileum), colon, or rectum, can be caused by polyps, inflammatory bowel disease, diverticulosis, cancer, vascular ectasias, or hemorrhoids.18 Occult bleeding is usually caused by slow, chronic blood loss that is not obvious and results in iron deficiency anemia as iron stores in the bone marrow are slowly depleted. Acute, severe GI bleeding is life threatening depending on the volume and rate of blood loss, associated disease, age, and effectiveness of treatment.
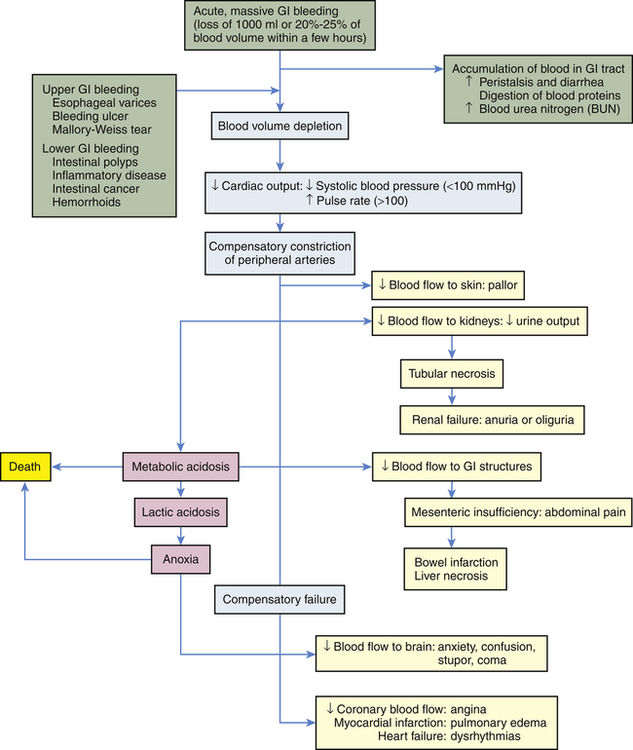
Physiologic response to gastrointestinal bleeding depends on the amount and rate of the loss (Figure 41-1). Changes in blood pressure and heart rate are the best indicators of massive blood loss in the gastrointestinal tract. Blood losses of 1000 ml or more over a short time cause a decrease in blood pressure and a corresponding increase in heart rate. With losses of 1000 ml or more, the heart rate is greater than 100 beats/minute and systolic blood pressure is less than 100 mmHg. During the early stages of blood volume depletion, the peripheral arteries and arterioles constrict to shunt blood to vital organs, including the brain (see Chapters 32 and 48). Signs of large-volume blood loss are postural hypotension (a drop in blood pressure that occurs with a change from the recumbent position to a sitting or upright position), lightheadedness, and loss of vision. If blood loss continues, hypovolemic shock develops. Diminished blood flow to the kidneys causes decreased urine output and may lead to oliguria (low urine output), tubular necrosis, and renal failure. Ultimately, insufficient cerebral and coronary blood flow causes irreversible anoxia and death.
The presentation of GI bleeding is summarized in Table 41-1. The accumulation of blood in the gastrointestinal tract is irritating and increases peristalsis, causing vomiting (hematemesis) or diarrhea, or both. If bleeding is from the lower GI tract, the diarrhea is frankly bloody. Bleeding from the upper GI tract also can be rapid enough to produce bright red stools (hematochezia), but generally some digestion of the blood components will have occurred, producing melena, black or tarry stools that are sticky and have a characteristic foul odor. The digestion of blood proteins originating from massive upper GI bleeding is reflected by an increase in blood urea nitrogen (BUN) levels (see Figure 41-1).
TABLE 41-1
PRESENTATIONS OF GASTROINTESTINAL BLEEDING
| PRESENTATION | DEFINITION |
| Acute bleeding | |
| Hematemesis | Bloody vomitus; either fresh, bright red blood or dark, grainy, digested blood with “coffee grounds” appearance |
| Melena | Black, sticky, tarry, foul-smelling stools caused by digestion of blood in the gastrointestinal tract |
| Hematochezia | Fresh, bright red blood passed from the rectum |
| Occult bleeding | Trace amounts of blood in normal-appearing stools or gastric secretions; detectable only with a guaiac test |
The hematocrit and hemoglobin values are not the best indicators of acute gastrointestinal bleeding because plasma and red cell volume are lost proportionately. As the plasma volume is replaced, the hematocrit and hemoglobin values begin to reflect the extent of blood loss. The interpretation of these values is modified to account for exogenous replacement of fluids and the hydration status of the tissues. Anemia associated with chronic GI bleeding is caused by iron depletion. Evaluation and treatment involves identifying and treating the source of the bleeding and replacing iron losses. Administration of blood products may be used for massive hemorrhage. Guidelines are available for the diagnosis and management of gastrointestinal bleeding.19–21
Disorders of Motility
Dysphagia
Pathophysiology
Functional dysphagia is caused by neural or muscular disorders that interfere with voluntary swallowing or peristalsis. Disorders that affect the striated muscles of the upper esophagus interfere with the oropharyngeal (voluntary) phase of swallowing. Typical causes of functional dysphagia in the upper esophagus are dermatomyositis (a muscle disease) and neurologic impairments caused by stroke, multiple sclerosis, Parkinson disease, amyotrophic lateral sclerosis, or myasthenia gravis.22
Achalasia is a rare disorder related to loss of inhibitory neurons in the myenteric plexus with smooth muscle atrophy in the middle and lower portions of the esophagus. The etiology is unknown but may be related to viral or autoimmune mechanisms.23 This leads to altered esophageal peristalsis and failure of the lower esophageal sphincter (LES) to relax, causing functional obstruction of the lower esophagus. Food accumulates above the obstruction, distends the esophagus, and causes dysphagia. Cough and aspiration can occur. As hydrostatic pressure increases, food is slowly forced past the obstruction into the stomach. Chronic inflammation from esophageal food retention can increase risk for esophageal cancer. Chronic esophageal distention requires dilation or surgical myomotomy of the lower esophageal sphincter (LES).24
Evaluation and Treatment
Knowledge of the individual’s history and clinical manifestations contributes significantly to a diagnosis of dysphagia. Videofluoroscopy and high-frequency ultrasound are used to visualize the contours of the esophagus and identify structural defects. High-resolution manometry with topography and intraluminal impedance monitoring documents the duration and amplitude of abnormal pressure changes associated with obstruction or loss of neural regulation.25 Esophageal endoscopy is performed to examine the esophageal mucosa, obtain biopsy specimens, or perform corrective surgery.
The individual is taught to manage symptoms by eating slowly, eating small meals, taking fluid with meals, and sleeping with the head elevated to prevent regurgitation and aspiration. Oral medications may need to be formulated so they can be swallowed.26 Tube feedings may be required for some individuals, particularly following stroke.27 Anticholinergic drugs may alleviate symptoms. Definitive treatments include mechanical dilation of the esophageal sphincter and surgical separation of the lower esophageal muscles with a longitudinal incision (myotomy) widening the passage into the stomach.28
Gastroesophageal Reflux Disease
Gastroesophageal reflux disease (GERD) is the reflux of acid and pepsin from the stomach to the esophagus that causes esophagitis. Risk factors for GERD include obesity, hiatal hernia, and drugs or chemicals that relax the LES (anticholinergics, nitrates, calcium channel blockers, nicotine).29 GERD may be a trigger for asthma or chronic cough.30 Gastroesophageal reflux that does not cause symptoms is known as physiologic reflux. In nonerosive reflux disease (NERD), individuals have symptoms of reflux disease but no visible esophageal mucosal injury (functional heartburn).31
Pathophysiology
The resting tone of the LES tends to be lower than normal from either transient relaxation or weakness of the sphincter in those who develop GERD. Vomiting, coughing, lifting, bending, or obesity increases abdominal pressure, contributing to the development of reflux esophagitis. Delayed gastric emptying contributes to reflux esophagitis by: (1) lengthening the period during which reflux is possible and (2) increasing the acid content of chyme. Disorders that delay emptying include gastric or duodenal ulcers, which can cause pyloric edema; strictures that narrow the pylorus; and hiatal hernia, which can weaken the LES.32
GERD causes inflammatory responses in the esophageal wall resulting in hyperemia, edema, tissue fragility, erosion, and ulcerations (Figure 41-2). Severity of inflammation is related to composition of gastric contents and length of exposure time.33 Fibrosis, basal cell hyperplasia, and elongation of papillae are common. Precancerous lesions (Barrett esophagus, see p. 1466) with progression to adenocarcinoma can be a long-term consequence.33,34
Clinical Manifestations
The clinical manifestations of reflux esophagitis are heartburn from acid regurgitation, chronic cough, asthma attacks (see Chapter 35), and laryngitis.30 Upper abdominal pain usually occurs within 1 hour of eating and can be relapsing and remitting. Symptoms can worsen if the individual lies down or if intra-abdominal pressure increases (e.g., as a result of coughing, vomiting, or straining at stool). Symptoms may be present when no acid is in the esophagus.35 Heartburn also may be experienced as chest pain, which requires ruling out cardiac ischemia. Edema, fibrosis (strictures), esophageal spasm, or decreased esophageal motility may result in dysphagia. Alcohol or acid-containing foods, such as citrus fruits, can cause discomfort during swallowing.
Evaluation and Treatment
Proton pump inhibitors are the most effective monotherapy. Other therapies include histamine-2 (H2) receptor antagonists, prokinetics, and antacids. Pain medication may be used in resistant cases. Elevation of the head of the bed 6 inches prevents reflux. Weight reduction and cessation of smoking also help to alleviate symptoms. Laparoscopic fundoplication is the most common surgical intervention when medical treatment fails.36,37
Eosinophilic esophagitis is a rare, idiopathic inflammatory disease of the esophagus characterized by esophageal infiltration of eosinophils associated with atopic disease, including asthma and food allergies. It occurs in adults and children. Dysphagia, food impaction, vomiting, and weight loss are common symptoms. Endoscopy with biopsy identifies the eosinophilc infiltration and differentiation from GERD. Treatment is symptomatic including elimination diets, proton pump inhibitors, and immunosuppression.38
Hiatal Hernia
Pathophysiology
Hiatal hernia is the protrusion (herniation) of the upper part of the stomach through the diaphragm and into the thorax.39 There are four types: sliding (type I); paraesophageal (type II); mixed (type III), which include elements of types I and II (Figure 41-3).40 In type IV the entire stomach and other abdominal organs slide into the thorax.
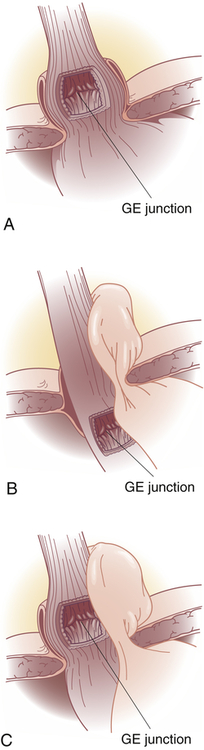
A, Type I—sliding hernia. The visceral peritoneum remains intact and restrains the size of the hernia in sliding hiatal hernia. B, Type II—paraesophageal or rolling hernia. The membrane becomes thinner or defective in paraesophageal hernia, allowing a true peritoneal sac to protrude into the posterior mediastinum where negative intrathoracic pressure causes it to enlarge. C, Type III—mixed hernia. GE, Gastroesophageal.
(From Townsend CM et al: Sabiston textbook of surgery, ed 19, Philadelphia, 2012, Saunders.)
Note: Type IV-complex paraesophageal hernia is not shown.
In type II, paraesophageal hiatal hernia (rolling hiatal hernia), herniation of the greater curvature of the stomach is through a secondary opening in the diaphragm (see Figure 41-3). A giant paraesophageal hiatal hernia develops when 30% to 60% of the stomach moves into the thorax. As the stomach protrudes through the opening into the thorax, it lies alongside the esophagus. The gastroesophageal junction remains below the diaphragm. With paraesophageal hernia, reflux is uncommon. The position of a portion of the stomach above the diaphragm, however, causes congestion of mucosal blood flow and can lead to gastritis and ulcer formation. A mechanical strangulation of the hernia is a major complication, and surgical correction is required. Strangulation occludes blood vessels and causes vascular engorgement, edema, ischemia, and hemorrhage. Type III, mixed hiatal hernia, is a combination of both types I and II and tends to occur in conjunction with several other diseases, including gastroesophageal reflux, peptic ulcer, cholecystitis (gallbladder inflammation), cholelithiasis (gallstones), chronic pancreatitis, and diverticulosis. Type IV is an aggravated form of type III.
Clinical Manifestations
Hiatal hernias are often asymptomatic. Generally, a wide variety of symptoms develop later in life and are associated with other gastrointestinal disorders, including GERD. Manifestations of the various types of hiatal hernia are difficult to distinguish. Symptoms include heartburn, regurgitation, dysphagia, and epigastric pain.41 Ischemia from hernia strangulation causes acute, severe chest or epigastric pain, nausea, vomiting, and GI bleeding.
Evaluation and Treatment
Treatment for sliding hiatal hernia is usually conservative. The individual can diminish reflux by eating small, frequent meals and avoiding the recumbent position after eating. Abdominal supports and tight clothing are avoided, and weight control is recommended for obese individuals. Antacids alleviate reflux esophagitis. Drugs that relax the LES (anticholinergic, nitrates, calcium channel blockers) are contraindicated because they delay gastric emptying. Laparoscopic surgery (i.e., fundoplication) may be performed for paraesophageal hiatal hernia or if medical management fails to control symptoms.42
Pyloric Obstruction
Pathophysiology
Pyloric obstruction (gastric outlet obstruction) is the narrowing or blocking of the opening between the stomach and the duodenum. This condition can be congenital (i.e., infantile hypertrophic pyloric stenosis, see Chapter 42) or acquired. Acquired obstruction is caused by peptic ulcer disease or carcinoma near the pylorus. Duodenal ulcers are more likely than gastric ulcers to obstruct the pylorus. Ulceration causes obstruction resulting from inflammation, edema, spasm, fibrosis, or scarring. Tumors cause obstruction by growing into the pylorus.43
Evaluation and Treatment
Obstructions resulting from ulceration often resolve with conservative management. Gastric drainage is used to decompress the stomach and restore normal motility. Gastric secretions that contribute to inflammation and edema can be suppressed with proton pump inhibitors or histamine-2 (H2) receptor antagonists. Fluids and electrolytes (sodium chloride and potassium) are given intravenously to effect rehydration and correct hypochloremia, alkalosis, and hypokalemia (see Chapter 3). Severely malnourished individuals may require parenteral hyperalimentation (intravenous nutrition). Surgery or stenting may be required to treat gastric carcinoma or persistent obstruction caused by fibrosis and scarring.44
Intestinal Obstruction and Ileus
Intestinal obstruction can be caused by any condition that prevents the normal flow of chyme through the intestinal lumen or failure of normal intestinal motility in the absence of an obstructing lesion (ileus). The small intestine is more commonly obstructed because of its narrower lumen. Common causes of intestinal obstruction are summarized in Table 41-2. Criteria for classifying intestinal obstruction are summarized in Table 41-3. Intestinal obstruction is classified by cause as simple or functional. Simple obstruction is mechanical blockage of the lumen by a lesion and is the most common type of intestinal obstruction. Paralytic ileus, or functional obstruction, is a failure of motility after gastrointestinal or abdominal surgery. Anesthetic agents, local inflammatory reactions, use of opioid analgesia, and hyperactivity of the sympathetic nervous system contribute to postoperative ileus.
TABLE 41-2
COMMON CAUSES OF INTESTINAL OBSTRUCTION
| CAUSE | PATHOPHYSIOLOGY |
| Herniation | Protrusion of the intestine through a weakness in the abdominal muscles or through the inguinal ring |
| Intussusception | Telescoping of one part of the intestine into another; this usually causes strangulation of the blood supply; more common in the ileocecal area in infants 10 to 15 months of age than in adults |
| Torsion (volvulus) | Twisting of the intestine on its mesenteric pedicle, with occlusion of the blood supply; often associated with fibrous adhesions in the small intestine; occurs most often in the large intestine in older adults |
| Diverticulosis | Inflamed saccular herniations (diverticula) of the mucosa and submucosa through the tunica muscularis of the colon; diverticula are interspersed between thick, circular, fibrous bands; most common in obese individuals older than 60 years |
| Tumor | Tumor growth into the intestinal lumen; adenocarcinoma of the colon and rectum is the most common tumoral obstruction; most common in individuals older than 60 years |
| Paralytic (adynamic) ileus | Loss of peristaltic motor activity in the intestine; associated with abdominal surgery, peritonitis, hypokalemia, ischemic bowel, spinal trauma, pneumonia, neuropathies, or myopathies; affects small and large intestines |
| Fibrous adhesions | Peritoneal irritation from surgery or trauma leads to formation of fibrin and adhesions that attach to intestine, omentum, or peritoneum and can cause traction and obstruction; most common in small intestine |
TABLE 41-3
CLASSIFICATION OF INTESTINAL OBSTRUCTION
| CRITERIA FOR CLASSIFICATION | DEFINITION |
| Onset | |
| Acute | Sudden onset; often caused by torsion, intussusception, or herniation |
| Chronic | Protracted onset; more commonly from tumor growth or progressive formation of strictures |
| Extent of Obstruction | |
| Partial | Incomplete obstruction of intestinal lumen |
| Complete | Complete obstruction of intestinal lumen |
| Location of Obstructing Lesion | |
| Intrinsic | Obstruction develops within intestinal lumen; examples: luminal edema or hemorrhage, foreign bodies (gallstones), tumors, or intraluminal fibrosis |
| Extrinsic | Obstruction originates outside the intestine; examples: tumors, torsion, fibrosis, hernia, intussusception |
| Effects on Intestinal Wall | |
| Simple | Luminal obstruction without impairment of blood supply |
| Strangulated | Luminal obstruction with occlusion of blood supply |
| Closed loop | Obstruction at each end of a segment of the intestine |
| Causal Factors | |
| Mechanical | Blockage of the intestinal lumen by intrinsic or extrinsic lesions; usually treated surgically |
| Functional (paralytic ileus) | Paralysis of the intestinal musculature as a result of accidental or surgical trauma, peritonitis, electrolyte imbalances, or spasmolytic agents; usually treated medically |
Simple obstruction of the small intestine from fibrous adhesions is the most common type of intestinal obstruction.45 Acute obstructions usually have mechanical causes, such as adhesions or hernias (Figure 41-4). Chronic or partial obstructions are more often associated with tumors or inflammatory disorders, particularly of the large intestine. Intussusception is rare in adults compared with the more frequent occurrence in infants. The most common causes of large bowel obstruction are colorectal cancer, volvulus (twisting), and strictures related to diverticulitis. Common causes of intestinal obstruction in children are presented in Chapter 42.
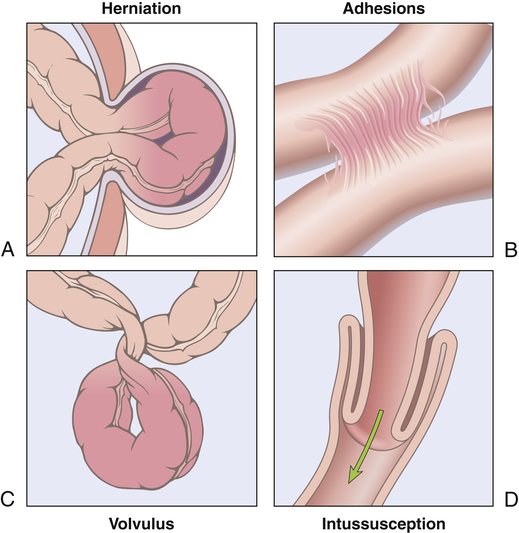
A, Hernia. B, Constriction from adhesions. C, Volvulus. D, Intussusception. (From Kumar V, Abbas AK, Aster J: Robbins basic pathology, ed 9, Philadelphia, 2013, Saunders.)
Pathophysiology
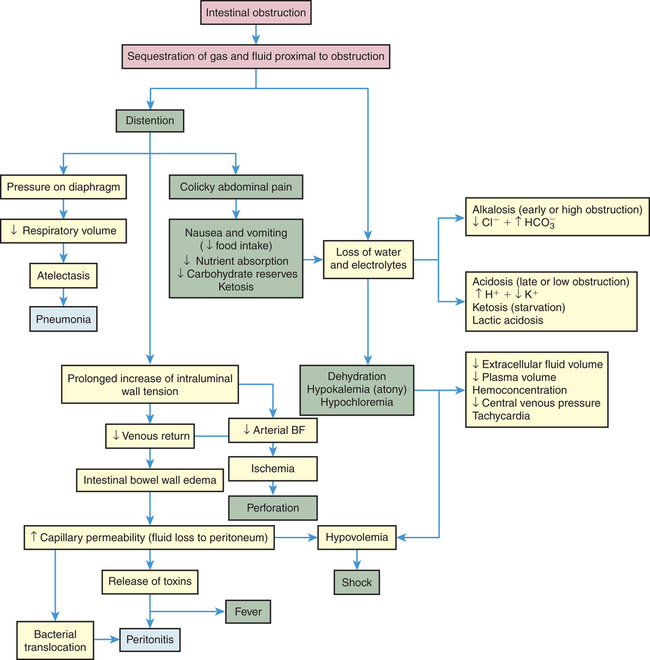
The consequences of intestinal obstruction are related to its onset and location, the length of intestinal tract proximal to the obstruction, and the presence and severity of ischemia. The major pathophysiologic alterations are presented in Figure 41-5. Postoperative paralytic ileus results from inhibitory neural reflexes associated with inflammatory mediators, and the influence of exogenous (meperidine) and endogenous opioids (endorphins) that affect the entire GI tract, including the stomach.46,47 Small intestine obstruction leads to accumulation of fluid and gas inside the lumen proximal to the obstruction. Fluids accumulate from impaired water and electrolyte absorption and enhanced secretion with net movement of fluid from the vascular space to the intestinal lumen. Gas from swallowed air, and to a lesser extent from bacterial overgrowth, contributes to the distention. Distention begins almost immediately, as gases and fluids accumulate proximal to the obstruction. Distention decreases the intestine’s ability to absorb water and electrolytes and increases the net secretion of these substances into the lumen. Within 24 hours, up to 8 L of fluid and electrolytes enters the lumen in the form of saliva, gastric juice, bile, pancreatic juice, and intestinal secretions. Copious vomiting or sequestration of fluids in the intestinal lumen prevents their reabsorption and produces severe fluid and electrolyte disturbances. Extracellular fluid volume and plasma volume decrease, causing dehydration. Hemoconcentration (decreased plasma volume) elevates hematocrit level and causes hypotension and tachycardia. Severe dehydration leads to hypovolemic shock.
If the obstruction is at the pylorus or high in the small intestine, metabolic alkalosis develops initially as a result of excessive loss of hydrogen ions that normally would be reabsorbed from the gastric juice. With prolonged obstruction or obstruction lower in the intestine, metabolic acidosis is more likely to occur because bicarbonate from pancreatic secretions and bile cannot be reabsorbed. Hypokalemia from vomiting and decreased potassium absorption can be extreme, promoting acidosis and atony of the intestinal wall. Metabolic acidosis also may be accentuated by ketosis, the result of declining carbohydrate stores caused by starvation. Lack of circulation permits the buildup of significant amounts of lactic acid, which worsen the metabolic acidosis. If pressure from the distention is severe enough, it occludes the arterial circulation and causes ischemia, necrosis, perforation, and peritonitis. Fever and leukocytosis are often associated with overgrowth of bacteria, ischemia, and bowel necrosis. Bacterial proliferation and translocation across the mucosa to the mesenteric lymph nodes or systemic circulation cause sepsis. The release of inflammatory mediators into the circulation causes remote organ failure (see Chapter 48).
Clinical Manifestations
Vomiting and abdominal distention vary, depending on the level and completion of the obstruction. Obstruction at the pylorus causes early, profuse vomiting of clear gastric fluid. Obstruction in the proximal small intestine causes mild distention and vomiting of bile-stained fluid. Obstruction in the distal small intestine causes more pronounced distention because a greater length of intestine is proximal to the obstruction. In this case, vomiting may not occur or may occur later and contain fecal material. Partial obstruction can cause diarrhea or constipation, but complete obstruction usually causes constipation only. Complete obstruction increases the number of bowel sounds, which may be tinkly and accompanied by peristaltic rushes and crampy, abdominal pain. Signs of hypovolemia and metabolic acidosis (see Chapter 3) may be observed as early as 24 hours after the occurrence of complete obstruction. Distention may be severe enough to push against the diaphragm and decrease lung volume. This can lead to atelectasis and pneumonia, particularly in debilitated individuals.
Evaluation and Treatment
Evaluation is based on clinical manifestations and includes ultrasound and radiography.48 Successful management requires early identification of the site and type of obstruction. Replacement of fluid and electrolytes and decompression of the lumen with gastric or intestinal suction are essential forms of therapy. Laparoscopic procedures can release adhesions. Immediate surgical intervention is required for strangulation and complete obstruction. Neostigmine, a parasympathomimetic, is used for colonic pseudo-obstruction and colonoscopic decompression may be required.49
Gastritis
Acute gastritis is usually caused by injury of the protective mucosal barrier by drugs, chemicals, or Helicobacter pylori infection (Figure 41-6). Nonsteroidal anti-inflammatory drugs (NSAIDs [ibuprofen, naproxen, indomethacin, and aspirin]) inhibit the action of cyclooxygenase-1 (COX-1), and are known to cause erosive gastritis because they inhibit prostaglandins, which normally stimulate the secretion of mucus and suppress inflammation. With the exception of aspirin, NSAIDs also cause gastric hypermotility, causing mucosal compression and injury.50 Alcohol, histamine, digitalis, and metabolic disorders such as uremia are contributing factors. H. pylori–associated acute gastritis causes inflammation, increased gastric secretion in antral gastritis, decreased gastric section in fundal gastritis, pain, nausea, and vomiting51 (Box 41-1).

Acute erosive gastritis is shown in the opened stomach. The mucosa appears hyperemic, and the foci of superficial ulceration are manifested as scattered, small, red areas termed erosions. (From Kumar V et al: Pathologic basis of disease, ed 7, Philadelphia, 2006, Saunders.)
Chronic fundal gastritis is the most rare and severe type and is associated with loss of T-cell tolerance and development of autoantibodies to gastric H+-K+ ATPase. Infection with H. pylori can trigger the immune response through molecular mimicry.52 The gastric mucosa degenerates extensively in the body and fundus of the stomach, leading to gastric atrophy. Loss of parietal cells diminishes secretion of hydrochloric acid and intrinsic factor. Because acid secretion is insufficient, the feedback mechanism that normally inhibits gastrin secretion is impaired, causing elevated plasma levels of gastrin. Chronic antral gastritis generally involves the antrum only and is more common than fundal gastritis. It is caused by H. pylori bacteria or chronic use of alcohol, tobacco, and nonsteroidal anti-inflammatory drugs. There are high levels of hydrochloric acid secretion with an increased risk of duodenal ulcers. H. pylori can also progress to autoimmune atrophic gastritis and involve the fundus, thus becoming pangastritis. In these cases there is greater risk for the development of gastric cancer.53
Signs and symptoms of chronic gastritis often do not correlate with the severity of the disease. Gastroscopic examination and biopsy may show a long-standing inflammatory process and gastric atrophy in an individual with no history of abdominal distress. The presence of antiparietal cell antibody and elevated plasma ghrelin level are specific for atrophic gastritis.54
H. pylori infection is evidence for H. pylori gastritis. In chronic fundal gastritis, failure to stimulate acid secretion confirms achlorhydria (diminished secretion of hydrochloric acid). The gastric secretions also can be evaluated for the presence of intrinsic factor. Pernicious anemia can develop because intrinsic factor is less available to facilitate vitamin B12 absorption. Individuals may report vague symptoms, including anorexia, fullness, nausea, vomiting, and epigastric pain. Gastric bleeding may be the only clinical manifestation of gastritis. Evaluation for gastric carcinoma is completed with chronic H. pylori infection.55 Symptoms can usually be managed with consumption of smaller meals, including a soft, bland diet; and avoidance of alcohol and NSAIDs. Combination antibiotics are used to treat H. pylori, and the emergence of antimicrobial resistance is a concern.56 Vitamin B12 is administered to correct pernicious anemia (see Chapter 28).57
Alkaline reflux gastritis is a stomach inflammation caused by reflux of bile and alkaline pancreatic secretions that contain proteolytic enzymes and disrupt the mucosal barrier in the remnant stomach. This form of gastritis occurs in 5% to 20% of individuals who have undergone gastrectomy or pyloroplasty. Clinical manifestations include nausea, bilious vomiting (vomiting in which the vomitus contains bile), and sustained epigastric pain that worsens after eating and is not relieved by antacids. Endoscopy shows a hemorrhagic and friable gastric mucosa. Antacids do not consistently improve symptoms. Avoidance of aspirin and alcohol may decrease gastric irritation, and a low-fat diet may limit bile secretion. Surgical correction may ultimately be required.58
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree