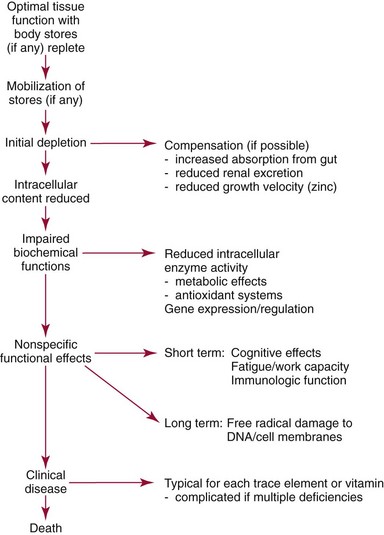
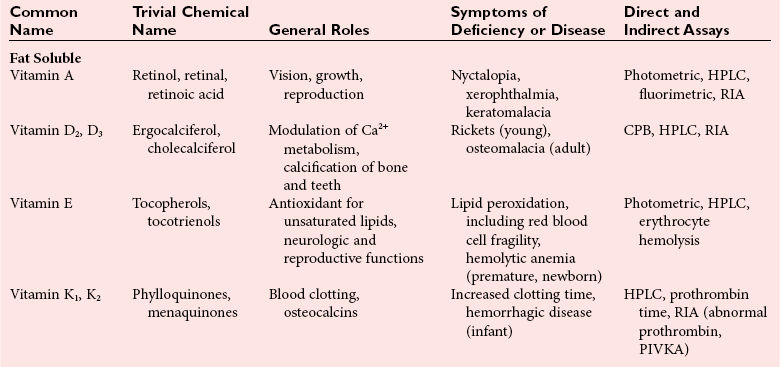
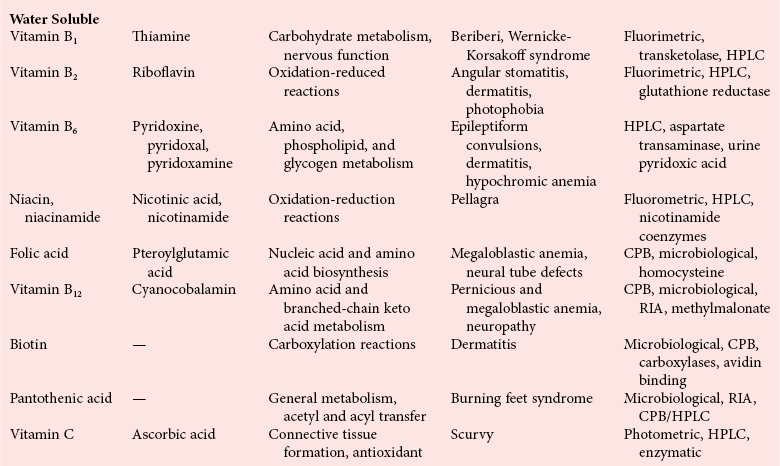
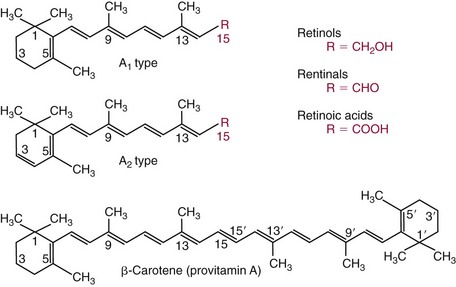
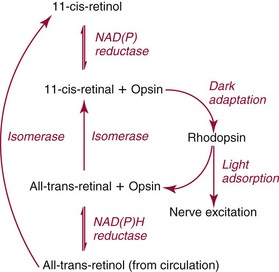
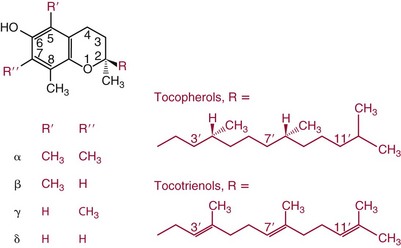
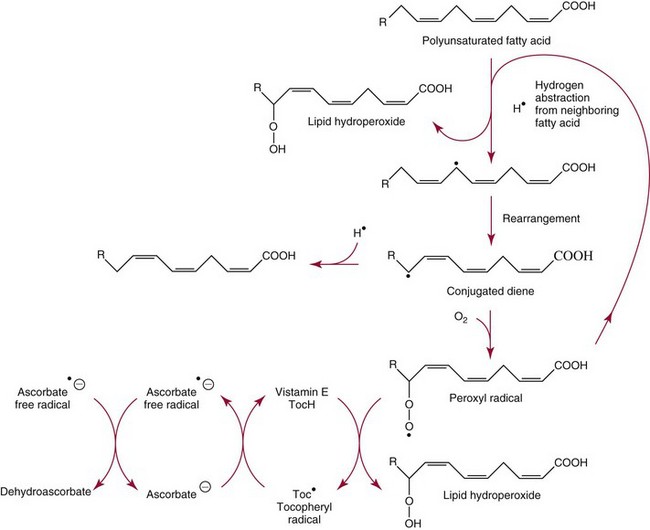
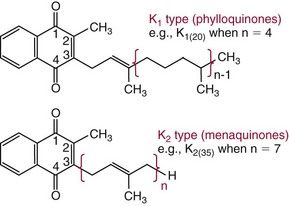
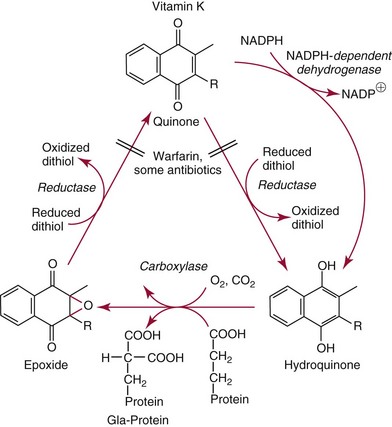
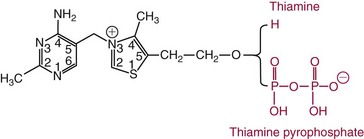
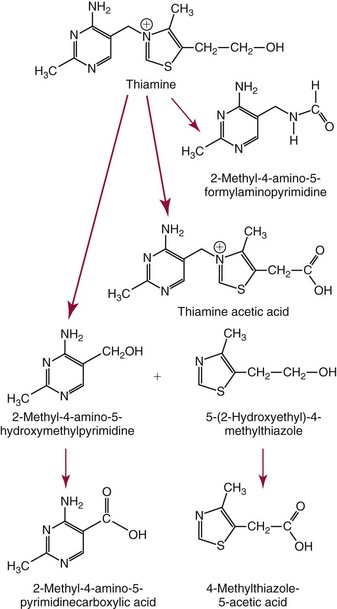
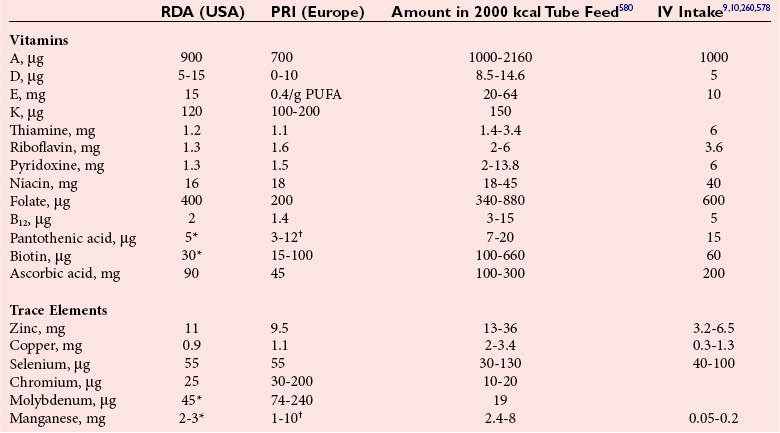
Chapter 31 Accurate assessment of supply and intake is a complex procedure. In practice, a crude estimate of intake is obtained from a careful clinical history taken by an experienced practitioner or from a food frequency questionnaire that summarizes the content of the individual’s diet over several days, depending on how frequently particular typical foods are consumed.60 A more accurate quantitative assessment usually requires a minimum of 3 days’ recording of a complete dietary diary, which is subsequently analyzed using a computer program with reference tables of the nutritional contents of most foods.450 Unfortunately, estimates of portion size, amounts consumed, and actual nutritional composition of the food consumed may be inaccurate. In addition, the disease process affects the amount actually consumed and absorbed, further reducing the accuracy of the estimate of nutritional intake. The requirements for most nutrients to maintain health have been characterized and made available in reports from the Institute of Medicine (IOM) of the National Academies (http://www.iom.e).186–190 However, the effects of disease may increase demands. For example, hypermetabolism, as a result of trauma or infection, increases the need for protein and energy and for the vitamin and trace element cofactors necessary for their metabolism.585 Increased losses from the gut, kidney, and skin, or through dialysis, may also increase the overall demand for these nutrients. An estimate of supply also is obtained from a careful dietary history, especially if performed by a dietitian, together with knowledge of any artificial nutritional supplements or therapy that may have been provided enterally or intravenously. Table 31-1 summarizes the Recommended Dietary Allowance (RDA) used in the United States and the Population Reference Intakes (from the European community) for vitamins and trace elements. These amounts are expected to be present in the normal diet of healthy adults. Table 31-1 also summarizes the amounts present in 2000 kcal of most tube feeds used in nutritional support. It is clear that the amounts used enterally are greater than the oral amounts recommended in health so as to meet increased needs resulting from preexisting deficiencies and increased ongoing requirements resulting from disease. The amounts recommended for supply during intravenous nutrition are also summarized in Table 31-1. For the trace elements, these amounts are generally less than the oral and/or enteral requirements to allow for reduced absorption enterally. For the vitamins, these amounts are usually greater than the oral and/or enteral requirements to allow for the effects of disease. TABLE 31-1 Oral and Intravenous Micronutrient Intakes for Adults PRI, Population reference intake (Europe)517; RDA, recommended dietary allowance (U.S.).186,188,190 Reference intakes for infants and children are age and weight dependent and are summarized in various sources.186,188,190,225,589 In an attempt to improve accuracy of assessment of nutritional status, clinicians often turn to the laboratory to obtain a result that may reflect the net balance of supply and demand.583,585 Clinical laboratorians need to be aware of when such tests are useful and how to place the results of laboratory tests into the context of the clinical situation of the patient. It is important to be aware of the limitations of laboratory tests, especially in acutely ill patients. In this brief overview, the nutrients used in nutritional assessment and in monitoring will be briefly discussed.* In clinical practice, only a few laboratory tests are of value in the assessment of protein-energy status583,587 and it is particularly important to recognize that serum protein concentrations are not helpful in sick patients with any form of inflammatory process (see Chapter 21). Although serum albumin is often measured and reported as an indicator of protein-energy status, factors such as increased transcapillary escape182 and reduced hepatic synthesis make it of little value as a nutritional marker. Serum albumin is, however, a valuable prognostic marker and is frequently used as part of prognostic indices.97 Short half-life proteins such as transthyretin (TTR) (prealbumin) also may be of value in patients with no inflammatory response.182,286 Although some workers have suggested that TTR may reflect protein-energy status in patients with an inflammatory response,149 it seems more likely that it helps to identify the patient who is at risk of becoming malnourished as a result of their illness.586 Assessment of nitrogen balance may be used to obtain information on recent protein-energy status. This requires careful 24-hour urine collection and estimation of total nitrogen content,335 or urea nitrogen (from which total nitrogen usually can be approximately estimated).208 With a reasonable calculation of protein (nitrogen) intake, intake minus urine output (together with some unmeasured losses) gives an estimate of nitrogen balance. In practice, however, such measurements are now rarely required except when (1) patients appear to have excessive losses, (2) they are failing to respond to what appears to be adequate nutritional support therapy, or (3) a novel therapy is being evaluated for its effectiveness in limiting catabolism or stimulating anabolism. Although requirements for vitamins and trace elements in health are known (see Table 31-1), the effects of illness on these requirements are poorly understood and quantified. However, it is now apparent that as an individual develops progressively more severe depletion in vitamin or trace element status, they pass through a series of stages with biochemical or physiologic consequences. The metabolic or physiologic penalty of such suboptimal nutritional status usually is not clear, but the assumption remains that suboptimal metabolism is likely to have detrimental effects [e.g., subclinical deficiency of folic acid is associated with an increase in serum homocysteine concentration, which has been proposed to be an independent risk factor for coronary artery disease (see Chapter 27)], although folate supplements do not reduce this risk.389 Similarly, subclinical deficiency of chromium may be associated with impaired glucose tolerance in certain types of diabetes.13 The time course for development of a subclinical deficiency state varies for each individual vitamin and trace element and depends on the nature and quantity of body stores. Moreover, the extent of depletion necessary before significant biochemical, physiologic, or histologic changes occur is poorly characterized. The consequences of an inadequate intake are clearly delineated in Figure 31-1, which shows progression from optimal tissue status through a period of initial depletion until a period of subclinical deficiency is reached with a variety of biochemical and nonspecific physiologic effects. In some cases, certain nonspecific histologic changes may put the individual at risk of tissue damage or neoplastic change. It is only with persistent mismatch of intake and demand that eventually a full blown clinical deficiency state develops. In practice, it is practically impossible to measure all biologically active antioxidants in human samples; therefore the concept of a “global” assessment of antioxidant capacity has proved attractive. Several methods have been developed for use in assessing this capacity and in conducting clinical research.600 Most of these methods use one of two main approaches to measurement: (1) quenched or delayed production of a stable, measurable radical species; or (2) the reductant properties of antioxidants against a radical cation or a metal ion.554 These are usually standardized with the water-soluble vitamin E analog Trolox. An example of the former is the total radical-trapping antioxidant parameter (TRAP) assay, which uses the stable radical species 2,2′-azinobis(3-ethylbenzthiazoline sulfonate) (ABTS+), and an example of the latter is the ferric reducing ability of plasma (FRAP) assay.47 Because these methods use different reaction principles, the same antioxidant can make different contributions to each assay, and it is for this reason that the use of more than one method is recommended for any study.503 Vitamins that have contributed to plasma antioxidant capacity include ascorbate (up to 24% of measured capacity), α-tocopherol (up to 10% of measured capacity), and β-carotene, although at typical plasma concentrations of around 0.5 µmol/L, the contribution of β-carotene—as with most fat-soluble vitamins—to a total antioxidant capacity of around 1000 µmol/L is minimal. A disadvantage of many methods of total antioxidant capacity measurement is the variable contribution of common plasma constituents, particularly albumin and urate, to the measured concentration.215 Changes in circulating concentrations of these molecules caused by acute-phase changes or changes in renal function can alter measured values without reflecting changes in antioxidant vitamin concentration. This problem is typically resolved by the use of the antioxidant gap, a derived value that subtracts the Trolox equivalence of albumin and urate from the measured total antioxidant capacity.422 Another drawback is the nonphysiologic conditions of study; hence new approaches to genomic and proteomic studies may allow a more comprehensive assessment.331 In an attempt to assess the functional adequacy of vitamins and trace elements involved in antioxidant pathways, products of oxidative metabolism can be measured. Those frequently used are malondialdehyde and F2 isoprostanes, both of which give an indication of oxidation of polyunsaturated fatty acids within cells.352 Increasing the provision of individual antioxidants or cocktails of vitamins and trace elements can lead to reduction in production of these metabolites, which can be measured in serum or urine. Such tests have not yet reached widespread application primarily because interpretation of results is difficult. Also, the appropriate amount of oxidative activity that is linked to particular outcomes has not yet been identified. This is especially important in that few clinical studies have demonstrated benefit from provision of increased quantities of antioxidants.46 Most studies have indicated that antioxidant supplements (usually vitamin C and/or vitamin E) have no beneficial effect or indeed may be harmful,63,90 although antioxidant-rich foods may be beneficial.691 Concentrations of vitamins and trace elements are measured most often in plasma or serum; this provides a reliable index of status for only a few of them (e.g., vitamin B12, vitamin D). For others (e.g., folate, selenium), their concentrations may reflect only the adequacy of recent intake. Excessive provision of elements, such as manganese and chromium, may be detected by high serum concentrations.579 For some vitamins or trace elements, serum measurement is limited in value, especially in seriously ill patients. In part, this is a result of the lack of correlation between the amount of nutrient in the plasma compartment and the amount within the intracellular compartment in most body tissue. For example, substantial stores of particular vitamins or trace elements may be present in individual tissue (e.g., vitamin A in the liver), but mobilization into the plasma is affected by the availability of appropriate binding proteins or by metabolism. Also, differences in the content of individual vitamins or trace elements have been noted between tissues, and the serum concentration will not reflect these differences. Furthermore and particularly important is the fact that the concentration in plasma can alter rapidly when a systemic inflammatory response syndrome (SIRS) [previously known as the acute-phase response (APR)] results from trauma or infection, leading to redistribution of metals between body compartments582; increased synthesis of metallothionein leads to uptake of zinc into the liver and increased synthesis of ferritin, causing uptake of iron.135,529 The result is a fall in plasma concentrations of both zinc and iron. These changes in plasma concentration clearly do not reflect changes in whole body status. Some patients may be relatively stable, with little SIRS after injury, infection, or other inflammatory disease. If this is the case, it may be possible to interpret the plasma concentrations of elements such as zinc, copper, and iron, or of vitamins such as vitamin C or B6, that would be affected by SIRS.372 Of particular relevance is the trend in concentration of a trace element or vitamin in relation to the magnitude of SIRS changes.211 Therefore, repeated measurement of a rapid response acute-phase protein, such as C-reactive protein (CRP), together with trace elements, may be helpful to include as part of the nutritional assessment. More commonly, certain types of cells may be obtained from blood samples and can provide useful information. For example, red cell folate is commonly used as a marker of folate status, and leukocyte vitamin C is a better marker of vitamin C status than plasma concentration.550 Because of the difficulty of preparation of pure populations of cells, cellular measurements usually are used only within a research environment. Table 31-2 provides a list of 13 known vitamins and vitameric groups essential to humans. Vitamin A is the nutritional term for the group of compounds with a 20-carbon structure containing a methyl-substituted cyclohexenyl ring (β-ionone ring) and an isoprenoid sidechain (Figure 31-2), with a hydroxyl group (retinol), an aldehyde group (retinal), a carboxylic acid group (retinoic acid), or an ester group (retinyl ester) at the terminal C15. Retinol, the principal vitamin A vitamer, can be oxidized reversibly to retinal—which shares all the biological activity of retinol—or further oxidized to retinoic acid, which shows some of its biological activity. The principal storage forms of vitamin A are retinyl esters, particularly palmitate. The term retinoids refers to retinol, its metabolites, and synthetic analogs with similar structure. Included in the vitamin A family are some dietary carotenoids (C40 polyisoprenoid compounds) that are classified as provitamin A because they are cleaved