CHAPTER 22
Erythropoietin-Stimulating Agents
TIMOTHY NGUYEN, PharmD, FASCP, BCPS, CCP
VIJAY LAPSIA, MD
SARA S. KIM, PharmD, BCOP
OVERVIEW
Erythropoietic stimulating agents (ESA) are recombinant and synthetic erythropoietin (EPO). They are structurally and biologically similar to endogenous EPO and are used in the management of various types of anemia. Drugs in this class are epoetin alfa, darbepoetin alfa, and peginesatide. ESAs work by stimulating the bone marrow to produce red blood cells. Epoetin alfa, sometimes referred to as recombinant human erythropoietin (rHuEPO), is an exogenous EPO manufactured by recombinant DNA technology, and it was approved by the Food and Drug Administration (FDA) in 1989. It contains a 165-amino acid sequence with three N-linked and one O-linked carbohydrate changes and has the same biological effects as endogenous EPO.1,2 It has a molecular weight of 30,400 daltons and is produced by mammalian cells.
Darbepoetin alfa (DA), a hyperglycosylated epoetin alfa analogue, contains five N-linked carbohydrate chains, two more than epoetin alfa, and has the same mechanism of action as rHuEPO.3,4 Compared with epoetin alfa, darbepoetin alfa has a threefold increased serum half-life and allows extended dosing intervals.4-6 The additional N-linked carbohydrate chains increased the molecular weight of darbepoetin from 30.4 to 37.1 daltons, and the carbohydrate contribution to the molecule corresponding increased from 40 percent to approximately 52 percent.7
Peginesatide is a synthetic pegylated peptide. It stimulates the EPO receptor similar to the endogenous hormone EPO and rHuEPO. Peginesatide is produced using chemistry rather than recombinant DNA technology. Its amino acid sequence is completely different from EPO and yet still able to activate the EPO receptor and stimulates erythropoiesis.8 In the summer of 2014, the manufacturer suspended peginesatide production due to post marketing reports of serious hypersensitivity reactions that may be life-threatening or fatal.
The FDA approved ESAs for the treatment of anemia resulting from chronic kidney disease, anemia in certain types of cancer patients receiving myelosuppressive chemotherapy, certain treatments for human immunodeficiency virus (HIV), and also to reduce the number of blood transfusions during and after certain major surgeries.8,19,20 ESAs are also reportedly being used off-label for various conditions, including myelodysplastic syndrome,9 critically ill patients,10,11 chronic heart failure,12 anemia of chronic disease,13 chronic hepatitis C virus infection,14 and anemia in low birth weight infants.15
PHARMACOKINETICS
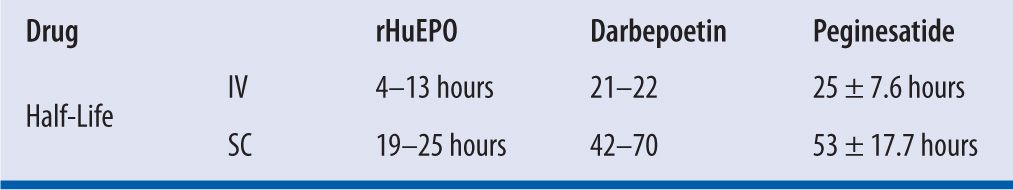
rHuEPO has a relatively short terminal half-life of 4–8 hours in humans, and it needs to be administered two to three times a week.16 Compared with rHuEPO, the EPO analogue darbepoetin-alfa carries two additional glycosylation sites that permit a higher degree of glycosylation. Consequently, darbepoetin alfa has a longer half-life of 25.3–48.8 hours in humans,5 and greater in vivo biological activity, which allows for less frequent administration.17 Unlike rHuEPO or darbepoetin alfa, peginesatide comprises a peptide sequence that is dimerized and linked to a two-branched 20-kDa PEG moiety, thus prolonging systemic circulation and reducing enzymatic degradation.18 This characteristic permits a once-monthly dosing schedule. (See Tables 22-1 through 22-4.)
