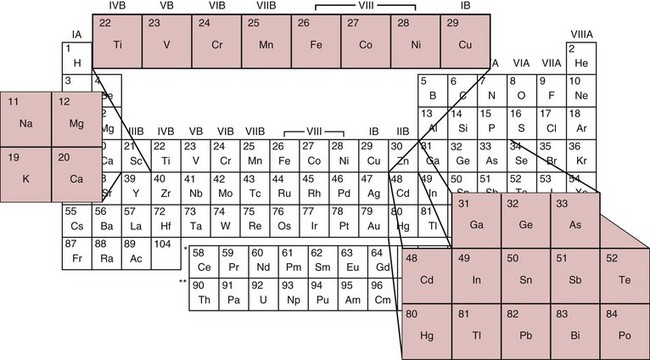
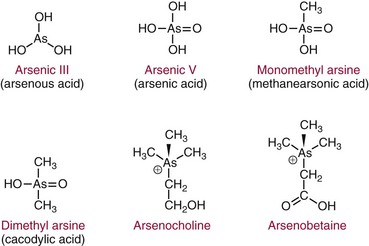
Chapter 36 Important questions to address when considering metal toxicity today are listed in Box 36-1. These questions are addressed generally in the first section of this chapter. In the second section, the unique characteristics of the more common metals known to be associated with toxicity are discussed. Readers are referred to The Handbook on the Toxicology of Metals101 for details on rare metal toxicities. As the twenty-first century begins, one would expect that metal toxicities would be thoroughly known and avoidable. However, humans frequently still encounter elemental toxins, and chronic, low-concentration exposure occurs more frequently in individuals than in large population groups. Concern continues regarding low-concentration exposure to lead and the effect such exposure has on mental development in the young. As is common in our environment, individuals are occasionally exposed because of lack of knowledge of the household products they are using. Many insecticides contain As as an active ingredient; careless use of these products has led to significant exposure. As is frequently identified as the cause of peripheral neuropathy among patients who have been unwittingly exposed. Ground water contaminated with As in the Bengal basin of Bangladesh, exceeding World Health Organization (WHO) safety limits because of leaching from bedrock, presents a serious health risk to the large population living in that region. Cadmium (Cd) is used to manufacture brightly colored paint pigments; painters who fail to use adequate respiratory protection while using abrasive materials to remove paint, or while spray painting with cadmium-containing products, can experience significant exposure. Cd is also significantly present in tobacco products.61 Studies indicate that apoptotic pathways are initiated by metals such as As, Cd, chromium (Cr), nickel (Ni), and beryllium (Be); and possibly lead (Pb), antimony (Sb), and cobalt (Co).108 The incidence of metal poisoning in a large population attributable to As, Cd, Pb, or mercury (Hg) poisoning appears to be of the same scale as the more common inborn errors of metabolism, such as neonatal hypothyroidism and phenylketonuria, and is the same order of magnitude as the incidence of adult-onset hemochromatosis, a disease for which mandatory screening has been suggested. Screening for these diseases is indicated because they are treatable, and treatment significantly reduces long-term morbidity.92 The same is true for metal toxicities. When identified early, disease caused by metal exposure is readily treatable with good outcomes. Conversely, if exposure is not identified and reduced, serious and sometimes irreparable damage to the nervous, renal, and cardiovascular systems can occur. In clinical practice, analysis of toxic elements should always be considered in the clinical work-up of the patient with (1) renal disease of unexplained origin, (2) bilateral peripheral neuropathy, (3) acute changes in mental function, (4) acute inflammation of the nasal or laryngeal epithelium, or (5) a history of exposure. Certain elements should be considered as the active, causative, or deficient agent in specific circumstances (Table 36-1). TABLE 36-1 Conditions That Involve Metal Toxicity Some metals are essential for life (see Chapter 31), but if an individual’s exposure exceeds a certain threshold, toxicity may develop. Some nonessential metals are toxic even at low concentrations. Review of the periodic table provides some insight into the determination of a metal’s potential toxicity (Figure 36-1). Employees are frequently monitored when working in an environment where exposure to toxic metals is a possibility.101 The most common form of monitoring involves quantification of airborne concentrations of metals in the production process. Threshold limit values (TLVs) for airborne concentrations and time interval exposure concentrations are defined by the U.S. National Institute for Occupational Safety and Health (NIOSH) to ensure worker safety. Workers may also be monitored by quantification of biological samples. The most common sample used is a random urine sample, and results are expressed in concentration units for the metal of interest per gram of creatinine to normalize for excretion volume variances. Cd, Cr, and Pb have defined urine excretion concentrations set by a U.S. federal agency to ensure worker safety.15,26,80 Additional technical and regulatory information about toxic metals is available at the Occupational Safety and Health Administration (OSHA) Website at http://www.osha.gov/SLTC/metalsheavy/index.html/ accessed June 2, 2011. The WHO