biologically to yield retinol. Although around 1000 compounds with carotenoid structure have been identified,395 only about 50 possess provitamin A activity, with the principal dietary compounds being β-carotene, α-carotene, and β-cryptoxanthin. Vitamin A compounds are yellowish oils or low-melting-point solids (depending on isomeric purity) that are practically insoluble in water but are soluble in organic solvents and mineral oil. Vitamin A is sensitive to oxygen and to ultraviolet light, which induces a greenish fluorescence with an absorbance peak at 325 nm. The structure for the most common and effective provitamin A, β-carotene, is given in Figure 31-2. This compound is an orange-to-purple, water-insoluble solid that is oxidized in air to inactive products. The other carotenes, cryptoxanthin and β-apocarotenals, are asymmetric with only one β-ionone ring and yield less vitamin A activity. Pre-formed vitamin A is obtained from animal-derived foods, such as liver, other organ meats, and fish oils. Other sources are full cream milk, butter, and fortified margarines. The provitamin A carotenoids are obtained from yellow to orange fruits and vegetables and from green leafy vegetables. Good sources are pumpkin, carrots, tomatoes, apricots, grapefruit, lettuce, and most green vegetables.385 The U.S. National Health and Nutrition Examination Survey (NHANES II) indicated that approximately 25% of the vitamin A requirement was provided by carotenoids and about 75% by pre-formed retinol.67 Pre-formed vitamin A, most often in the form of retinyl ester, or carotenoids are subject to emulsification and mixed micelle formation by the action of bile salts before they are transported into the intestinal cell. Here the retinyl esters are moved across the mucosal membrane and hydrolyzed to retinol within the cell to be then re-esterified by cellular retinol-binding protein II and packaged into chylomicra, which then enter the mesenteric lymphatic system and pass into the systemic circulation.445 A small amount of the ingested retinoid is converted into retinoic acid in the intestinal cell. The efficiency of absorption of pre-formed vitamin A is high at between 70% and 90%.593 Carotenoids, also in micellar form, are absorbed into the duodenal mucosal cells by passive diffusion. The efficiency of absorption of carotenoids is much lower than for vitamin A—between 9% and 22%461—and is subject to a large number of variables, including carotenoid type, the amount in the meal, matrix properties, nutrient status, and genetic factors.621 Once inside the mucosal cell, β-carotene is principally converted to retinal by the enzyme β-carotene-15,15′-dioxygenase. The retinal is converted by retinal reductase to retinol and esterified. β-Carotene can also be cleaved eccentrically to β-apocarotenals, which can be further degraded to retinal or retinoic acid. The newly synthesized retinyl esters, from both pre-formed vitamin A and carotenoids, along with exogenous lipids and nonhydrolyzed carotenoids, then pass with chylomicrons via the lymphatic system to the liver, where uptake by parenchymal cells again involves hydrolysis. In the liver, retinol is bound with retinol-binding protein (RBP) (MW ≅ 21,000 Da) and transthyretin (thyroxine-binding prealbumin) (MW ≅ 55,000 Da) in a 1 : 1 : 1 complex of sufficient size to prevent loss by glomerular filtration and is returned to the circulation, or it may be stored as esters within the stellate cells. Delivery of retinol to the tissue is controlled by the availability of the vitamin A–protein complex in the circulation, although this control mechanism can be bypassed by large doses of retinol. The participation of retinal in vision is considered the most important physiologic function of vitamin A. All-trans-retinol is the predominant circulating form of vitamin A. Cells of the retina isomerize this to the 11-cis alcohol that is reversibly dehydrogenated to 11-cis retinal. This sterically hindered geometrical isomer of the aldehyde combines as a lysyl-linked Schiff base with suitable proteins (e.g., opsin) to generate photosensitive pigments, such as rhodopsin. Illumination of such pigments causes photoisomerization and the release of all-trans-retinal and the protein, a process that couples the large conformational change with ion flux and optic nerve transmission. The all-trans-retinal is isomerized to the 11-cis isomer, which combines with the liberated protein to reconstitute the photo pigment in a visual cycle, as shown in Figure 31-3. The pyridine nucleotide–dependent dehydrogenase (reductase) can also reduce the all-trans-retinal to all-trans-retinol. Other functions of vitamin A include its role in reproduction, growth, and embryonic development, as well as in immune function; many of these functions are mediated through the binding of retinoic acid to specific nuclear receptors that regulate genomic expression. In normal growth, and in maintenance of the integrity of epithelial cells, retinoic acid acts through the activation of retinoic acid receptors (RARs) and retinoid X receptors (RXRs) in the nucleus to regulate various genes that encode for structural proteins, enzymes, extracellular matrix proteins, and RBPs and receptors.142,387 In vertebrate embryonic development, the vitamin A requirement (mediated via RARs and RXRs) begins at the time of formation of the primitive heart circulation and specification of the hindbrain,709 and is later required for normal development of the limbs, heart, eyes, and ears.151 Vitamin A deficiency impairs innate immunity by impeding normal regeneration of mucosal barriers damaged by infection, and by diminishing the function of neutrophils, macrophages, and natural killer cells. Vitamin A is also required for adaptive immunity and plays a role in the development of both T-helper cells and B cells. Retinol and its metabolites and synthetic retinoids provide protective effects against the development of certain types of cancer by blocking tumor promotion, by inhibiting proliferation, by inducing apoptosis, by inducing differentiation, or by performing a combination of these actions.433,458 Finally, synthetic retinoids have been used successfully, both topically and systemically, to treat severe acne and other skin disorders of abnormal keratinization. Some caution is required regarding the use of vitamin A or β-carotene supplements in the general population. Although synthetic retinoids are of value in treating certain forms of leukemia,433 they appear to provide no benefit in reducing the incidence of gastrointestinal cancer and indeed may increase the incidence of lung cancer and mortality in certain other cancers.63,474 Historical studies in adult humans have suggested that intakes of retinol of 500 to 600 µg/d are required to maintain adequate blood concentrations and to prevent all deficiency symptoms. The relative contributions of β-carotene and other provitamin A carotenoids toward achieving this goal have undergone much revision as our knowledge has developed. In the older system of international units (IU), now largely redundant, a ratio for equivalence of activity of 1 : 2 : 4 for retinol:β-carotene : other provitamin A carotenoids was used, but this was superseded in 1967 by the retinol equivalent (RE), devised by a Food and Agriculture/World Health Organization Expert Committee and proposing an equivalence ratio of 1 : 6 : 12. However, studies using stable isotopes of β-carotene653 led the Food and Nutrition Board of the U.S. Institute of Medicine to recommend the retinol activity equivalent (RAE) as the basis of calculation of retinol intake. In this system, a ratio equivalence of 1 : 12 : 24 is recommended (i.e., 12 µg β-carotene or 24 µg mixed carotenoids has the same biological activity as 1 µg retinol). With this system, current RDAs for vitamin A are 900 µg RAE for men 19 years and older; 700 µg RAE for women 19 years and older, with up to 770 µg RAE/d in pregnancy and up to 1300 µg RAE/d in lactation; 300 to 900 µg RAE for children 1 to 18 years, dependent on age and sex; and an adequate intake (AI) of 400 µg RAE at 0 to 6 months and 500 µg RAE from 7 to 12 months for infants.190 The recommended provision of vitamin A to adults during intravenous nutrition (IVN), whether this is partial or total parenteral nutrition (TPN), is 1000 µg retinol. This is usually provided as retinol palmitate and may be supplied with other fat-soluble vitamins in a mixture dissolved in a fat emulsion for intravenous feeding, or may be designed to be compatible with a mixture of all vitamins suitable for addition to other water-soluble nutrients.578 Vitamin A deficiency primarily affects infants and children, and its prevalence is subject to World Health Organization (WHO) surveillance.692 Risk factors include poverty, low birth weight, poor sanitation, malnutrition, infection, and parasitism. Because hepatic accumulation of vitamin A occurs during the last trimester of pregnancy, preterm infants are relatively vitamin A deficient at birth. Providing a daily oral intake of vitamin A that meets the RDA of 400 µg RAE is therefore important. Infants with birth weights of less than 1500 g (those under 30 weeks’ gestation) have virtually no hepatic vitamin A stores and are at risk of vitamin A deficiency. Various workers have observed that (1) bronchopulmonary dysplasia (BPD), a debilitating, chronic lung disease that mimics some histologic features of vitamin A deficiency, is common in premature infants; (2) intramuscular injections of 630 µg RAE every 2 days can reduce the incidence of BPD; (3) blood concentrations of vitamin A decline during TPN, often reaching concentrations of 10 to 15 µg/dL (normal, 20 to 65 µg/dL) unless adequate supplements are given; and (4) vitamin A (retinol) delivered in TPN solutions may be adsorbed into the inner walls of plastic administration sets; however, this loss can be minimized by the use of ethylene vinyl acetate rather than polyvinyl chloride.225,577 Fat malabsorption, particularly caused by celiac disease or chronic pancreatitis, and protein-energy malnutrition predispose to vitamin A deficiency. Liver disease diminishes RBP synthesis, and ethanol abuse leads to both hepatic injury and competition with retinol for alcohol dehydrogenase, which is necessary for the oxidation of retinol to retinal and retinoic acid.353 Vitamin A deficiency may lead to anemia, although the precise mechanism is not known.568 Although vitamin A metabolism is tightly regulated, toxic effects of hypervitaminosis A have occurred as a result of ingestion of excess vitamin, or as a side effect of inappropriate therapy.485,682 Hypervitaminosis A occurs after liver storage of retinol and its esters exceeds 3000 µg/g tissue, with ingestion of more than 30,000 µg/d for months or years, or if plasma vitamin A concentrations exceed 140 µg/dL (4.9 µmol/L). The elderly are more susceptible to vitamin A toxicity at lower doses, as exposure to retinyl esters is longer because of delayed postprandial clearance of lipoproteins.537 Symptoms of acute toxicity from a single massive dose present as abdominal pain, nausea, vomiting, severe headaches, dizziness, sluggishness, and irritability, followed within a few days by desquamation of the skin and recovery. Chronic toxicity from moderately high doses taken for protracted periods is characterized by bone and joint pain, hair loss, dryness and fissures of the lips, anorexia, benign intracranial hypertension, weight loss, and hepatomegaly. Administration of doses up to threefold the RDA for several years resulted in classic histologic changes of hepatotoxicity in 41 patients.214 Osteoporosis and hip fracture are associated with vitamin A intakes only twice the RDA.485 Infants given excess vitamin A over months to years can develop intracranial features, typically bulging fontanelle, and skeletal abnormalities at doses of 5500 to 6750 µg/d.487 Epidemiologic and experimental evidence has supported the view that high vitamin A intake in humans, acting via 13-cis-retinoic acid, is teratogenic.343 The critical period of susceptibility is the first trimester of pregnancy, and primary abnormalities derive from the cranial neural crest (CNC) cells. A 1995 study of almost 23,000 pregnant women found that those who ingested more than 4500 µg/d of pre-formed vitamin A were at greater risk of delivering infants with malformations of CNC cell origin than were women consuming less than 1500 µg/d.532 A further intriguing association, supported in part by epidemiologic studies, is that observed between excessive vitamin A intake and reduction in bone mineral density (BMD). Studies of Scandinavian women show that consistent loss of BMD at four sites was associated with increased intake of pre-formed vitamin A.408 Intake amounts of vitamin A exceeding 1500 µg/d were associated with these changes, although studies in the United States have showed no increase in bone mineral loss at pre-formed vitamin A intakes up to 2000 µg/d.274 Although measurement of the plasma concentration of vitamin A is the most convenient and widely used assessment of vitamin A status, it is not an ideal indicator because it does not decline until liver stores become critically depleted, which is thought to occur at a concentration of approximately 20 µg/g liver. Early chemical methods, which may remain in use if high-performance liquid chromatography (HPLC) is not available, include the Carr-Price photometric method, which uses antimony trichloride in chloroform as the reagent, and the later Neeld-Pearson method, which uses trifluoroacetic acid to produce a blue pigment with the conjugated double bonds of vitamin A (and the carotenoids). To improve specificity and sensitivity, later methods used solvent extraction and other separation techniques with fluorometric or spectrophotometric measurement. HPLC has brought enhanced (1) specificity, (2) lowered limits of detection (<0.07 µmol/L), (3) accuracy (using primary standards, reference materials, and quality assurance schemes), and (4) reproducibility (between batch coefficients of variation of 10% or better).632 Both normal-phase and reversed-phase techniques have been used.123,403 Because retinol circulates in plasma as a 1 : 1 : 1 complex with RBP and TTR, both of these hepatically produced proteins have been measured as indicators of vitamin A status. RBP has been measured by radial immunodiffusion or nephelometry (see Chapter 21), but its circulating concentration may be limited by inadequate dietary protein, energy, or zinc, all of which are necessary for RBP synthesis. Another confounding factor in the assessment of vitamin A status is the effect of the SIRS. Both RBP and TTR are negative acute-phase proteins; thus inflammatory changes will result in transient falls in both proteins and plasma retinol. To distinguish inflammatory from nutritional causes of reduced plasma retinol concentrations, it may be necessary to measure CRP.564 Because circulating retinol concentrations do not always correlate with total body stores of vitamin A, indirect tests have been used to assess these stores. The relative-dose-response test, described first by Loerch and associates,366 requires two blood samples to be collected—one before and one 5 hours after a physiologic dose of vitamin A. In vitamin A–depleted subjects, a rapid, large, and sustained rise in serum retinol concentration contrasts with a lower, more shallow rise in vitamin A–sufficient subjects. A modified relative-dose-response test [using 3,4-didehydroretinyl (DR) acetate rather than retinyl acetate and measuring the DR : retinol ratio after 5 hours] has been used by other workers to assess the vitamin A status of preschool Indonesian children622 and also in a population of well-nourished American children.623 Quantitative assessment of total body stores may be made by using deuterated retinol dilution techniques, but these require long periods (about 3 weeks) for equilibration. Ribaya-Mercado and colleagues developed a predictive mathematical formula that does not require serum isotope equilibration, allowing blood sampling 3 days after isotope dosing.520 Guidance reference intervals for serum vitamin A are 20 to 40 µg/dL (0.70 to 1.40 µmol/L) for 1- to 6-year-old children; 26 to 49 µg/dL (0.91 to 1.71 µmol/L) for 7- to 12-year-old children; 26 to 72 µg/dL (0.91 to 2.51 µmol/L) for 13- to 19-year-old adolescents; and 30 to 80 µg/dL (1.05 to 2.80 µmol/L) for adults.365 Values above 30 µg/dL (1.05 µmol/L) are associated with appreciable reserves in the liver and correlate well with vitamin A intake. Within the reference interval, values for men are generally about 20% higher than those for women. By HPLC, the reference interval for serum β-carotene is 10 to 85 µg/dL (0.19 to 1.58 µmol/L).633 Elevated concentrations are found in hypothyroid patients, in whom conversion to vitamin A is decreased, and in patients with hyperlipemia associated with diabetes mellitus. Vitamin D plays an essential role as a hormone in the control of calcium and phosphorous metabolism. It is discussed in detail in Chapter 52. Vitamin E is the nutritional term for the group of naturally occurring tocopherols and tocotrienols that have biological activity similar to RRR-α-tocopherol (formerly D-α-tocopherol).416 Both groups have a common 6-chromanol nucleus substituted with methyl groups at positions 2 and 8 and with a phytyl tail of isoprenoid units at position 2. The isoprenoid chain is saturated in the tocopherols but is unsaturated at positions 3′, 7′, and 11′ for tocotrienols (Figure 31-4). The Greek letter prefixes α, β, γ, and δ indicate the presence or absence of methyl groups at positions 5 and 7. The tocopherols have three asymmetric carbon atoms in the isoprenoid chain, giving eight optical isomers. The naturally occurring tocopherols occur as the RRR forms, whereas the synthetic compounds are of the racemic SR forms. Synthetic vitamin E contains about 12.5% of RRR-α-tocopherol, together with seven other tocopherol isomers that are less biologically active. Tocopherol and tocotrienols are viscous oils at room temperature, soluble in fat solvents and insoluble in aqueous solutions, although there exists a water-soluble analog (Trolox-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid). Also, tocopherol and tocotrienols are stable to acid and heat in the absence of oxygen, but are labile to oxygen in alkaline solutions and to ultraviolet light. The principal sources of dietary vitamin E are oils and fats, particularly wheat germ oil and sunflower oil, grains, and nuts.35 Meats, fruits, and vegetables contribute little vitamin E. Gamma-tocopherol is the major form of vitamin E in many plant seeds in the U.S. diet, but it is present at only one quarter to one tenth of the concentration of α-tocopherol in human plasma.303 In the presence of bile, vitamin E is absorbed from the small intestine. Most forms of vitamin E are absorbed nonselectively and are secreted in chylomicron particles along with triacylglycerol and cholesterol. Some of this chylomicron-bound vitamin E is transported and delivered to the peripheral tissue (mainly adipose tissue) with the aid of lipoprotein lipase. The liver takes up the chylomicron remnants where α-tocopherol is incorporated into very low-density lipoproteins (VLDLs) by α-tocopherol transfer protein (α-TTP), enabling further distribution of α-tocopherol throughout the body. Plasma vitamin E is further delivered to the tissue by low-density lipoprotein (LDL) and high-density lipoprotein (HDL).303 The specificity of α-TTP for α-tocopherol is probably responsible for its preferential storage in most tissue. Vitamin E is excreted via the bile, in the urine as tocopheronic acid and its β-glucuronide conjugate, as carboxyethyl hydroxychromans (CEHC), and by unknown routes.561 The future direction of vitamin E research has been reviewed.85 Historically, vitamin E has been recognized as necessary for neurologic and reproductive functions, for protection of the red cell from hemolysis, and for prevention of retinopathy in premature infants.85 Inhibition of free radical chain reactions of lipid peroxidation is the most thoroughly defined role of vitamin E.302 This occurs mainly within the polyunsaturated fatty acids of membrane phospholipids. Tocopherols and tocotrienols inhibit lipid peroxidation largely because they scavenge lipid peroxyl radicals faster than the radical can react to adjacent fatty acid sidechains or membrane proteins. The resultant tocopheryl or tocotrienyl radicals may then react with additional peroxyl radicals to produce tocopherones (nonradicals), or they may be regenerated by transfer of an electron to ascorbate to form the ascorbyl radical. Thus vitamins E and C act synergistically to reduce lipid peroxidation (Figure 31-5).115 Moreover, intracellular and extracellular concentrations of vitamin C may critically control the amount of biologically active vitamin E within the cell membrane.59 Some epidemiologic surveys have shown an association between reduced vitamin E intake (and other dietary factors) and increased incidence of chronic disease, particularly cardiovascular disease and cancer, although intervention studies have produced mixed results. The Cambridge Heart Antioxidant Study606 showed a significant 47% reduction in nonfatal myocardial infarction (MI) in the vitamin E treatment (400 or 800 IU/d) arm of a placebo-controlled study involving subjects with existing heart disease, whereas the Gruppo Italiano Studio Sopravvivenza Infarcto (GISSI) trial,231 also involving secondary prevention, produced a small but insignificant reduction in risk of death, nonfatal MI, or stroke in those given 300 mg/d of synthetic vitamin E versus the control group. In 2002, the U.K. Heart Protection Study of 20,536 subjects with existing coronary disease, other occlusive disease, or diabetes showed that supplementation with vitamin E (600 mg), vitamin C (250 mg), and β-carotene (20 mg) over a period of 5 years produced no significant reduction in any type of vascular disease, cancer, or other major outcome, when compared with placebo.256 A meta- analysis of clinical trials suggests that response to vitamin E may be dose dependent; trials using 400 mg or less showed very slight or no benefit, whereas those using more than 400 mg/d showed a significant increase in all-cause mortality. Other studies have confirmed the lack of effect on heart disease133 and in slowing the rate of cognitive decline.313 Any influence of vitamin E on disease progression may therefore be seen in primary prevention rather than secondary intervention, but growing concern has arisen about the possible harmful effects of high-dose supplements. Vitamin E has no proven effect in reducing the incidence of various cancers63 Beyond its antioxidant properties, α-tocopherol inhibits protein kinase C and 5-lipoxygenase and activates protein phosphatase 2A and diacylglycerol kinase at the post-translational amount. Some genes (coding for CD36, α-TTP, α-tropomyosin, and collagenase) are affected by α-tocopherol at the transcriptional concentration. α-Tocopherol also induces inhibition of cell proliferation, platelet aggregation, and monocyte adhesion, which are thought to be the result of direct interaction of α-tocopherol with cell components.85,521 α-Tocopherol reduces inflammatory mediator production.98 Properties independent of α-tocopherol have been ascribed to γ-tocopherol. These include inhibition of cyclo-oxygenase activity, thus conferring anti-inflammatory properties, and the natriuretic property of its main metabolite, γ-CEHC [2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman], not shared by α-CEHC.303 The requirement for vitamin E is related to the polyunsaturated fatty acid content of cellular structures and therefore depends on the nature and quantity of dietary fat that affect such composition. Hence the minimum adult requirement for vitamin E is not certain but is probably not more than 3 to 4 mg (4.5 to 6 IU) of RRR–α-tocopherol/d for those who ingest a diet containing the minimum of essential fatty acids (3% of calories).188 Because vitamin E activity is derived from a series of tocopherols and tocotrienols in usual mixed diets, calculations are used that are based on their abundance and activity relative to the biologically most active RRR–α-tocopherol. The milligrams of β-tocopherol are multiplied by 0.5, those of γ-tocopherol by 0.1, and those of α-tocotrienol by 0.3. Their sum plus milligrams of α-tocopherol accounts for the milligrams of α-tocopherol equivalents. It has been estimated that a range of 7 to 13 mg of α++-tocopherol equivalents (10 to 20 IU) can be expected in balanced diets supplying 1800 to 3000 kcal. This intake will maintain plasma concentrations of total tocopherols within the reference interval of 0.5 to 1.2 mg/dL, which ensures an adequate concentration in all tissue.58 Some investigators claim that the ratio of circulating α-tocopherol to total lipids (or triglycerides or β-lipoproteins) is a more accurate indicator of tissue vitamin E status than circulating α-tocopherol alone. In the year 2000, the RDA for vitamin E for adults was increased by 50% from 10 to 15 mg/d by the U.S. Food and Nutrition Board.188 Most European reference intakes are related to the polyunsaturated fatty acid intake.517 Changes in the United States were accompanied by some debate, with critics arguing that this amount could not be met by the usual North American diet.273,636 For infants up to 6 months, an AI of 4 mg/d was proposed, for infants 7 to 12 months an AI of 5 mg/d, and for children 1 to 18 years the RDA was set at 6 to 15 mg/d, dependent upon age.188 Another departure in the newer recommendations was that the daily requirement must be met by RRR-α-tocopherol alone, as the other forms of vitamin E are not converted to α-tocopherol and are poorly recognized by the α-tocopherol transfer protein in the liver. The recommended amount of vitamin E to be supplied intravenously to adults as α-tocopherol is 10 mg.260 This is rather lower than the oral provision, but takes into account the fact that it is completely delivered into the bloodstream. Premature and low birth weight infants are particularly susceptible to development of vitamin E deficiency, because placental transfer is poor and infants have such limited adipose tissue where much of the vitamin is normally stored.58 Signs of deficiency include (1) irritability, (2) edema, and (3) hemolytic anemia. Anemia reflects the shortened life span of erythrocytes with fragile membranes; it does not respond to iron therapy, which may aggravate the condition. Although symptoms of vitamin E deficiency are rare in children and adults, deficiency can occur in some conditions. Fat malabsorption states, such as cystic fibrosis and chronic cholestasis in children, can cause neuropathy597 and hemolytic anemia,683 as can the genetic disorder abetalipoproteinemia (within which vitamin E is transported).438 Mutations of the gene coding for α-TTP lead to very low plasma α-tocopherol concentrations and cause neurologic symptoms, including cerebellar ataxia.560 Plasma concentrations may be normalized only by administering large amounts (up to 2 g/d) of vitamin E. Low concentrations of vitamin E may exist without clinical signs, and may occur acutely as a result of oxidative stress, as in major trauma or SIRS.59 Excess vitamin E intake usually is achieved only by dietary supplementation. Such supplementation is contraindicated in subjects with coagulation defects caused by vitamin K deficiency and in those receiving anticoagulant drugs. The U.S. Food and Nutrition Board has recommended a tolerable upper limit of 1000 mg/d of vitamin E for adults 19 years and older, based on the absence of hemorrhagic toxicity in animal models,188 although this has been challenged on the grounds that in those regularly taking aspirin, this intake may be associated with increased risk of bleeding.273 A comprehensive review of tolerance and safety of vitamin E suggested that intakes up to 3000 mg/d were safe, and reversible side effects of gastrointestinal symptoms, increased creatinuria, and impairment of blood coagulation are seen at intakes of 1000 to 3000 mg/d.318 However, as noted earlier, long-term use of intakes greater than 400 mg/d may cause increased mortality. Assessment of vitamin E status has been achieved by functional methods such as (1) protection of erythrocyte hemolysis on addition of peroxide,167 (2) inhibition of lipid peroxidation products [malondialdehyde, thiobarbituric acid–reactive substances (ethane or pentane)],651 or (3) direct measurement of vitamin E concentration in tissues (erythrocytes, lymphocytes, or platelets) or serum. Early direct methods used photometric or fluorometric measurement often based on the Emmerie-Engel procedure, in which tocopherol is oxidized to tocopheryl quinone by FeCl3, and the resultant Fe2+ is coupled with α,α′-dipyridyl to form a red color. Later, chromatographic methods were used, including thin layer and gas liquid, which had the ability to separate the tocopherols and the tocotrienols, but these methods were labor intensive and time consuming. HPLC is currently the method of choice for quantitation of tocopherols in serum, as it offers the advantages of accuracy (through the use of primary standards) and reproducibility (between-batch coefficients of variation of 7% or better) and the ability to quantitate multiple analytes, including vitamin A and some carotenoids, in a single analytical run.703 Both α- and γ-tocopherols are the principal vitamers seen, although others may be detected with minor modifications to the analytical conditions. Guidance reference intervals for serum or plasma (heparin) vitamin E are 0.1 to 0.5 mg/dL (2.3 to 11.6 µmol/L) for premature neonates; 0.3 to 0.9 mg/dL (7 to 21 µmol/L) for children (1 to 12 years)365; 0.6 to 1.0 mg/dL (14 to 23 µmol/L) for adolescents (13 to19 years); and 0.5 to 1.8 mg/dL (12 to 42 µmol/L) for adults.656 Compounds in the vitamin K series are 2-methyl-1,4-napthoquinones, which are substituted with sidechains at carbon 3. Phylloquinon (K1 type) synthesized in plants and menaquinones (K2 type) of bacterial origin are the two principal natural classes of vitamin K (Figure 31-6). The principal vitamin K1 (phylloquinone) bears a saturated, phytol, 20-carbon sidechain derived from four isoprenoid units; this is the main K vitamin produced by plants and is the major dietary form for humans.575 K2 shows greater variation, but an all-trans-farnesylgeranylgeranyl, 35-carbon chain of 7 isoprenoid units is typical; these are produced in humans by the large bowel bacterial mass, although their contribution to vitamin K status remains a matter of dispute. Several synthetic analogs and derivatives have been used in human nutrition; most relate to or derive from menadione (K3), which lacks a sidechain substituent at position 3, but can be converted to menaquinone (MK) (e.g., MK-4, where 4 is the number of isoprenoid sidechains) through addition of the sidechain in the liver. The K vitamins are insoluble in water but dissolve in organic fat solvents. They are destroyed by alkaline solutions and reducing agents and are sensitive to ultraviolet light. The main dietary sources of the phylloquinones are green vegetables, margarines, and plant oils, whereas some menaquinones can be obtained from cheese, other milk products, and eggs.563 Within metabolically active and vitamin K–using tissue, especially liver, a microsomal vitamin K cycle exists (Figure 31-7). The vitamin (quinone) is normally reduced by a thiol-sensitive flavoprotein system to the hydroquinone, which then can couple to the oxygen and carbon dioxide with the use of γ-carboxylation of glutamyl residues in specific proteins (e.g., prothrombin).176 The 2,3-epoxide of vitamin K that is subsequently formed is reduced to the starting vitamin K quinones—a process that can be antagonized by such vitamin K antagonists as warfarin. The essential and most thoroughly defined role of vitamin K is as a cofactor to vitamin K–dependent carboxylase, an enzyme necessary for the post-translational conversion of specific glutamyl residues in target proteins to γ-carboxyglutamyl (Gla) residues. This γ-carboxylation increases the affinity of these proteins for calcium.51 The antihemorrhagic function of vitamin K depends on the formation of the Gla proteins prothrombin (factor II), proconvertin (factor VII), plasma thromboplastin component (factor IX), and Stuart factor (factor X), which, together with two other hemostatic vitamin K–dependent proteins, proteins C and S, and Ca2+, initiate a process to form thrombin that then catalyzes the conversion of fibrinogen to a fibrin clot.449 Proteins that contain γ-carboxyglutamyl are also abundant in bone tissue, with osteocalcin accounting for up to 80% of the total γ-carboxyglutamyl content of mature bone. Epidemiologic studies71,178 have shown an association between low vitamin K intakes and hip fracture risk, but not BMD measurements. Intervention studies have shown that vitamin K can increase BMD in osteoporotic subjects and can reduce fracture rates,590 although these studies have used menaquinone-4 in pharmacologic rather than physiologic doses. The improvement in bone markers was accompanied by a significant fall in the concentration of undercarboxylated osteocalcin in treated groups. Evidence indicates that vitamins K and D may act synergistically in maintaining bone density.293,575 A further major Gla protein, matrix Gla protein (MGP)—containing five residues of γ-carboxyglutamic acid—is found in vascular smooth muscle, bone, and many soft tissues (heart, kidney, and lungs). It is thought that MGP accumulates at sites of calcification, including calcified aortic valves and bone, and is a potent inhibitor of calcification. In experimental studies with mice lacking the gene coding for MGP, calcification of the arteries was observed that led to hemorrhagic death of the animals as a result of blood vessel rupture.377 Several other Gla proteins have been identified, and putative roles have been assigned576 Although the human gut bacteria synthesize large quantities of menaquinones, and such compounds are found in the liver in concentrations up to 10 times those of phylloquinones, absorption of these compounds has been difficult to demonstrate, and dietary restriction of vitamin K leads to evidence of inadequacy, as demonstrated by undercarboxylation of vitamin K–dependent proteins.176 Thus dietary reference intakes for vitamin K have been revised by the Food and Nutrition Board of the U.S. Institute of Medicine. Current recommendations are 120 µg/d for men older than 18 years; 90 µg/d for women older than 18 years, including those pregnant or lactating; 30 to 75 µg/d for children 1 to 18 years, dependent on age; 2.0 µg/d for infants up to 6 months; and 2.5 µg/d for infants between 7 and 12 months, with the latter requirements met by mature breast milk.190 Dietary intake of phylloquinone in North American and most European populations studied has been estimated at around 150 µg/d for subjects older than 55 years and around 80 µg/d for younger subjects, although intakes in the Netherlands have been reported to be two to three times higher than these estimates.563 In the United States, whether vitamin K should be included in preparations of vitamins for use in TPN is controversial. Although this has been standard in Europe for many years,581 the long-standing recommendation from the American Medical Association was not to include vitamin K, because this would complicate the provision of adequate warfarin therapy in those patients who require anticoagulation.9 However, the 2003 requirements of the U.S. Food and Drug Administration (FDA) specified that vitamin K should be included in vitamin supplements for both infants and adults, making the judgment that the physiologic and practical benefits of regular provision outweigh any problems in readjusting warfarin dosage.260 The recommended intravenous (IV) adult dose is 150 µg/d, which is provided as phytonadione. Although vitamin K deficiency in the adult is uncommon, the risk is increased for fat malabsorption states such as (1) bile duct obstruction, (2) cystic fibrosis, and (3) chronic pancreatitis and liver disease.337 Risk is also increased by the use of drugs that interfere with vitamin K metabolism, such as the coumarin anticoagulants (e.g., warfarin) and antibiotics containing the N-methylthiotetrazole sidechain (e.g., cephalosporin).574 Other at-risk groups are hospitalized patients with poor nutrient intakes or those receiving TPN, when fat-soluble vitamin supplements may not fully meet requirements. Conversely, ingestion of supraphysiologic doses of vitamins A and E has been reported to induce vitamin K deficiency, probably through competitive mechanisms.473 Defective blood coagulation and demonstration of abnormal noncarboxylated prothrombin are at present the only well-established signs of vitamin K deficiency. The use of high doses of naturally occurring vitamin K (K1 and K2) appears to have no untoward effect; however, menadione (K3) treatment can lead to the formation of erythrocyte cytoplasmic inclusions known as Heinz bodies and hemolytic anemia.441 With severe hemolysis, increased bilirubin formation and undeveloped capacity for its conjugation may produce kernicterus in the newborn. Because no adverse effects associated with vitamin K consumption from food or supplements have been reported in humans or animals, the U.S. Institute of Medicine has reported that a quantitative risk assessment cannot be performed, and thus an upper limit (UL) cannot be derived for vitamin K.190 A wide range of biochemical and functional tests are available for vitamin K status.576 Because of its relatively low plasma concentration (approximately 50 times lower than vitamin D and at least 103 times lower than vitamin A or E), vitamin K has long presented an analytical challenge. For this reason, vitamin K status has traditionally been assessed by functional methods, primarily by its effect on clotting time. The prothrombin time (PT) is assessed by adding a portion of tissue thromboplastin to recalcified plasma and measuring the clotting time against a normal control sample. In vitamin K deficiency, the PT may rise above 30 seconds (normal, 10 to 14 seconds), and at least 2 seconds beyond the control time. Attempts at cross-laboratory standardization led to the introduction of the International Normalized Ratio (INR), by which PT can be expressed as a fraction of the control time. A more sensitive (1000-fold) assessment of vitamin K status with respect to prothrombin can be made by the immunoassay of des-γ-carboxy prothrombin, or undercarboxylated prothrombin, PIVKA-II (protein induced by vitamin K absence or antagonism).66 PIVKA-II has proved to be a useful marker of subclinical vitamin K deficiency. Another measurement of deficient γ-carboxylation, plasma undercarboxylated osteocalcin, has been shown to correlate individually with PIVKA-II and plasma phylloquinone concentrations and has a better correlation with plasma phylloquinone than PIVKA-II.599 In this study of biochemical indices of vitamin K nutritional status in a healthy adult population, and in a later one looking at changes in response to dietary phylloquinone,598 the urinary γ-carboxyglutamic acid : creatinine ratio was measured by derivatization, HPLC separation, and fluorometric detection and was shown to be sensitive to changes in dietary phylloquinone intake. This marker may have advantages in epidemiologic surveys as a less invasive sample. Direct measurement of plasma phylloquinone is probably the best indicator of vitamin K status and has been shown to correlate with intake.598 HPLC methods have been reviewed573 and typically require 0.5 to 2.0 mL of serum or plasma. Protein precipitation and lipid extraction (often into hexane) followed by solvent evaporation, preparative HPLC (to isolate vitamin K from other lipids), re-evaporation of the vitamin K–rich fraction, dilution in the mobile phase, and further HPLC, with electrochemical or fluorometric detection299 often after postcolumn reduction, are required. Typical between-batch imprecision values are coefficients of variation (CVs) of 11 to 18% with limits of detection lower than 50 pmol/L. An External Quality Assessment Scheme (EQAS) is available in the United Kingdom. The structure of thiamine (vitamin B1) [3-(4-amino-2-methyl-pyrimidyl-5-methyl)-4-methyl-5-(β-hydroxyethyl)thiazole] is that of a pyrimidine ring, bearing an amino group, linked by a methylene bridge to a thiazole ring (Figure 31-8). The thiazole has a primary alcohol sidechain at C5, which can be phosphorylated in vivo to produce thiamine phosphate esters, the most common of which is thiamine pyrophosphate (TPP) [also known as thiamine diphosphate (cocarboxylase)]. Monophosphate and triphosphate esters also occur. The basic vitamin is isolated or synthesized and handled as a solid thiazolium salt (e.g., thiamine chloride hydrochloride). Thiamine is somewhat heat labile, particularly in alkaline solutions, where base attacks occur at C2 of the thiazolium ring. Thiamine absorption occurs primarily in the proximal small intestine342 by a saturable (thiamine transporter) process at low concentration (1 µmol/L or lower) and by simple passive diffusion beyond that, although percentage absorption diminishes with increased dose. Absorbed thiamine undergoes intracellular phosphorylation, mainly to the pyrophosphate, but at the serosal side, 90% of transferred thiamine is present in the free form.522 Thiamine uptake is enhanced by thiamine deficiency and is reduced by thyroid hormone, diabetes, and ethanol ingestion. The gene for the specific thiamine transporter has been identified, and the transporter cloned.183 Thiamine is carried by portal blood to the liver. The free vitamin occurs in the plasma, but the coenzyme, TPP, is the primary cellular component. Approximately 30 mg is stored in the body, with 80% as pyrophosphate, 10% as triphosphate, and the rest as thiamine and its monophosphate. About half of body stores are found in skeletal muscle, with much of the remainder in heart, liver, kidneys, and nervous tissues (including the brain, which contains most of the triphosphate). The three tissue enzymes known to participate in the formation of phosphate esters are (1) thiaminokinase (a pyrophosphokinase), which catalyzes formation of TPP and adenosine monophosphate (AMP) from thiamine and adenosine triphosphate (ATP); (2) TPP-ATP phosphoryl-transferase (cytosolic 5′-adenylic kinase),317 which forms the triphosphate and adenosine diphosphate from TPP and ATP; and (3) thiamine triphosphatase, which hydrolyzes TPP to the monophosphate. Although thiaminokinase is widely distributed in the body, phosphoryl transferase and the membrane-associated triphosphatase are found mainly in nervous tissue. With the use of labeled thiamine probes, a study of thiamine metabolism at normal loads produced an estimated half-life of thiamine of 9.5 to 18.5 days, and showed a large number of breakdown products in the urine.19 Several of these urinary catabolites are shown in Figure 31-9.
Vitamins and Trace Elements
Nutritional Assessment and Monitoring
Protein-Energy Status
Vitamin and Trace Element Status
Markers of Antioxidant Status
Analytical Factors
Plasma Concentrations
Tissue Concentrations
Vitamins
Vitamin A
Chemistry
Dietary Sources
Absorption, Transport, Metabolism, and Excretion
Functions
Requirements and Reference Nutrient Intakes
Intravenous Supply
Deficiency
Toxicity
Laboratory Assessment of Status
Reference Intervals
Vitamin D
Vitamin E
Chemistry
Dietary Sources
Absorption, Transport, Metabolism, and Excretion
Functions
Requirements and Reference Nutrient Intakes
Intravenous Supply
Deficiency
Toxicity
Laboratory Assessment of Status
Reference Intervals
Vitamin K
Chemistry
Dietary Sources
Absorption, Transport, Metabolism, and Excretion
Functions
Requirements and Reference Nutrient Intakes
Intravenous Supply
Deficiency
Toxicity
Laboratory Assessment of Status
Vitamin B1—Thiamine
Chemistry
Absorption, Transport, Metabolism, and Excretion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Vitamins and Trace Elements