| TABLE 22-1 | ESAs Pharmacokinetics Data8,19,20 |
| TABLE 22-2 | ESAs Dosing Information8,19,20 |
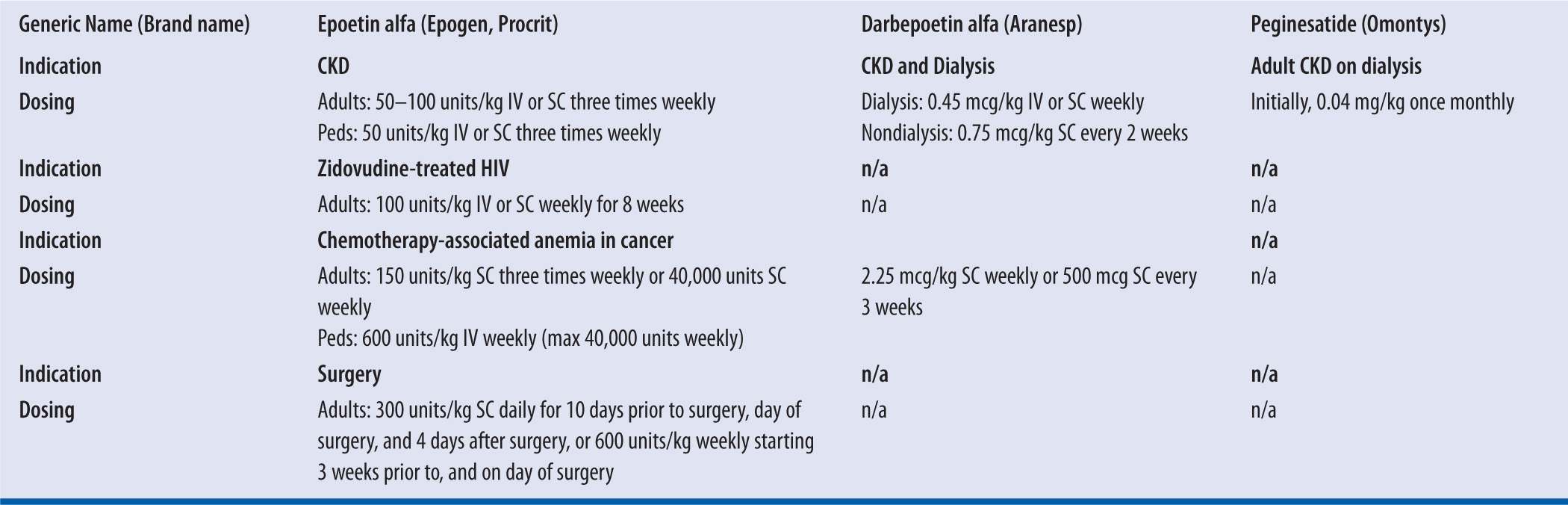
IV, intravenous; kg, kilogram; SC, subcutaneous
| TABLE 22-3 | Adult Dosing Conversion Between EPO and DA8,19 |
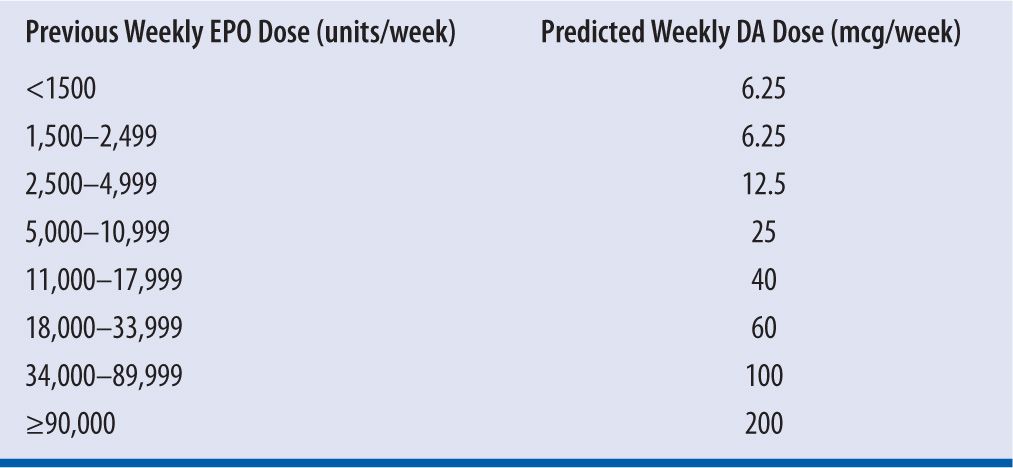
EPO, rHuEPO; DA, darbepoetin alfa
| TABLE 22-4 | Adult Dosing Conversion from EPO, DA, to Peginesatide8,19,20 |
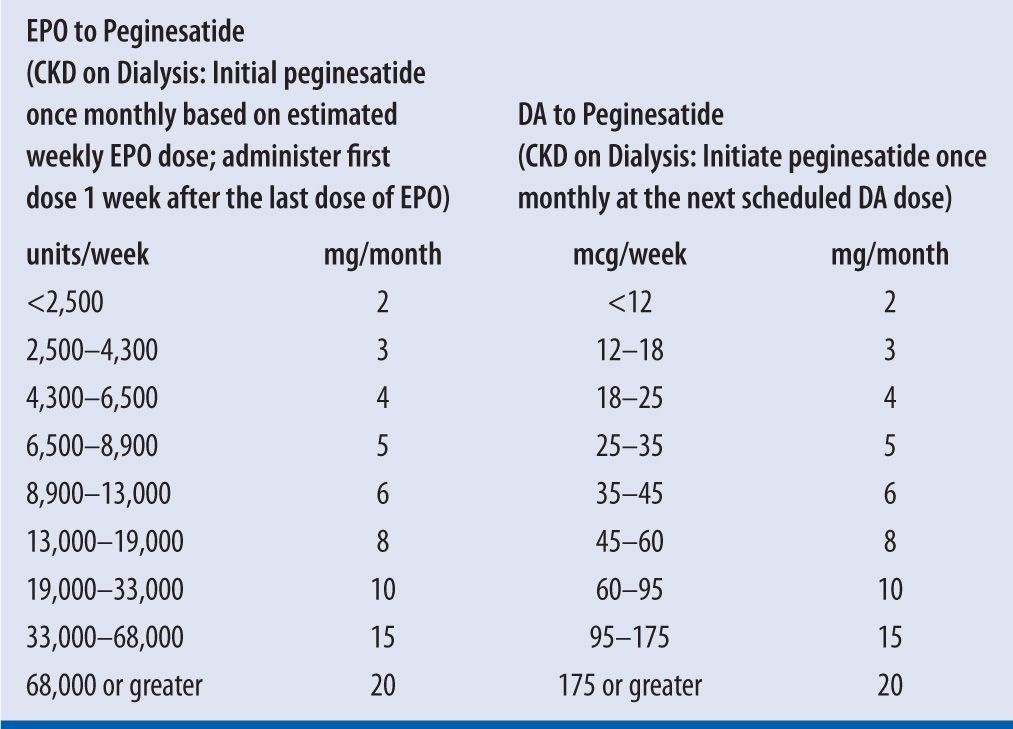
CKD, chronic kidney disease; EPO, rHuEPO; DA, darbepoetin alfa
DOSING CONSIDERATIONS
Adequate iron supply is necessary for maintaining an optimal response to ESA therapy. Prior to and during ESA therapy, a patient’s iron stores, including transferrin saturation (TSAT) and serum ferritin, should be evaluated. Virtually all patients will eventually require supplemental iron to increase or maintain TSAT to levels that will adequately support erythropoiesis stimulated by ESA. The FDA recommends using the lowest dose possible to avoid RBC transfusions.
rHuEPO administered subcutaneously (SC) is more efficacious than intravenous (IV) administration. The required dose of rHuEPO administered via IV is usually 25 percent to 30 percent higher than SC administration to achieve a similar erythropoietic response. The reason is thought to be due to a rapid decline in rHuEPO concentration (i.e., below the threshold necessary for erythropoiesis) after IV administration.21 In contrast, dosage requirements of darbepoetin-alfa do not appear to differ between the IV and SC routes of administration. The dose of DA is apparently equivalent between the IV and SC administration.
Dose reduction or increase by approximately 25 percent is suggested to maintain goal Hb levels. Significant variability in responsiveness in various patient populations makes it wise to individualize patient treatment (Table 22-5).
| TABLE 22-5 | Monitoring |
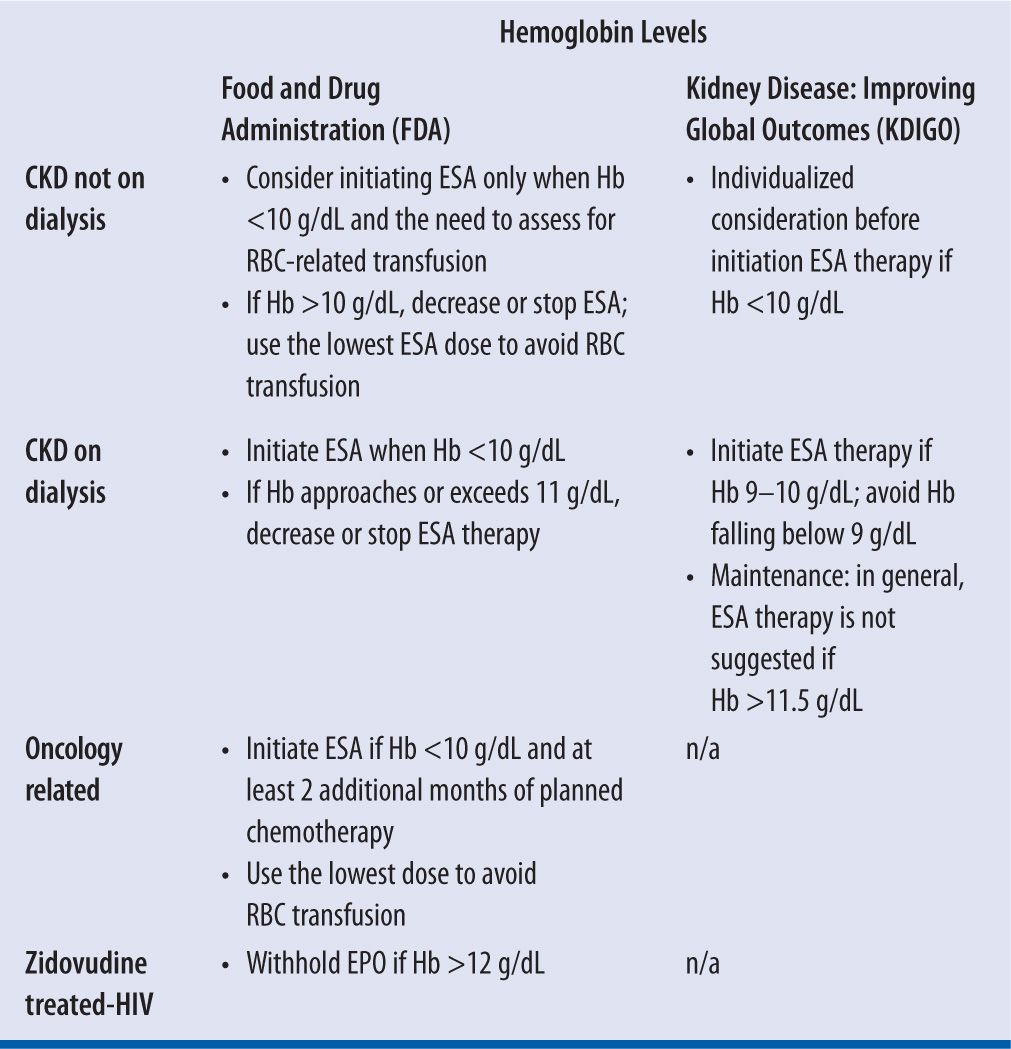
In normal subjects, plasma EPO levels range from 0.01 to 0.03 units/mL and increase 100- to 1,000-fold during hypoxia or anemia.22 Responsiveness to rHuEPO therapy in HIV-infected patients is dependent on the endogenous serum EPO level prior to treatment. Patients with endogenous serum EPO levels ≤500 mUnits/mL and receiving a dose of zidovudine ≤4,200 mg/week may respond to rHuEPO therapy. Patients with endogenous EPO levels >500 mUnits/mL do not appear to respond to rHuEPO therapy.19 In patients with CKD, serum EPO level is expected to be low, therefore it has no diagnostic value. Neither does it influence the starting dose or any adjustment in dosing of ESAs in such patients; these agents may have some use, however, in patients with anemia secondary to chronic illness. In addition, regression of left ventricular hypertrophy (LVH) is a known benefit of initiation of treatment with ESAs.
The FDA also stated that health care professionals who prescribe ESAs for anemia in cancer patient for chemotherapy-induced anemia must (1) complete a online training module that covers the use of ESAs; (2) be enrolled in the ESA APPRISE (Assisting Providers and Cancer Patients with Risk Information for the Safe Use of ESAs) Oncology program following completion of the training module and obtain provider enrollment number; (3) sign the patient/health care professional acknowledgment form prior to initiation of ESA therapy; and provide a copy of the signed consent form to the patient.23
Other monitoring parameters include hemoglobin (Hb), iron indices, folic acid, cyanocobalamin levels, and red blood cells profiles.
SIDE EFFECTS
All ESAs prescribed must be part of a Risk Evaluation and Mitigation Strategy (REMS) to ensure the safe use of these drugs.
The FDA mandated that the patients be provided with material and guidance to understand the risks associated with ESAs. For patients with cancer, these risks include the following:
1. ESA may cause tumors to grow faster.
2. ESA use may be associated with earlier death.
3. ESAs may cause some patients to develop blood clots and serious heart problems such as a heart attack, heart failure, or stroke.
For this reason, ESA therapy is not indicated for treatment of chemotherapy-induced anemia (CIA) if the patient is receiving chemotherapy for curative intent.
Patients with or without cancer should be informed that the use of ESAs can increase the risk of stroke, heart attack, heart failure, blood clots, and death. All patients receiving ESAs are instructed to read the medication guide to understand the benefits and risks of using an ESA, and to discuss with their health care professional any questions they may have. Also, patients with cancer will need to sign an acknowledgment form that states that they have talked with their health care professional about the risks associated with ESAs. This form must be signed before patients begin a course of ESA treatment. Patients without cancer will be asked to get blood tests while using ESAs to help guide the course of therapy and lower the risk of serious adverse events.
Side effects include vascular-access thrombosis, seizures, hypertension, and others. ESAs are rated pregnancy category C. In addition, some patients have developed anti-EPO antibodies due to immunogenicity, which can reduce the benefit of taking epoetin alfa.17 ESA-related pure red cell aplasia (PRCA) is a rare but potentially life-threatening condition. The presence of neutralizing antibodies has been observed and most cases have been associated with darbepoetin and rHuEPO given SC in patients with CKD, and treated for hepatitis C infection with interferon and ribavirin.17,19
CONTRAINDICATIONS/WARNINGS
CONTRAINDICATIONS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