and OSHA have defined blood concentrations for Pb that are designed to warn employers when workers are overexposed.113 Safety limits for other metals have been set by professional organizations, such as the American Conference of Governmental Hygienists.36 Analytical techniques used to measure metals in biological fluid includes (1) atomic absorption spectrometry with flame (AA-F) or electrothermal atomization furnace (AA-ETA), (2) inductively coupled plasma emission spectroscopy (ICP-ES), (3) inductively coupled plasma mass spectrometry (ICP-MS), and (4) high-performance liquid chromatography–inductively coupled plasma mass spectrometry (LC-ICP/MS). These tecniques are specific, sensitive, and provide the clinical laboratory with the capability to measure a broad array of metals at clinically significant concentrations. For example, ICP-MS is used to measure several metals simultaneously.13,88,100 Photometric assays have been used in the past but require large volumes of sample and have limited specificity. Spot tests are also available but should be considered obsolete because they are error prone, often yielding false-positive results. Certain metals are known to be toxic when humans are exposed to elevated concentrations; five metals are listed in the top 20 of the 2007 CERCLA (Comprehensive Environmental Response, Compensation, and Liability Act) Priority List of Hazardous Substances.21 They include As (No. 1), Pb (No. 2), Hg (No. 3), Cd (No. 7), and Cr (No. 18). Other metals of concern include aluminum (Al), Be, Co, copper (Cu), gadolinium (Gd), iron (Fe), manganese (Mn), Ni, platinum (Pt), selenium (Se), silicon (Si), silver (Ag), and Ti. The Agency for Toxic Substances and Disease Registry (ATSDR) provides toxicologic profiles for many of these metals on its Website.2 These hazardous substances are ranked on the basis of their frequency of occurrence, toxicity, and potential for human exposure. Several of these metals are also considered essential trace elements and are discussed in Chapter 31. Risk assessments for essentiality versus toxicity for Cr, Cu, iodine (I), Fe, Mn, molybdenum (Mo), Se, and zinc (Zn) have been performed by several U.S. governmental and private organizations.52,98 In 1972, Alfrey and colleagues first described an encephalopathy that was observed in patients undergoing prolonged hemodialysis for renal failure.1 The disease was characterized by abnormal speech, myoclonic jerks, and convulsions. Patients with these signs also showed a predominance of osteomalacic fractures. Subsequently, it was found that exposure of patients in renal failure to Al (1) laden dialysis water, (2) containing oral phosphate binders, and (3) laden albumin administered during dialysis is the primary cause of these signs of Al toxicity. Aluminum is also a developmental toxicant if administered parenterally.37 Under normal physiologic conditions, the usual daily dietary intake of Al is 5 to 10 mg, which is completely excreted. This excretion is accomplished by avid filtration of Al from the blood by the glomerulus of the kidney. Patients in renal failure lose this ability and are candidates for Al toxicity. The dialysis process is not highly effective at eliminating Al and can be a significant source of exposure. Furthermore, it is a common practice to administer Al-based gels orally to patients in renal failure to reduce the amount of phosphate absorbed from their diet to avoid excessive phosphate accumulation.104 A small fraction of this Al may be absorbed and patients in renal failure accumulate this Al. Following dialysis, albumin may be administered to replace that which is removed during dialysis. Some albumin products have high Al content resulting from the pharmaceutical purification process of passing the product through Al silicate filters. Aluminum (1) accumulates in blood if not filtered by the kidney; (2) avidly binds to proteins, such as transferrin; and (3) rapidly distributes throughout the body. Deposition of it in bone interrupts physiologic calcium exchange; the calcium in bone becomes unavailable for resorption into blood, a process under the physiologic control of parathyroid hormone (PTH) and 1,25-dihydroxy vitamin D (see Chapter 52). The normal physiologic action of PTH on bone is blunted in patients with renal failure because their renal cells are not synthesizing the 1,25-dihydroxy vitamin D required for normal PTH action. It is typical for patients in renal failure to have high serum PTH values and low serum calcium; this represents secondary hyperparathyroidism, the normal physiologic response to vitamin D deficit. Deposition of Al at the bone mineralization front and binding to parathyroid calcium receptors interfere with this physiologic process. The usual parathyroid response to these conditions decreases secretion of PTH. The result is lower-than-expected serum PTH concentration for the degree of renal disease present. In addition, a biochemical profile that characterizes Al overload disease has been defined.87 In human subjects with normal renal function, serum Al concentration is typically lower than 6 µg/L, but patients in renal failure invariably have serum Al concentrations significantly higher. Clinical guidelines published in 200691 suggest that patients with no signs or symptoms of osteomalacia or encephalopathy are likely to have serum Al concentrations <20 µg/L and PTH whole molecule concentrations 150 to 300 ng/L, typical for secondary hyperparathyroidism associated with renal failure.119 Patients with signs and symptoms of osteomalacia or encephalopathy typically have serum Al concentrations >60 µg/L, and PTH concentrations <65 ng/L indicate Al-related bone disease. Patients with serum Al concentrations >20 and <60 µg/L were identified as candidates for likely onset of Al-related bone disease; these patients required aggressive efforts to reduce their daily Al exposure. Efforts to reduce Al intake include (1) switching from Al-containing phosphate binders to calcium-containing phosphate binders, (2) ensuring that dialysis water contains less than 10 µg/L of Al, and (3) ensuring that albumin used during postdialysis therapy is Al-free. Interest in the role of Al in Alzheimer’s disease (AD) was raised when Perl observed that Al accumulates in the neurofibrillary tangle of patients with AD.107 He concluded that the focal accumulation of Al had an association with neurofibrillary degeneration in the hippocampal neurons that might play a role in the development of AD. Although a cause-and-effect relationship between accumulation of Al in brain and AD has yet to be conclusively demonstrated,11A,112 studies have clearly shown an increased concentration of Al in the brain.90 It is possible that accumulation of Al in the neurofibrillary tangle of AD patients is a secondary finding associated with the disease but not directly related to the cause. Also, the neurofibrillary tangle has a higher than normal affinity for Al that may explain increased accumulation of Al in brain tissue of Alzheimer’s patients. Aluminum-related bone disease has been diagnosed and treated with deferoxamine, an avid chelator of both iron and Al.31 The deferoxamine infusion test is useful for the ultimate diagnosis of Al overload disease, and the drug has demonstrated utility for treating acute Al overload.31,40 Preanalytical considerations related to collection of specimen for Al analysis are significant. Most of the common evacuated blood collection devices used in phlebotomy today have rubber stoppers that are made of Al silicate. Puncture of the rubber stopper for blood collection is sufficient to contaminate the sample with Al and produce an abnormal concentration of Al. Typically, blood collected in standard evacuated blood tubes will be contaminated by 20 to 60 µg/L of Al; this is readily demonstrated by collecting blood from a healthy volunteer into a standard evacuated phlebotomy tube. Special evacuated blood collection tubes are required for Al testing.94 These tubes are readily available from commercial suppliers and should always be used. Failure to pay attention to this issue can result in the generation of abnormal results because of sample contamination, which leads to misinterpretation and misdiagnosis. Sb compounds have been known since ancient Egyptian times and were used as cosmetics by the women of that era. In the sixteenth century, Sb preparations were thought to be wonder drugs that in the nineteenth century were prescribed for a number of conditions. Today antimony compounds are used to treat parasitic diseases such as leishmaniasis, schistosomiasis, and bilharziasis.18,126 Antimony is not an essential metal. Workplace exposure to Sb dust over a period of years leads to pneumoconiosis. The size of the dust particles of Sb trioxide significantly increases the occurrence of pneumoconiosis, with smaller particles being more dangerous.126 The workers at greatest danger are those in underground facilities and metal production. Smoking may also contribute to respiratory problems. Symptoms of acute exposure include (1) a metallic taste, (2) headache, (3) nausea, and (4) dizziness; and after a short interval, (5) vomiting, (6) diarrhea, and (7) intestinal spasms. In chronic intoxication, adverse health effects include (1) cardiac arrhythmias, (2) upper respiratory and ocular irritation, (3) spontaneous abortion, (4) premature birth, and (5) dermatitis.35 Lymphocytosis, eosinophilia, and a reduction in leukocyte and platelet counts are also seen and indicate damage to the liver and spleen. Evidence supports increased risk for the development of lung cancer in Sb smelter workers, but the effect may be multifactorial and may be due, for example, to the presence of arsenic in the work environment.30 It is important to remember that when intoxication occurs with metallic Sb, the effect is caused not only by Sb, but also by the lead, arsenic, and other metals that may accompany it. As is perhaps the best known of the metal toxins, having gained notoriety from its extensive use by Renaissance nobility as an antisyphilitic agent and an antidote against acute arsenic poisoning; long-term administration of low arsenic doses protects against acute poisoning by massive doses—an historic example of hepatic enzyme induction. This agent was memorably used in the well-known tale “Arsenic and Old Lace”73 as a means of terminating undesirable acquaintances. Currently, As is still a dangerous toxicant as evidenced by the Bangladesh incident wherein several hundred persons were poisoned by drinking ground water contaminated with As leaching from bedrock. As discussed earlier, As is listed as the No. 1 toxicant on the 2007 U.S. CERCLA Priority List of Hazardous Substances,21 and it is still used extensively in insecticides. Arsenic exists in numerous toxic and nontoxic forms.93 The toxic forms include (1) the inorganic species As3+, also denoted as As(III); (2) the more toxic As5+, also known as As(V), and their partially detoxified metabolites; (3) monomethylarsine (MMA); and (4) dimethylarsine (DMA). Detoxification occurs in the liver as As5+ is reduced to As3+ and then is methylated to MMA and DMA. As a result of these detoxification steps, As3+ and As5+ are found in the urine shortly after ingestion, whereas MMA and DMA are the species that predominate longer than 24 hours after ingestion. Urinary As3+ and As5+ concentrations peak in urine at approximately 10 hours and return to normal 20 to 30 hours after ingestion. Urinary MMA and DMA concentrations normally peak at about 40 to 60 hours and return to baseline 6 to 20 days after ingestion.93 In a large U.S. population study, for all participants aged >6 years, dimethylarsinic acid and arsenobetaine had the greatest contribution to the quantity of total urinary arsenic. Aarsenobetaine was the primary contributor to high total urinary arsenic concentrations.16,33,45 The half-life of inorganic As in blood is 4 to 6 hours, and the half-life of the methylated metabolites is 20 to 30 hours. Blood concentrations of As are elevated for only a short time after administration, after which As rapidly disappears into the large body phosphate pool. Abnormal blood As concentrations in the 5 to 50 ng/mL range are detected after exposure.55 The structures of these and related As species are shown in Figure 36-2. Nontoxic forms of As are present in many foods. Arsenobetaine and arsenocholine are the two most common forms of organic As that are found in food.59 The foods that most commonly contain significant concentrations of organic As are shellfish and other predators in the seafood chain (e.g., cod, haddock). In the large U.S. population study, for all participants aged >6 years, dimethylarsinic acid and arsenobetaine had the greatest contribution to the total urinary arsenic. Arsenobetaine was the primary contributor to high total urinary arsenic concentrations.16 Arsenic excretion in normal people who have ingested arsenobetaine-containing foods is >120 µg per 24-hour specimen. Following ingestion, arsenobetaine and arsenocholine undergo rapid renal clearance to become concentrated in the urine. Arsenobetaine and arsenocholine are completely excreted within 1 to 2 days after ingestion, and no residual toxic metabolites are present. The apparent half-life of organic As is 4 to 6 hours. Consumption of seafood before collection of a urine sample for As testing is likely to result in an elevated concentration of As in the urine; this can be clinically misleading. The toxicity of As is due to three different mechanisms, two of which are related to energy transfer. Arsenic avidly binds to dihydrolipoic acid, a necessary cofactor for pyruvate dehydrogenase. Absence of the cofactor inhibits the conversion of pyruvate to acetyl coenzyme A—the first step in gluconeogenesis. Arsenic competes with phosphate for reaction with adenosine diphosphate (ADP), resulting in formation of the lower-energy adipic acids (ADPAs) rather than adenosine triphosphate (ATP). Arsenic also binds with any hydrated sulfhydryl group on protein, distorting the three-dimensional configuration of the protein, thus causing it to lose activity. British antilewisite (BAL) is an effective antidote for treating As intoxication; the active agent in BAL is dimercaprol, a sulfhydryl-reducing agent. This suggests that the primary mechanism of action of the toxicity of As is related to sulfhydryl binding. Arsenic also interferes with the activity of several enzymes of the heme biosynthetic pathway.49 Arsenic is also a known carcinogen24 as evidence suggests increased risk of bladder, skin, and lung cancers, as well as lung cancer associated with smoking, following consumption of water with high As contamination.11,115 To distinguish among toxic inorganic species and nontoxic organic species of As of seafood origin, high-performance liquid chromatography (HPLC) techniques that separate the various species of As in biological fluids and tissues have been developed.16 A typical finding in a urine specimen with total 24-hour excretion of As of 350 µg/24 hours is that more than 95% is present as the organic nontoxic seafood species, and less than 5% is present as the inorganic toxic species. Such a finding indicates that the elevated total As concentration was likely due to ingestion of seafood. Hair analysis is frequently used to document time of As exposure.76 Arsenic circulating in the blood will bind to protein by formation of a covalent complex with sulfhydryl groups of the amino acid cysteine. Because As has a high affinity for keratin, which has high cysteine content, the As concentration in hair or nails is greater than in other tissue. Several weeks after exposure, transverse white striae, called Mees’ lines, may appear in the fingernails; this event is caused by denaturation of keratin by metals such as As, Cd, Pb, and Hg. Because hair grows at a rate of approximately 0.5 cm/mo, hair collected from the nape of the neck can be used to document recent exposure. Axillary or pubic hair is used to document long-term (6 months to 1 year) exposure. Hair As >1 µg/g dry weight indicates excessive exposure. In one study, the highest hair As observed was 210 µg/g dry weight in a case of chronic exposure that was the cause of death.63 Blood is the least useful specimen for identifying As exposure. Blood As concentrations are elevated for only a short time after administration55 and rapidly disappear into the large body phosphate pool, because the body treats As like phosphate, incorporating it wherever phosphate would be incorporated. Absorbed As is rapidly circulated and distributed into tissue storage sites. Abnormal blood As concentrations are detected for only a few hours (<4 hours) after ingestion. This test is useful only to document an acute exposure when the As is likely to be >20 ng/mL for a short period of time. Typically, serum As is <40 ng/mL. Arsenic has been accurately analyzed by ICP-MS. The specimen is prepared in dilute acid containing gallium as an internal standard and is aspirated directly into the argon plasma. Mass response from the argon plasma is monitored for As (75 m/z), gallium (70 m/z), and 16O35Cl (51 m/z) to allow for correction for 40Ar35Cl (75 m/z) interference. The operator must be aware of the potential for interference from argon chloride. A correction is made by accounting for chloride by measuring 51 m/z and subtracting that residual from 75 m/z.100 Urine is the sample of choice for As analysis because As is excreted predominantly by the kidney, where it becomes concentrated. The general population is exposed to low concentrations of Be through food and drinking water; these exposures are of no clinical consequence. The major route by which Be enters the body is via the respiratory tract, and industrial exposure usually occurs from inhalation and ingestion of Be dust. Inhaled Be compounds are cleared very slowly from the lungs. Soluble compounds are absorbed to a much greater degree than other such as Be oxide, which are much less soluble. Beryllium salts are strongly acidic when dissolved in water, and this is thought to have a major toxic effect on human tissue. Absorbed Be accumulates in the skeleton. Renal clearance is very slow. Beryllium inhibits a variety of enzyme systems, including alkaline phosphatase, acid phosphatase, phosphoglycerate mutase, hexokinase, and lactate dehydrogenase.114 Acute exposure to Be is rare, is usually caused by an industrial accident or explosion, and typically results in chemical pneumonitis. Chronic Be exposure in the workplace has led to occupational health concerns because of its potential to cause a progressive and potentially fatal respiratory condition called chronic Be disease (CBD). This disease, also known as berylliosis, is characterized by the formation of granulomas resulting from an immune reaction to Be particles in the lung.34,67 To reduce the number of workers currently exposed to beryllium in the course of their work at the U.S. Department of Energy (DOE) facilities or among its contractors, the DOE has established a chronic beryllium disease prevention program (CBDPP) to minimize the concentrations of, and the potential for exposure to, beryllium, and has put forth medical surveillance requirements to ensure early detection of the disease (http://www.hss.energy.gov/healthsafety/wshp/be/ accessed June 2, 2011). Studies have suggested that the size of the Be particles affects not only the site of deposition but also the amount deposited. This in turn may influence the clearance rate and thus the time of contact between the immune cells and Be.74 Several years ago, researchers noted that blood and lung cells from CBD patients proliferated when exposed to Be in culture. This assay has been refined and is offered as the Be lymphocyte proliferation test (BeLPT). Unfortunately, because of the nature of the test and the variability from lab to lab, the BeLPT has been known to produce false-negative and problematic results.34,86 Efforts are under way by several groups to standardize the assay. Despite these issues, the BeLPT in bronchoalveolar cells is part of the current “gold standard” diagnosis for CBD.114 Quantification of Be in serum or urine is not useful in making this diagnosis. Air analysis (TLV) is the preferred method of exposure evaluation.75 The toxicity of Cd resembles that of As, Hg, and Pb in that it attacks the kidney; renal dysfunction with proteinuria of slow onset (over a period of years) is the typical presentation. Chronic exposure to Cd causes accumulated renal damage.15,33,101 Breathing the fumes of Cd vapors leads to nasal epithelial deterioration and pulmonary congestion resembling chronic emphysema. Cadmium toxicity is expressed via formation of protein-Cd adducts that change the conformational structure of the protein, causing it to denature. This protein denaturation occurs at the site of highest concentration—in the alveoli if exposure is due to dust inhalation, and in the proximal tubule of the kidney because this is a major route of excretion. NIOSH regulations mandate that employees exposed to Cd in the workplace must be monitored using quantification of urine Cd and creatinine, with the results in µg of Cd expressed per gram of creatinine.95 This is based on the finding that renal damage caused by Cd exposure is detected by increased Cd excretion relative to creatinine. Cadmium excretion >3 µg Cd/g of creatinine indicates significant exposure to Cd. Results >15 µg Cd/g of creatinine are considered indicative of severe exposure. Urine Cd is a more specific measure of Cd exposure than are other markers of renal function, such as β2-microglobulin, retinol-binding protein, and N-acetyl glucosaminidase.33,95 Normal blood Cd concentration is less than 5 ng/mL, with most concentrations in the interval of 0.5 to 2 ng/mL. Moderately increased blood Cd (3 to 7 ng/mL) may be associated with tobacco use.61 Acute toxicity is observed when the blood concentration exceeds 50 ng/mL. Usual daily excretion of Cd is less than 3 µg/d. Collection of urine samples using a rubber catheter has been known to result in elevated results because rubber contains trace amounts of Cd that are extracted as urine passes through it. Brightly colored plastic urine collection containers should be avoided because the pigment in the plastic may be Cd-based. Cadmium concentrations also increase with age and may be involved with senescence.9 Cadmium is usually quantified by atomic absorption spectrometry, but it has been accurately quantified by ICP-MS.100
Toxic Metals
Assessment of Metal Poisoning
Prevalence of Metal-Based Toxicity
Diagnosing Toxicity
Metal
Condition
Aluminum
Dialysis, encephalopathy, or dementia
Arsenic
Bilateral pain radiating from feet to legs or peripheral neuropathy, or unexplained impaired renal function
Cadmium
Impaired renal function in aerosol painters
Copper-zinc deficiency
Impaired wound healing
Gadolinium
Nephrogenic systemic fibrosis
Lead
Children younger than 2 years living in older homes, or unexplained gastric upset, anemia, or impaired renal function at any age
Mercury
Acute changes in behavior, impaired speech, visual field constriction, hearing loss, and somatosensory disorders
Manganese
Onset of parkinsonism younger than age 50
Selenium (deficiency)
Patients undergoing total parenteral nutrition
Thallium
Acute hair loss
Zinc (deficiency)
Burn patients exhibiting erythema
Classification of Metals
Occupational Monitoring
Analytical Methods
Specific Metals
Aluminum
Antimony
Arsenic
Beryllium
Cadmium
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine