CHAPTER 7
Unfractionated Heparin and Low-Molecular Heparins
LIZ G. RAMOS, BS, PharmD, BCPS
AMY L. DZIERBA, PharmD, FCCM, BCPS
UNFRACTIONATED HEPARIN
OVERVIEW
Unfractionated heparin (UFH) is considered an indirect parenteral anticoagulant, as it has little or no intrinsic anticoagulant activity and works by potentiating the effect of antithrombin (AT), by UFH binding to it, and thereby inhibiting various activated clotting factors.1
UFH is a glycosaminoglycan found in the secretory granules of mast cells.2 UFH is a heterogeneous mixture of various lengths and properties. Each heparin molecule is made up of alternating D-glucuronic acid and N-acetyl-D-glucosamine residues with varying molecular size from 5,000 to 30,000 daltons (mean 15,000 daltons).3-5 The anticoagulant effect of UFH is mediated through a specific pentasaccharide sequence on the heparin molecule that binds to AT, which causes a conformational change to antithrombin.3 This UFH-AT complex inhibits the activity of factors IXa, Xa, XIIa, and thrombin (IIa). Only one-third of the heparin molecule possesses this unique pentasaccharide sequence with affinity to antithrombin. This complex is 100 to 1,000 times more potent as an anticoagulant compared to antithrombin alone.6 Through its action on thrombin, this UFH-AT complex also inhibits factors V and VIII. Not only does UFH prevent the growth of formed thrombus, it may also have effects on the patient’s own thrombolytic system.7 The factors that are most sensitive to this complex are IIa and Xa. Only molecules that contain >18 pentasaccharides can bind to both antithrombin and thrombin simultaneous. Conversely, molecules with as few as 5 pentasaccharides can inhibit factor Xa.8 In addition, heparin binds to platelets, thereby inhibiting platelet function by either inducing or inhibiting platelet aggregation, which may contribute to the bleeding effects of heparin by a mechanism independent of its anticoagulation effect.
Commercially available UFH preparations are derived from porcine intestinal mucosa.9
PHARMACOKINETICS
Due to its large molecular size and anionic structure, UFH is not absorbed reliably from the gastrointestinal tract when taken orally.2 Intramuscular (IM) injection is discouraged, given its erratic absorption. In addition, IM administration may result in hematomas. Therefore, the preferred route of UFH administration is either by a continuous intravenous (IV) infusion or by subcutaneous injection. It is recommended to give UFH as an IV bolus if an immediate anticoagulant effect is required rather than via a subcutaneous route, because its anticoagulant effect is seen in one to two hours.10
The bioavailability of UFH is dose dependant, which ranges from 30 percent at lower doses to as high as 70 percent at the higher doses. Therefore, if the subcutaneous route is chosen to deliver a dose, this dose should be higher than the usual intravenous dose.11,12 The bioavailability and anticoagulant activity of UFH is limited by the binding of UFH to a number of circulating plasma proteins such as platelet factor-4, macrophages, fibrinogen, lipoprotein, and endothelial cells, which may account for the inter- and intrapatient variability.13 The circulating plasma proteins levels can rapidly change in acutely ill patients or patients with active thrombosis. The volume of distribution of UFH is similar to blood volume (60 mL/kg) and binds extensively to low-density molecules. UFH does not cross the placenta and does not distribute in breast milk.10
The half-life of UFH is also dose-dependent and can range from 30 to 90 minutes or more in patients receiving high doses. Heparin clearance consists of a rapid saturable phase and slower first-order process. In the saturable phase, heparin binds to endothelial cells and macrophages, and once bound it is internalized and eliminated from the circulation. The slower nonsaturable phase is the renal elimination of heparin.14,15 Therefore, a disproportionate anticoagulant response may occur at therapeutic doses with the duration and intensity of anticoagulation rising nonlinearly with increasing dose. At therapeutic doses, a large proportion of heparin is cleared through the rapid saturable, dose-dependent mechanism. In addition, the apparent half-life of heparin increases from approximately 30 minutes after an IV bolus of 25 units/kg, to 60 minutes with an IV bolus of 100 units/kg, to 150 minutes with a bolus of 400 units/kg.8
UFH is used to treat various cardiovascular disorders including the prevention and treatment of arterial and venous thromboembolism, treatment of unstable angina, acute myocardial infarction, cardiac and vascular surgery, coronary angioplasty, stent placement and is also used as an adjunctive medication during thrombolysis.16 UFH still remains the anticoagulant of choice for any interventional or surgical procedures, despite the availability of newer agents on the market. It also remains the anticoagulant of choice for patients during pregnancy, given its favorable pharmacokinetics.
DOSING
The dose and route of UFH is dependent on the indication, the therapeutic goals and the patient’s response. For the prevention of venous thromboemolism, the recommended UFH dose is 5,000 units subcutaneous every 8 to 12 hours17 and a weight-based intravenous continuous infusion is preferred when immediate and full anticoagulation is required.8 The efficacy of heparin in the initial treatment of VTE is critically dependent on dose.
In a randomized trial by Raschke and colleagues,18 patients received heparin at fixed doses (5,000-unit bolus followed by 1,000 units/h by infusion) or adjusted doses using a weight-based nomogram (starting dose, 80 units/kg bolus followed by 18 units/kg/h by infusion). This trial showed that while patients receiving UFH via a weight-based nomogram received higher doses within the first 24 hours than those given fixed doses of heparin, the rate of recurrent thromboembolism was significantly lower with the weight-based UFH regimen. The 2012 guidelines from the American College of Chest Physicians8 recommend that the initial parenteral heparin dosing for VTE be administered as weight-based (80 units/kg IV bolus and 18 units/kg/h IV infusion). If continuous intravenous heparin administration is not possible, then it is recommended to administer UFH subcutaneously SC via two options: (1) an initial IV bolus of 5,000 units followed by 250 units/kg SC twice daily19; or (2) an initial SC dose of 333 units/kg followed by 250 units/kg SC twice daily thereafter.20 For the treatment of acute coronary syndromes, the recommended doses are much lower than that used for VTE. The American College of Cardiology21 recommends a heparin bolus of 60 to 70 units/kg (maximum 5,000 units) followed by an infusion of 12 to 15 units/kg/h (maximum 1,000 units/h) for unstable angina and non-ST-segment elevation myocardial infarction. If UFH is used in combination with a fibrinolytic agent for the treatment of ST-segment elevation myocardial infarction, the recommended dose is 60 units/kg (maximum 4,000 units) as a bolus and the infusion is 12 units/kg/h (maximum of 1,000 units/kg/h).22
MONITORING
The risk of heparin-associated bleeding increases as the UFH dose increases23,24 and if used in conjunction with other antithrombotic agents such as fibrinolytic agents25 or glycoprotein IIb/IIIa inhibitors.26 This risk also increases when patients have had recent surgery or other invasive procedures or have other comorbidities that can worsen hepatic function. The dose of UFH is adjusted based on the activated partial thromboplastin (aPTT), while the evidence is weak to maintain a “therapeutic range” (which is hospital-specific and only based on a subgroup analysis). A strong correlation is found, however, between subtherapeutic aPTT value and recurrent VTE, but a relationship between a supratherapeutic aPTT value and bleeding is not as clear.27 The activated clotting time can be used to monitor the higher heparin doses given to patients undergoing percutaneous coronary interventions or cardiopulmonary bypass surgery. The recommended aPTT ratio that has been associated with a reduced risk of recurrent VTE is between 1.5 and 2.5 times control.28
As stated before, this range has not been confirmed by any randomized trials, but has been accepted as the standard. The therapeutic aPTT ranges vary from hospital to hospital as it is dependent on the various reagents and instruments used to measure the aPTT. Therefore, no weight-based heparin nomogram is the same from hospital to hospital, nor can they be applied to all reagents; each hospital must determine there own anti-Xa assay dependent on the reagent being used. One study established a therapeutic range for an aPTT ratio of 1.5 to 2.5 that corresponded to a heparin level of 0.2 to 0.4 units by protamine titration and a heparin level of 0.3 to 0.7 units measured by an anti-Xa assay.28 Again, given the variability between aPTT and anti-Xa assays between each laboratory, more research is needed to identify which is the best monitoring tool for UFH. Therefore, in 2012, the American College of Chest Physicians8 recommended that each lab calibrate specifically for each reagent/coagulometer in determining aPTT values and correlating these values with therapeutic UFH levels. Prior to initiating therapeutic UFH, it is recommended to obtain a baseline PT, aPTT, CBC with platelet count, and subsequent aPTT levels every six hours after initiation and for any dosing changes, until therapeutic aPTT values have been achieved.
ADVERSE EFFECTS
A well-known adverse effect of UFH is thrombocytopenia in addition to hemorrhagic complications. The relationship between supratherapeutic aPTT and bleeding has not been clearly delineated.27 The occurrence of bleeding complications in patients receiving UFH ranges from 1.5 to 20 percent with an increased risk in patients with preexisting risk, which includes renal or liver disease, malignancy, and age greater than 65 years, to name a few. Thrombocytopenia is defined as platelet count of <150,000/mm3. Of the two types of heparin-induced thrombocytopenia (HIT), the first type of HIT presents within the first 2 days after exposure to heparin, and the platelet count normalizes with continued heparin therapy. It is well known that this type of HIT is a nonimmune disorder, and occurs with the direct effect of heparin on platelet activation. The second type of HIT is an immune-mediated disorder that typically occurs 4–10 days after exposure to heparin, and it has life-threatening prothrombotic complications if not quickly identified.29
Immune-mediated HIT should be suspected when a patient has a fall in platelet count while receiving heparin—particularly if the fall is more than 50 percent of the baseline count, even if the platelet count >150,000/mm3—and is evidenced by skin lesions at heparin injection sites. To help clinicians with determining the probability that a patient has this type of HIT, the 4Ts score has been developed and the most studied.30-32 This pretest clinical scoring system, helps clinicians in the diagnosis of HIT, but should never be used alone. Other nonhemorrhagic side effects are uncommon and include skin reactions that can progress to necrosis, alopecia, and hypersensitivity reactions manifested by chills, fever, pruritus, or anaphylactoid reactions. Other adverse effects that are seen with UFH therapy include elevations of serum transaminases, but are usually transient, and osteoporosis. Osteoporosis is caused by binding of heparin to osteoblasts, which has been reported in patients receiving long-term (>6 months) large daily doses of UFH.
REVERSAL OF ANTICOAGULANT EFFECT OF UFH
Given all the new anticoagulants coming to market, UFH has one advantage over these new agents in that protamine can rapidly reverse its anticoagulant effect. Protamine sulfate is a basic protein derived from fish sperm that binds to heparin to form a stable salt. The recommended dose is 1 mg of protamine sulfate to neutralize approximately 100 units of heparin.8 For example, if a patient bleeds immediately after receiving an IV bolus of 4,000 units of heparin then this patient should receive about 40 mg of protamine sulfate. Protamine sulfate is quickly cleared from circulation with a half-life of about 7 minutes.
As stated previously, the half-life of IV heparin is about 60–90 minutes when heparin is given as an IV infusion. The calculated dose of protamine that needs to be administered should take into account the amount of heparin only given in the previous few hours. If a patient is receiving UFH as a continuous IV infusion at 2,000 units/h, then you require approximately 50 mg of protamine sulfate to neutralize the heparin that was given in the past 2–2.5 hours. It is important that the correct dose of protamine be used to reverse UFH, because protamine can exert its own anticoagulant effect if used in doses larger than required. Additional protamine adverse effects include hypotension or bradycardia, which can be minimized by administering protamine no faster than 5 mg/min.9
LOW-MOLECULAR-WEIGHT HEPARIN
OVERVIEW
The development of low-molecular-weight heparin (LMWH) from either chemical or depolymerization of UFH has increased clinical options for the management of thromboembolic disorders. LMWHs primarily exert their anticoagulant effect by inactivating active factor X with less affinity for thrombin.8 Each LMWH has a different amount of antifactor Xa activity; therefore, each preparation should be considered an individual drug that cannot be used interchangeably.
It is well-established that LMWHs are approximately one-third the molecular weight and exhibit decreased binding to macrophages, endothelial cells, platelets, and platelet factor 4.8 These differences offer several advantages of LMWHs when compared to UFH. These advantages include a more predictable pharmacokinetic response, improved subcutaneous bioavailability, longer half-life, and lower incidence of HIT. As a result, LMWHs have largely replaced UFH for the prevention and treatment of venous thromboembolism (VTE) and in the management of unstable angina and non-ST elevation myocardial infarction (NSTE-MI).
PHARMACOKINETICS
After SC administration, the bioavailability of LMWH approaches 100 percent.33 Low-molecular-weight heparins predominately concentrate in the plasma and highly vascular tissues with little distribution in fat tissue.34 The half-life of LMWHs is approximately 3–6 hours after SC administration, significantly longer as compared to UFH. Antifactor Xa activity persists longer than antifactor IIa activity, reflecting the more rapid clearance of longer heparin chains.35 The peak anticoagulant effect is observed with LMWHs 3–5 hours after administration.8 Even though UFH is mainly cleared by a cellular mechanism, LMWHs are strongly dependent on the renal route for elimination. Renal impairment will lead to a reduction in clearance with a subsequent increase in elimination half-life and augmented anticoagulant activity leading to an increased risk of bleeding.
MONITORING
Routine monitoring of traditional measures of coagulation is not necessary for the vast majority of patients receiving LMWHs as a result of the predictable anticoagulant response. Baseline coagulation factors along with a complete blood count and serum creatinine should be obtained at the initiation of a LMWH and periodically throughout therapy.8 Monitoring of plasma anti-Xa activity is the recommended test if monitoring is desired; however, it is not essential in patients with stable and uncomplicated conditions.36,37 Peak anti-Xa level, drawn four hours after SC administration, is dependent on the drug and dosing interval. Target anti-Xa levels for twice daily treatment of VTE with enoxaparin are 0.6–1.0 units/mL.37,38 Target anti-Xa levels for once daily administration of enoxaparin, dalteparin, or tinzaparin are >1.0 units/mL, 1.05 units/mL, and 0.85 units/mL, respectively.37,39
GENERAL DOSING
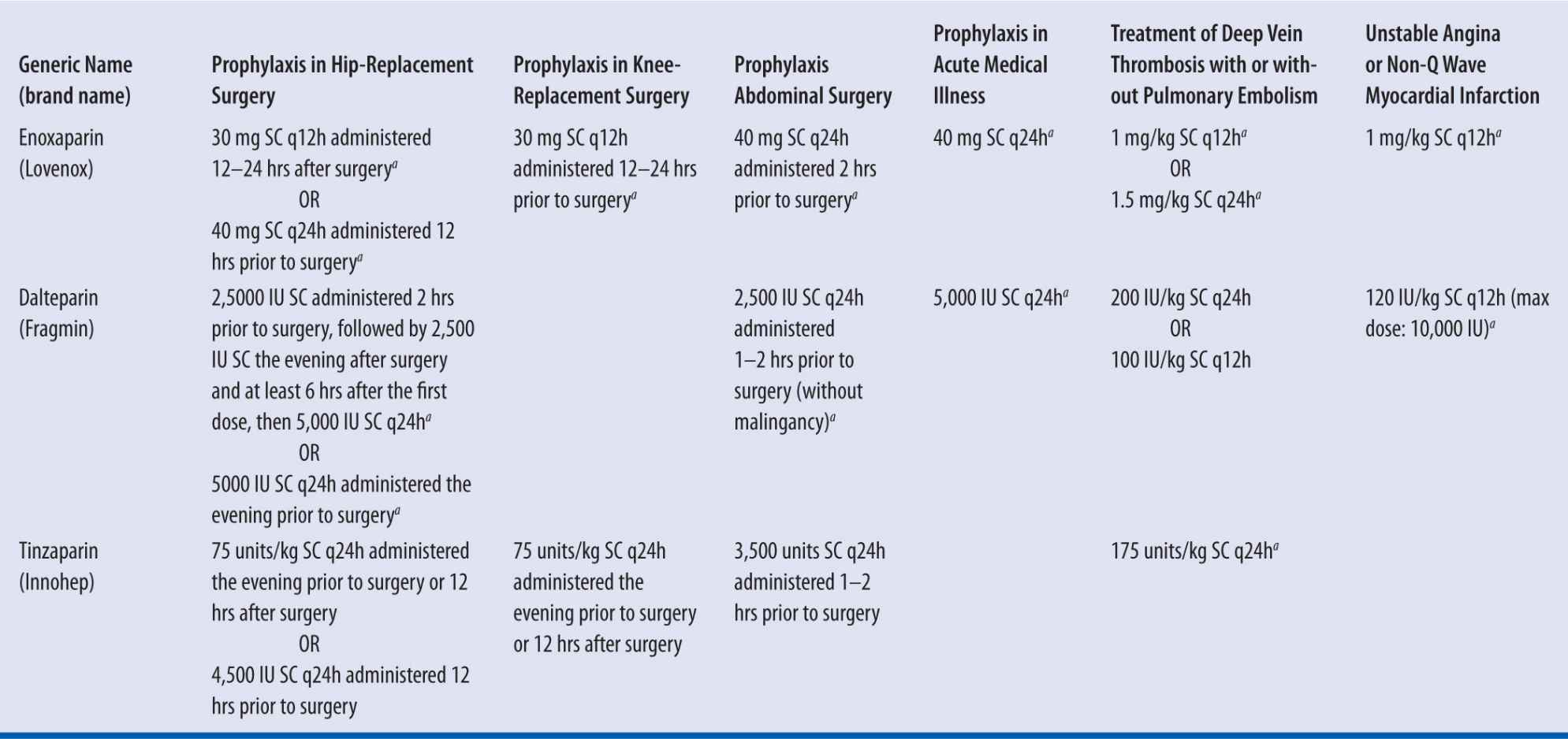
Fixed or weight-based dosing for LMWH is based on product and indication (see Table 7-1). For each product, doses should be based on actual body weight. It is important to note that enoxaparin dosing is expressed in milligrams, whereas dalteparin and tinzaparin are expressed in units of antifactor Xa activity.40-42
| TABLE 7-1 | Dosing and Indications of LMWH |
aFDA approved dose
Dosing Considerations in the Obese Patient
Obese patients, defined as individuals with a body mass index (BMI) of greater than 30 kg/m2, have a lower proportion of lean body mass as compared to their total body weight.43 Controversy surrounds the appropriate dosing in this patient population because of a paucity of data on the optimal dosing strategy of LMWHs.
Two retrospective subgroup analyses of obese patients receiving a fixed prophylactic dose of enoxaparin or dalteparin did not demonstrate a significant difference in VTE occurrence compared with placebo in nonobese patients.44,45 However, unadjusted prophylactic doses of LMWHs may be insufficient to prevent VTE in this patient population because of an inverse correlation between total body weight and antifactor Xa activity.46 A prospective study of bariatric surgical patients demonstrated a lower incidence of DVT in patients receiving a higher dose of enoxaparin without an increase in bleeding rate.47 A small nonrandomized trial demonstrated effectiveness without increased bleeding when enoxaparin was dosed per weight for the prevention of thromboembolism in medically ill, morbidly obese patients.48 Based on these and other clinical studies, increasing VTE prophylactic doses of enoxaparin or dalteparin may be appropriate in obese patients, especially the morbidly obese bariatric surgical patient.46,47,49,50
Small studies including obese patients treated for VTE using enoxaparin or dalteparin have demonstrated that dosing regimens based on actual body weight were effective without an increase in bleeding events.51,52
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree