CHAPTER 21
Warfarin
VALERY L. CHU, BS, PharmD, BCACP, CACP, AE-C
HELENE C. MALTZ, BS, PharmD, BCPS
OVERVIEW
Following the isolation of hemorrhagic agents from spoiled sweet clover hay in the 1930s and development of 3-phenyacetyl ethyl, 4-hydroxycoumarin as a rat poison in 1948, warfarin has been the primary oral anticoagulant used in North America since its approval for medical use in 1954.1 Even following the availability of new oral anticoagulant classes, it is widely used for its various indications including prophylaxis and treatment of venous thrombosis and pulmonary embolism, prophylaxis and treatment of thromboembolic complications of atrial fibrillation and heart valve replacement, and postmyocardial infarction (MI) reduction in the risk of death, recurrent MI, and thromboembolic events such as stroke or systemic embolization.2
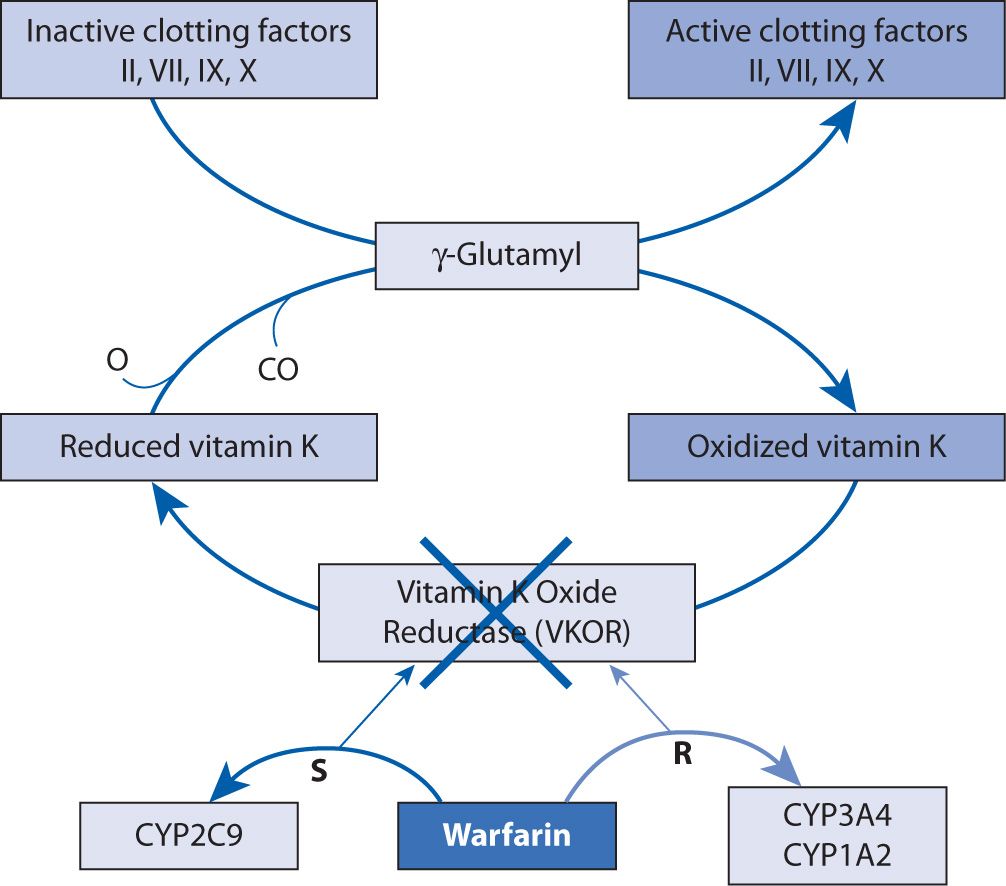
Warfarin, a vitamin K antagonist (VKA), inhibits the C1 subunit of vitamin K epoxide reductase (VKORC1) complex, preventing the regeneration of vitamin K1 epoxide. This interferes with the carboxylation and activation of vitamin K-dependent clotting factors, factors II, IV, IX, and X (Figure 21-1).2-4 At the same time, carboxylation of the natural anticoagulants proteins C and S is inhibited as well.4 This effect can be reversed with the administration (pharmacologically or nutritionally) of vitamin K1, which is also called phytonadione.4 Prolongation of the prothrombin time (PT) is seen with warfarin therapy, and a standardized measurement of this effect, the international normalized ratio (INR), is utilized for warfarin monitoring because it has been shown to correlate with efficacy.2,5,6
FIGURE 21-1. Mechanism of action of warfarin.
PHARMACOKINETICS
Warfarin has nearly 100 percent oral bioavailability, achieving peak concentrations within 4 hours of administration.2 Its volume of distribution is small, 0.14 L/kg, and is limited by 99 percent protein binding, primarily to albumin.2,4 Commercially available warfarin products are equal, racemic mixtures of the R and S enantiomers.4 The S-warfarin enantiomer is five times more potent. It is primarily metabolized by cytochrome P-450 (CYP) 2C9, and in part by CYP 2C19. The less potent R-warfarin enantiomer is metabolized by CYP 1A2 and CYP 3A4.7 The products of metabolism are inactive and 92 percent excreted in the urine.2 The pharmacokinetic half-life of warfarin is 36–42 hours2,4; therefore, ≥4 days is required to achieve steady-state concentrations of any given warfarin dose. However, the chief pharmacodynamic effect of warfarin is caused by its inhibition of factor II (thrombin). The full effect of warfarin is therefore determined by the half-life of factor II (60–72 hours), so complete factor II inhibition can take 10 days or more to achieve.4 It is for the same reason that when warfarin is initiated, its antithrombotic effect is not realized until at least 5 days of treatment has elapsed. Therefore, overlap with another form of anticoagulation with a faster onset, historically unfractionated or low molecular weight heparin, is required for the first 5 days and until the INR produced by warfarin therapy is in the therapeutic range for 2 consecutive days.4,7 Since complete factor II inhibition can take 10 days or more to achieve, and close monitoring of the extent and rate of anticoagulation achievement is warranted during initiation.
ADVERSE EFFECTS
The major adverse effect associated with warfarin use is bleeding, including fatal intracranial and gastrointestinal (GI) hemorrhages.2 High-intensity anticoagulation, older age, variable coagulation control, history of GI bleeds, hypertension, cerebrovascular disease, anemia, malignancy, trauma, renal impairment, liver function impairment, and genetic predisposition to over-anticoagulation are risk factors for bleeding. Because of the possibility of bleeding, it is imperative that any modifiable risk factors are eliminated or reduced prior to initiation of warfarin therapy, appropriate monitoring of anticoagulation is performed, and the benefit of anticoagulation outweighs the risk for each patient.2,4,6
Other serious adverse effects include tissue necrosis early in warfarin therapy and systemic atheroemboli or cholesterol micro-emboli presenting as “purple toe syndrome.” Less serious reactions include vasculitis, elevations in liver enzymes, GI complaints, and allergic reactions, which may be related to the dyes used in the tablets.2,8
DOSING STRATEGIES
Initiation of warfarin therapy can follow two general approaches: the use of a dose considered to be a prediction of the ultimate maintenance dose or use of a higher dose initially (often given the inaccurate moniker of “loading dose”) in an attempt to more rapidly identify the anticipated dose of warfarin to achieve target INR. Estimations of eventual maintenance doses must account for vast inter- and intrapatient variability discussed in the following section. It is also possible, through the use of various nomograms, to quickly titrate a given warfarin dose upward to the necessary maintenance dose based on the early INR response. It is essential that qualified, experienced clinicians are available to interpret the early INR response appropriately to improve patient outcomes.9-11
Several nomograms for dosing warfarin in the initiation stages of therapy have been developed. In 1999, a comparison of 5 mg and 10 mg initiation doses used to treat inpatients with heterogeneous indications found that patients in the 5 mg group were more likely to achieve stable anticoagulation within days 3–5 than those receiving the 10 mg dose (relative risk, 2.22, 95% confidence interval (CI) 1.30–3.70 [P <0.003]). Over-anticoagulation was less likely in the 5 mg group, although this difference did not reach statistical significance.6 Conversely, a randomized clinical trial in 2003 of outpatients with venous thromboembolism and a mean age of approximately 55 years found that those receiving a 10 mg initial dose reached a therapeutic INR 1.4 days earlier than those receiving a 5 mg dose (P <0.001) and a higher percentage of patients on the 10 mg dose were therapeutic by day 5 (83% vs 46%, P <0.001). No differences in bleeding or INRs greater than 5 were noted between the groups.12 Therefore, the 9th Antithrombotic Therapy and Prevention of Thrombosis: American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines recommend the second approach with an initial dose of 10 mg daily for the initial 2 days for otherwise stable outpatients with no risk factors for excessive warfarin sensitivity, followed by dosing adjustments made based on INR measurements.10 Were hospitalized, elderly patients with comorbidities to receive the same dosage, it may result in over-anticoagulation, so more conservative doses may be warranted in such cases.
DOSING VARIABILITY
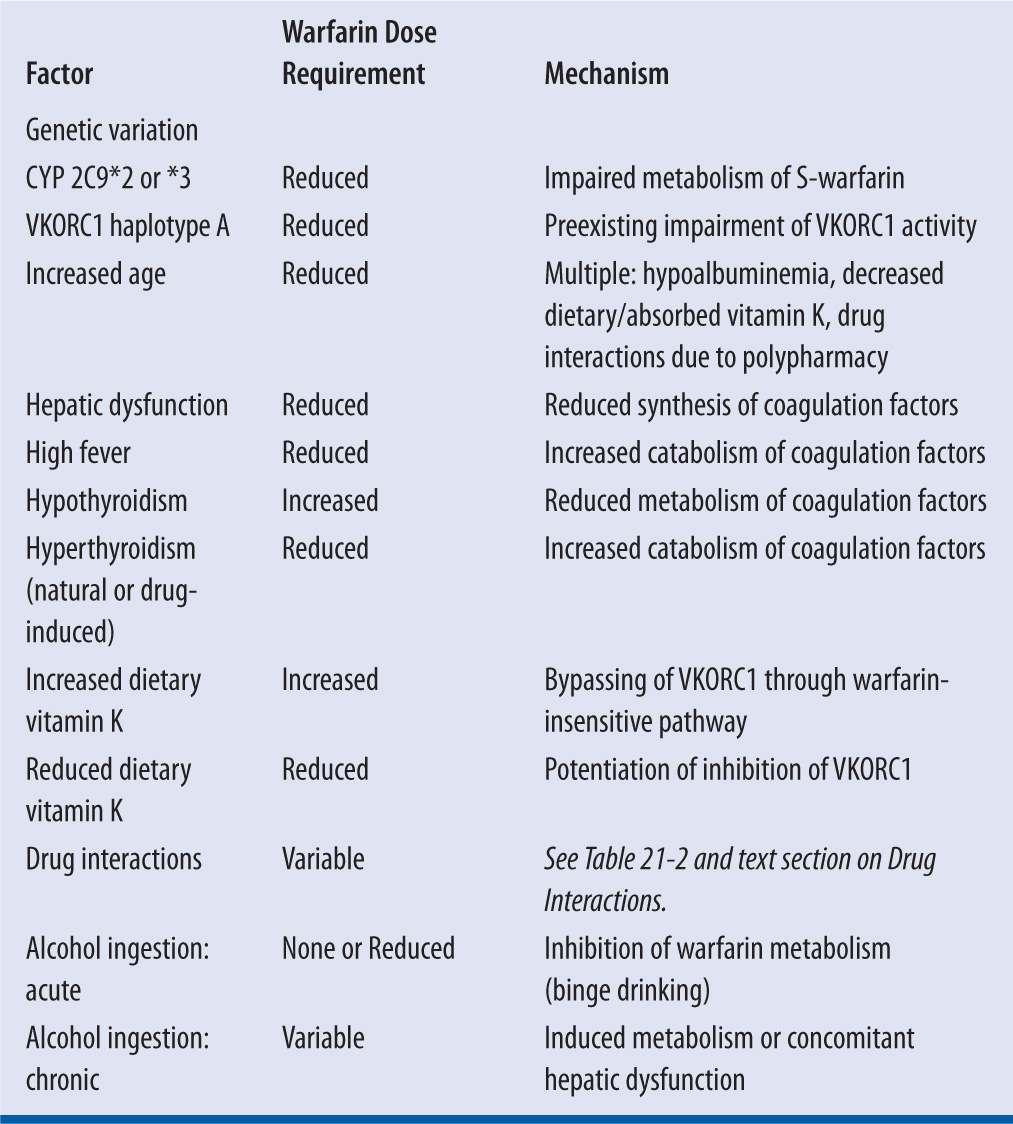
While warfarin therapy is individualized through monitoring of the INR response to specific dosage regimens in each patient, the selection of an initial dose, as a prediction of the resultant maintenance dose, is complicated by wide intra- and interpatient variability. Up to 10-fold interpatient variability has been reported,5,13 and many factors, both predictable and unpredictable, are responsible for the disparity (Table 21-1).4,5
| TABLE 21-1 | Factors That Increase or Reduce Warfarin Dose Requirements2,4,5,7,14 |
GENETICS
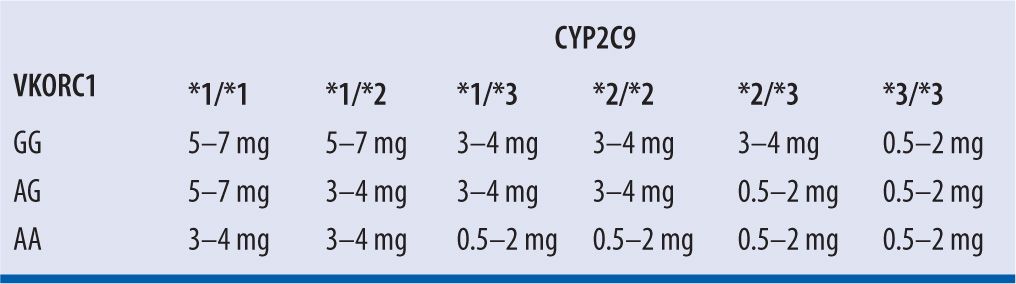
Because of significant genetic variability in CYP 2C9 enzymatic activity, individuals with CYP 2C9*2 and CYP 2C9*3 variant alleles have reduced enzymatic clearance of S-warfarin. Variability in VKORC1, resulting in baseline ineffective coagulation, exists as well.2 The Food and Drug Administration (FDA) suggests that genetic variations can be considered when a dosing regimen is selected, with expected maintenance doses based on genotype presentation provided by Bristol-Myers Squibb, manufacturer of Coumadin® brand of warfarin (Table 21-2)2; however, the current ACCP guidelines do not recommend genetic testing prior to warfarin initiation.10 Studies assessing the application of pharmacogenetic data to the selection of a warfarin dose have been conducted. The International Warfarin Pharmacogenetics Consortium13 conducted a validation cohort of 1,009 subjects and found that an algorithm incorporating variations in CYP 2C9 and VKORC1 was significantly better at correctly predicting the warfarin dose for patients requiring ≤21 mg (low dose) or ≥49 mg (high dose) of warfarin per week (49.3% vs. 33.3% and 24.8% vs. 7.2%, both P <0.001, respectively) as compared to a clinical algorithm without genetic information. However, no significant benefit was seen for patients requiring intermediate weekly doses. The authors propose that patients requiring low or high weekly doses of warfarin would benefit from such an algorithm since they may otherwise be over- or under-anticoagulated. On the other hand, in a prospective, observational study of 214 subjects, Li et al.5 found that even though accounting for genetic variations can assist with dose requirement prediction, in most cases the same information can be gleaned from close, expert monitoring of early INR response. Furthermore, not all variability is reflected by genetic testing and clinical evaluation of INR response would, therefore, be required in any case.
| TABLE 21-2 | Suggested Maintenance Warfarin Daily Doses Based on Genotypes2 |
AGE
It is widely accepted that the elderly have increased sensitivity to warfarin, requiring lower weekly doses than younger individuals.14 In a prospective and retrospective cohort including 2,359 subjects ≥80 years of age, Garcia et al.14 found that the average weekly warfarin dose decreased by 0.4 mg per year (95% confidence interval (CI), 3.8–5.3; P <0.001). The mechanism for this effect is not completely clear but may involve a combination of hypoalbuminemia, reduced vitamin K intake or GI absorption, and drug interactions due to polypharmacy.14 Furthermore, the time to achieve an anticoagulation effect is delayed by approximately 24–36 hours in the elderly.15 The elderly are also more susceptible to bleeding complications with warfarin use, and reversal of anticoagulation takes longer in the elderly as well.14
The standard nomograms often used for initiating warfarin therapy may overestimate the dose required for the elderly, placing them at risk for bleeding complications. Therefore, age-adjusted nomograms have been developed, including one by Gedge et al.15 In an age-stratified, randomized prospective study of 120 individuals over the age of 65, the investigators found that while their age-adjusted, low-dose warfarin induction regimen took longer to achieve a therapeutic INR than the comparator nomogram16 (patients 65–75 years of age: 4.6 ± 1.6 days vs. 3.8 ± 0.8 days, P = 0.03; patients >75 years of age: 4.5 ± 1.4 days vs. 3.5 ± 0.7 days, P = 0.03), use of the age-adjusted regimen resulted in a slightly longer duration of time spent in the therapeutic range (patients 65–75 years of age: 3.0 ± 1.3 days vs. 2.7 ± 1.3 days, P = 0.03; patients >75 years of age: 2.9 ± 1.1 days vs. 2.4 ± 1.3 days, P = 0.04) and significantly fewer INRs >4.5 in the first 8 days.
Roberts et al.17 hypothesized that the standard 5 mg starting doses, considered nonloading initiation doses at the time the study was conducted, may overshoot the actual maintenance dose requirement of elderly patients. They found that 73 patients (30 of whom were >75 years of age) dosed with an age-adjusted nomogram were able to quickly achieve a stable therapeutic INR without significant over-anticoagulation (INR >4.0). A nonsignificant trend of patients with albumin levels <3 gm/dL requiring lower doses was seen as well.
CLINICAL CONDITIONS
The clinical status of any individual can also change depending on comorbid conditions, concomitant medications, alterations in dietary vitamin K intake, and alcohol use. Hepatic dysfunction can ultimately result in reduced production of coagulation factors, causing an enhanced response to warfarin and increased risk of bleeding.4 A similar effect is seen with high fevers or hyperthyroidism, both hypermetabolic states that enhance degradation of coagulation factors.4 The metabolism of coagulation factors can be reduced in the hypothyroid state, requiring increased doses. This effect can disappear once the hypothyroidism is corrected and a euthyroid state is achieved.18
Inconsistent vitamin K intake can affect warfarin’s therapeutic effect. Excessive intake can counteract the desired effect of warfarin because dietary vitamin K can bypass VKORC1 through a warfarin-insensitive pathway. A diet deficient in vitamin K, which can result from decreased overall intake as seen with sick patients given antibiotics that deplete vitamin K-producing bacteria in the GI tract, fat malabsorption, or intravenous diets lacking adequate vitamin K supplementation, can be associated with increased sensitivity to warfarin’s effects.4 Because the INR represents the therapeutic effect of a given warfarin dosage regimen in a specific patient, and dosage adjustments are made to titrate warfarin to the desired INR, the actual quantity of vitamin K consumed, whether it is low, moderate, or high, is not relevant as long as the intake remains stable. With consistency comes a more predictable warfarin dose-INR response; therefore, large variations in the amount of vitamin K ingested should be avoided.
DRUG INTERACTIONS
Alterations in response to warfarin may result from interactions with numerous medications. Several distinct and overlapping mechanisms are responsible for these interactions as well as the inability to predict the outcome of the interactions precisely in many cases.7 Medications can affect the metabolic clearance of warfarin by affecting CYP 2C9, 2C19, 1A2, and/or 3A4. Inhibition of CYP 2C9 is most significant as it affects the more potent S-warfarin.
Interactions involving the GI tract can occur as well. Some medications may bind to warfarin in the GI tract, preventing or reducing its absorption and resultant efficacy. Broad-spectrum antibiotics, especially if used for a long duration, can impair endogenous vitamin K synthesis through disruption of the normal flora of the GI tract, potentiating the effect of warfarin.7
Pharmacodynamic interactions can result from coadministration of warfarin with agents that also impair hemostasis (antiplatelet or anticoagulant agents) or increase hemorrhagic potential (gastric irritants). The cumulative consequence of these additive effects is an increased bleeding risk.7
Finally, because warfarin is highly protein-bound, displacement interactions can enhance warfarin’s effects until equilibrium with serum concentrations can be reestablished.4,7
Given the known pharmacokinetic and pharmacodynamic mechanisms by which warfarin may interact with other agents, it is possible to anticipate potential drug-drug interactions (Table 21-3). Among the numerous published reports of interactions with warfarin, most are single case reports with confounding factors. Holbrook et al. performed an exhaustive review of the available evidence and summarized the likelihood of interactions occurring with select agents, but noted that lack of published data did not preclude the possibility of an interaction, especially in instances of theoretical mechanisms for interaction.7
| TABLE 21-3 | Select Drug Interactions with Warfarin7,19-21 |
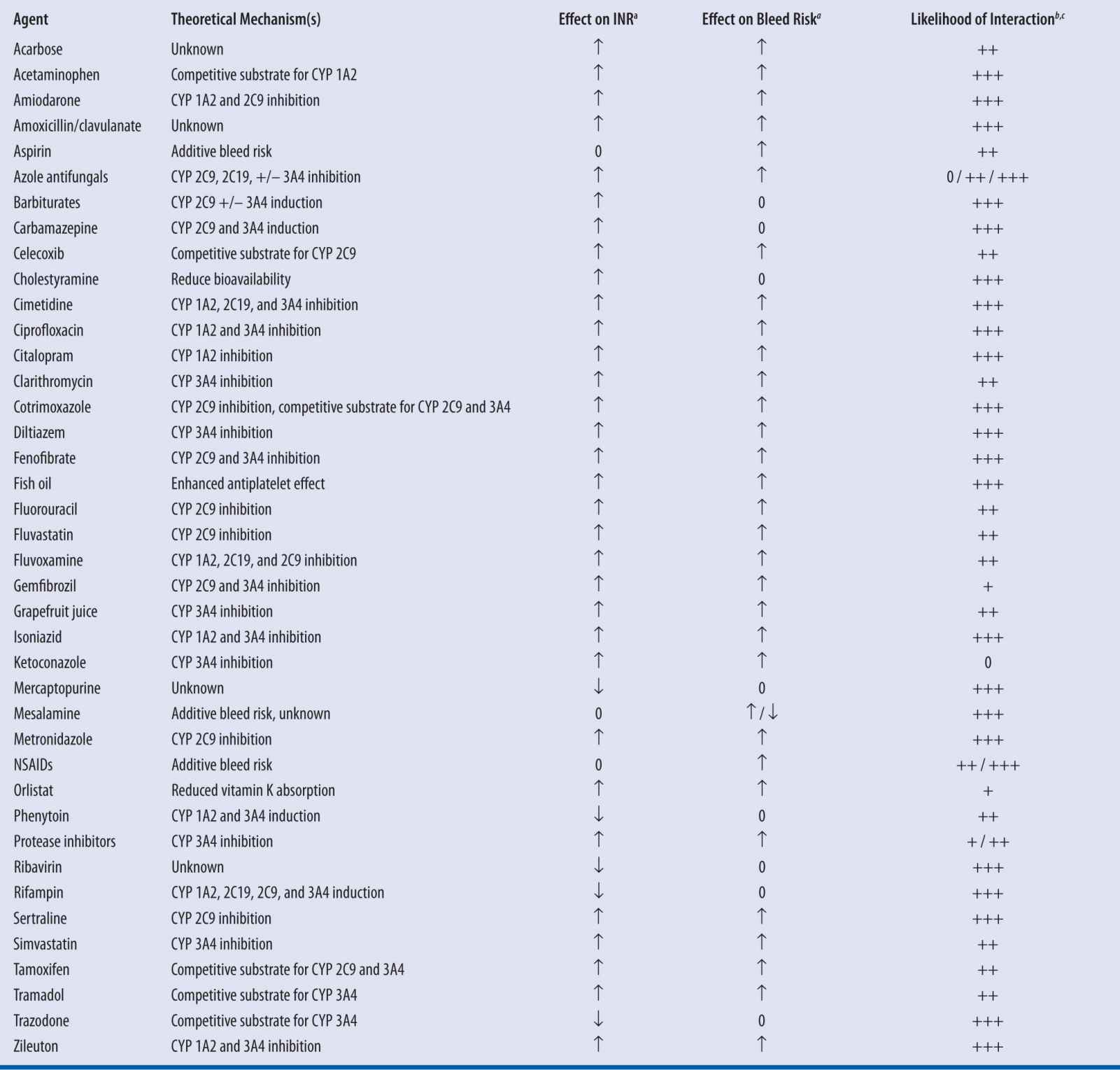
a↑, Increased; ↓, Decreased; 0, No effect
bEstimation based on systematic evaluation of published reports by Holbrook and colleagues7
c+++, Highly probable; ++, Probable; +, Possible7
Even though in theory it is best to avoid possible interactions, especially those considered major and highly probable, a risk versus benefit assessment in some situations may favor the use of potentially interacting medications. For interactions that may alter warfarin level and the INR, the INR test serves as a useful tool to measure the magnitude of the interaction so that in most cases, interactions are manageable with more frequent measurement of the INR, close monitoring for signs and symptoms of bleeding, and the reestablishment of an appropriate warfarin maintenance dose accounting for the interacting medication.7
MONITORING
Warfarin prolongs the PT, which reflects the extent of the inhibition of factors II, VII, and X. The test relies on the addition of calcium and thromboplastin to citrated plasma. However, due to variability in the potency of different batches of thromboplastin, the INR, a means of standardizing the PT values, is now used in lieu of PT. The INR calculation converts the ratio of a patient’s PT to that of the mean normal PT accounting for the responsiveness of the specific thromboplastin used in the laboratory conducting the assay of the VKA effect on coagulation factors. This responsiveness is the International Sensitivity Index (ISI) and the formula used is as follows4:
INR = (patient PT/mean normal PT)ISI
The ACCP guidelines recommend an INR between 2.0 and 3.0 for venous thromboembolic and atrial fibrillation indications and an INR between 2.5 and 3.5 for mitral valve replacement. Because the target range is the condition at which optimization of desired efficacy converges with acceptable toxicity risk, the time in target range (TTR) should be maximized.10
Historically, even though inpatients are generally monitored daily, for outpatient therapy, the INR is monitored once or twice weekly until the warfarin dose is determined and INR stability is achieved. This monitoring regimen is followed by testing every 4 weeks. The current ACCP guidelines recommend monitoring the INR at intervals of up to 12 weeks for patients with consistently stable INRs.10,11 This recommendation is partially based on a randomized, noninferiority trial of 250 patients at a stable warfarin dose for 6 months.22 The authors found no significant difference in TTR between the 4-week group and the 12-week group (74.1% ± 18.8% vs. 71.6% ± 20.0%; noninferiority P = 0.020). Significantly fewer dosage adjustments were necessary in the 12-week group and no significant differences in bleeding or thromboembolic complications. One caveat to this recommendation is that contact with anticoagulation staff was maintained at 4-week intervals, even for those with INR monitoring every 12 weeks.
A study conducted by Witt et al.23 describes clinical factors that can predict which patients may be more likely to have highly stable INRs and may be monitored on a less frequent basis. These factors included age over 70 and the absence of diabetes mellitus, heart failure, or concomitant estrogen therapy.
Should single outlying INRs occur during routine monitoring, a repeat INR should be conducted within 1 to 2 weeks to confirm whether dosage adjustments are clinically necessary.10
BRAND VERSUS GENERIC FORMULATIONS
Warfarin is a medication with a narrow therapeutic range, with the possibility of slight increases or decreases in therapeutic effect resulting in over-anticoagulation and bleeding or under-anticoagulation and thrombotic complications. Warfarin also requires individualized, careful titration of the dose to patient-specific therapeutic INR. Because of the serious consequences of slight variations in bioavailability for such medications, concern over the bioequivalence of different formulations of warfarin, including the differences between brand name and generic products, has arisen. A recent systematic review of relevant literature was conducted by Dentali and colleagues24 to assess whether significant consequences occur following switches between brand-name and generic warfarin. They found that overall, generic products that met FDA requirements for bioequivalence had no clinically significant differences in safety or efficacy when compared with brand-name products; INR results as well as thromboembolic and bleeding outcomes were evaluated. The authors recommended close monitoring should switches between products occur and switches should be infrequent so additional monitoring should not be necessary if patients remain at a stable warfarin dose. Available dose strengths are consistent between brand-name and generic manufacturers, as are the colors of the tablets (Table 21-4).
| TABLE 21-4 | Warfarin Dosage Strengths and Colors2 |

REVERSAL OF ANTICOAGULATION
EMERGENT REVERSAL FOR BLEEDING OR INCREASED BLEEDING RISK
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree