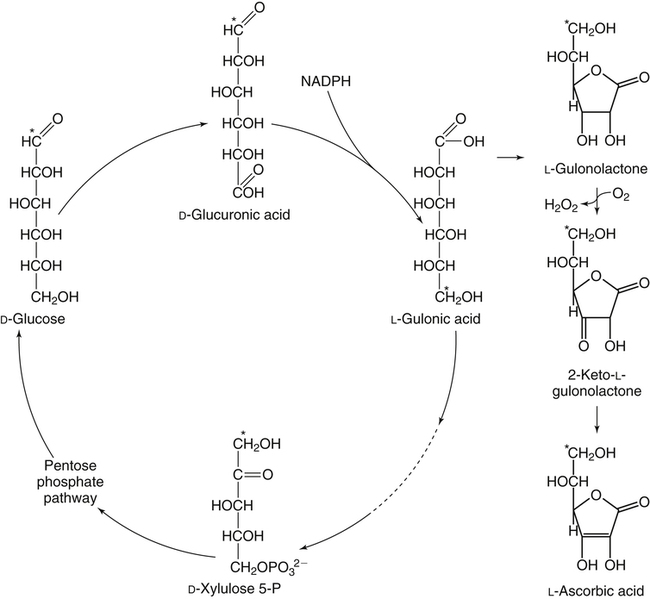
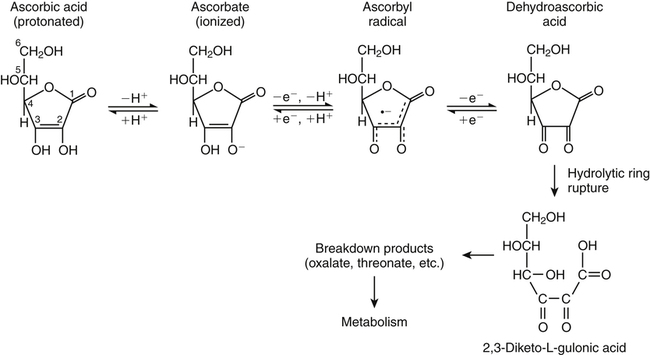
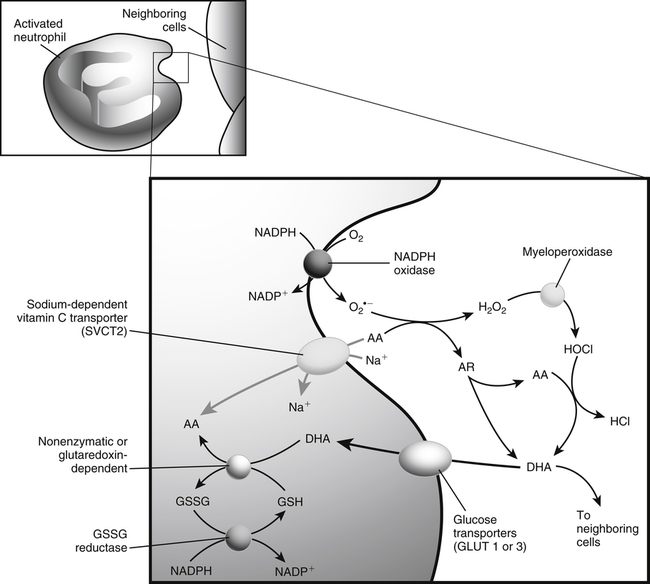
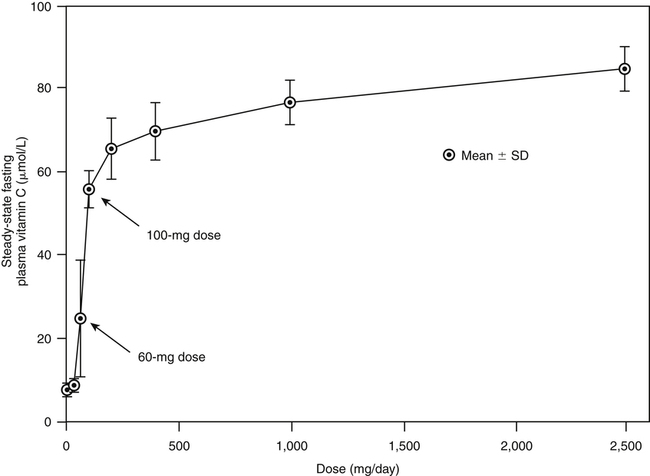
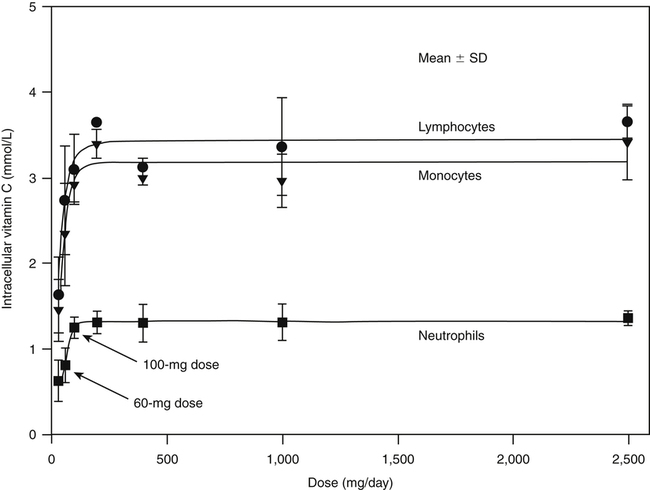
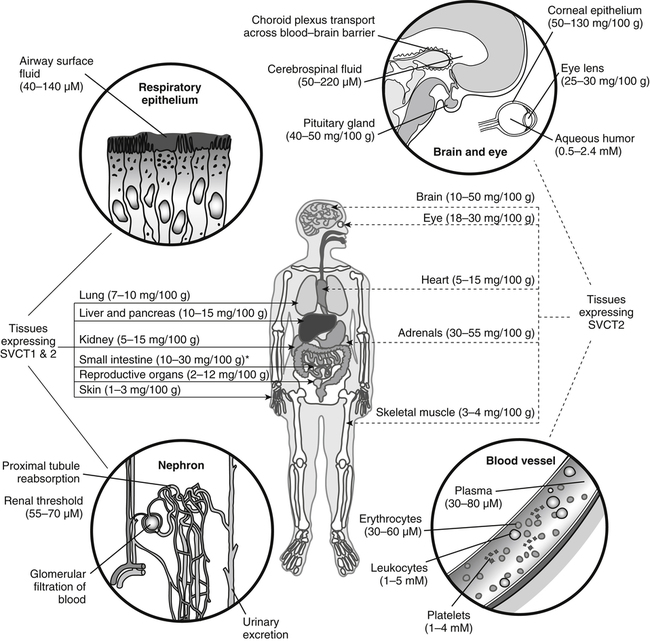
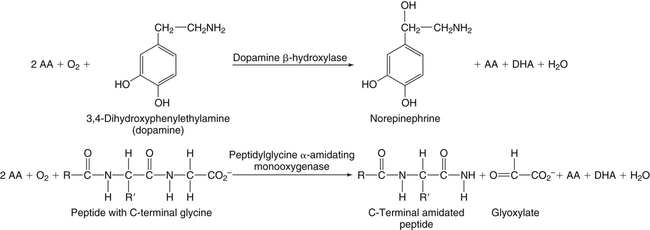
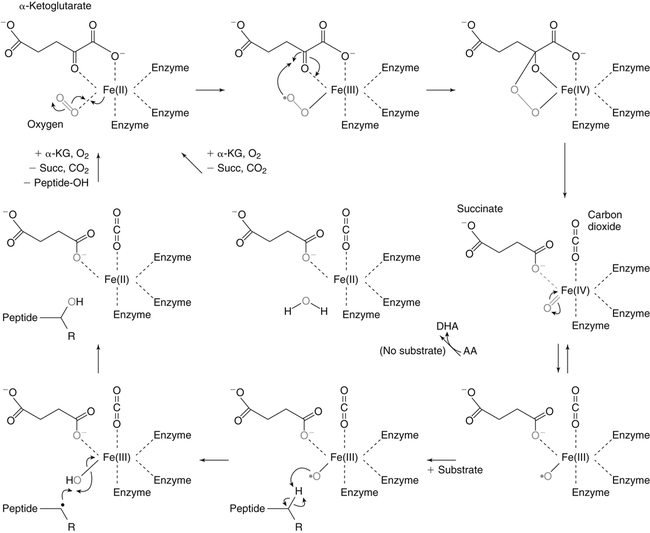
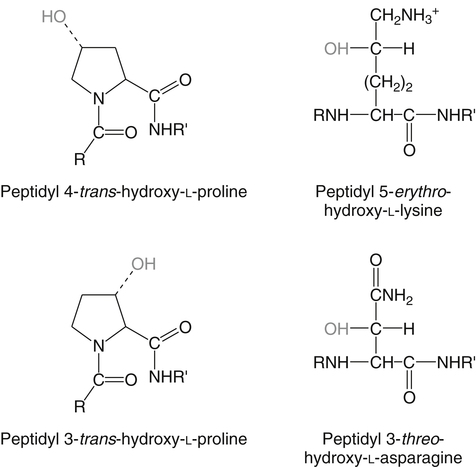
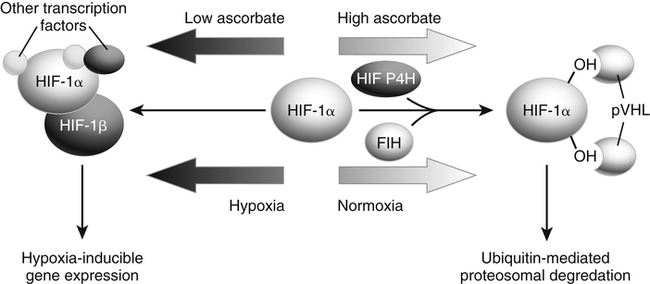
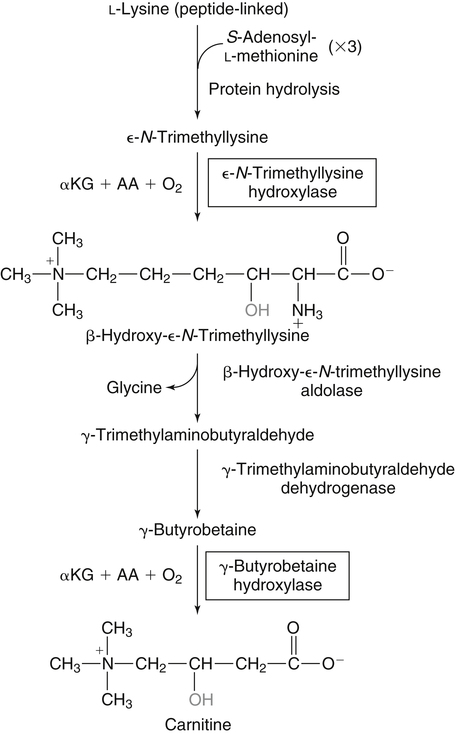
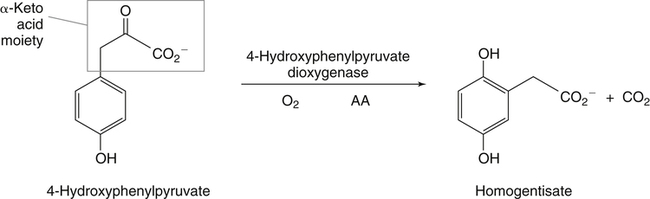
Alexander Michels, PhD and Balz Frei, PhD∗ Vitamin C is an essential micronutrient in the human diet. The electron donating or reducing capacity of ascorbate, the ionized form of ascorbic acid, allows it to participate in various enzymatic and nonenzymatic reactions that encompass all of its known biological functions. These include, for example, several enzymatic hydroxylation reactions in the biosynthesis of collagen, carnitine, and norepinephrine, as well as various nonenzymatic reactions that may protect cells against damaging free radicals and reactive oxygen species. Although vitamin C, also known as ascorbic acid or ascorbate, was discovered early in the twentieth century, our knowledge of vitamin C transport and distribution in the human body and its metabolism and biological functions remains incomplete. Long known for its role in the prevention of the deficiency disease scurvy, emerging research suggests an important role for vitamin C in the prevention of chronic diseases, particularly cardiovascular diseases, and novel applications of vitamin C in the treatment of cancer. As our understanding of the role of vitamin C in biology evolves, dietary intake recommendations to promote human health will need to be reevaluated. The reduced form of vitamin C, ascorbic acid, is a six-carbon lactone synthesized from glucose by many animals (Figure 27-1). Most mammals synthesize ascorbic acid in the liver, but in reptiles and some birds synthesis occurs in the kidneys (Chatterjee, 1973). Vitamin C synthesis is coupled to glycogenolysis, and thus is downregulated in animals on carbohydrate-restricted diets or when glycogen is depleted during fasting (Banhegyi et al., 1997). Several species are unable to synthesize ascorbic acid, including humans and other simian primates, guinea pigs, Indian fruit bats, capybaras, and various bird and fish species. Humans lack gulonolactone oxidase, the terminal enzyme in the biosynthetic pathway of ascorbic acid. The gene encoding the enzyme in these species has undergone substantial mutation so that no protein is produced (Nishikimi et al., 1994). Animals unable to synthesize ascorbic acid must ingest it to survive, so ascorbic acid is a vitamin for humans. At neutral pH ascorbic acid exists primarily as the ascorbate monoanion (Figure 27-2), although a small amount of the dianion also exists. For this reason, the term ascorbate is used synonymously with ascorbic acid, although in many cases the ascorbate anions are the reacting molecules. The fully protonated molecule, ascorbic acid, only exists in low pH environments. Although the term vitamin C typically refers to the reduced forms of the molecule (ascorbic acid or ascorbate), it can also be understood as the combination of these reduced forms and oxidized forms (ascorbyl radical and dehydroascorbic acid). Ascorbate acts as an electron donor (reducing agent), which accounts for all of its known biochemical and molecular functions. Two electrons of the 2-ene-2,3-diol (ene-diol) structural element of ascorbate are available for donation (see Figure 27-2). The full oxidation of ascorbic acid to dehydroascorbic acid reflects the ascorbate donation of both of these electrons to one or more acceptor molecules. However, the full oxidation of ascorbate in a single step is an unlikely occurrence because electrons are usually lost sequentially (Halliwell, 1996), with formation of the intermediate free radical called ascorbyl radical (sometimes also termed semidehydroascorbic acid, monodehydroascorbic acid, or ascorbate free radical). Because of resonance stabilization of the unpaired electron across the ene-diol and 1-oxo groups (see Figure 27-2), the ascorbyl radical reacts poorly with other molecules (Buettner and Jurkiewicz, 1996). The reactivity of ascorbate with many potentially harmful radical species and the relative stability of its free-radical form make ascorbate an ideal electron donor and strong antioxidant in biological systems. These roles of ascorbic acid are discussed later in this chapter. The ascorbyl radical, formed by partial oxidation of ascorbate, can be reduced to ascorbate, although the enzymes that catalyze this reduction have not been fully characterized. Ascorbyl radical reducing activities have been observed in membranes of mitochondria, endoplasmic reticulum (ER) and erythrocytes (May et al., 2001a), which may be a component of a transmembrane electron transfer system (see later section, Vitamin C Transport and Tissue Distribution). The ascorbyl radical can also be reduced by the cytosolic selenium-containing enzyme thioredoxin reductase (May et al., 1998). Furthermore, two molecules of ascorbyl radical react readily with each other in a nonenzymatic dismutation reaction that reduces one molecule of ascorbyl radical to ascorbate, while the other molecule is oxidized to dehydroascorbic acid (Bors and Buettner, 1997). Dehydroascorbic acid may exist in one of several forms, and it is likely that the dominant form in vivo is the bicyclic hemiketal (Corpe et al., 2005). The term dehydroascorbic acid is used widely in the scientific literature; the designation “dehydroascorbate” is incorrect, because dehydroascorbic acid is not ionized under physiological conditions. Although dehydroascorbic acid is more stable than the ascorbyl radical, dehydroascorbic acid degradation at physiological pH occurs within minutes. At acidic pH, especially below pH 4, dehydroascorbic acid stability improves markedly. The breakdown of dehydroascorbic acid occurs through hydrolysis, with irreversible rupture of the ring to yield 2,3-diketo-L-gulonic acid (see Figure 27-2). Although 2,3-diketo-L-gulonic acid metabolism is not well characterized, its metabolic products include oxalate, threonate, threose, xylonate, lyxonate, and L-erythrulose (Banhegyi and Loewus, 2004). These breakdown products of vitamin C may enter the pentose phosphate pathway, gluconeogenesis, or other metabolic pathways (Banhegyi et al., 1997). It has been reported that carbons from vitamin C are released as carbon dioxide from cells or expired by animals (Baker et al., 1975). Additionally, murine and human erythrocytes can produce lactate when provided ascorbic acid (Braun et al., 1997), suggesting that ascorbate breakdown products can enter normal pathways of carbohydrate metabolism. Like the ascorbyl radical, dehydroascorbic acid is rapidly reduced in vivo, either by one-electron reduction to the ascorbyl radical or two-electron reduction to ascorbate, referred to as “ascorbate recycling.” Recycling mechanisms exist even in animals that synthesize ascorbic acid (Banhegyi et al., 1997), allowing them to recycle ascorbate that has been oxidized in the body or dehydroascorbic acid that has been ingested in the diet. Dehydroascorbic acid reduction in biological systems is accomplished through several pathways that ultimately require NADPH as the electron-donating agent (Arrigoni and De Tullio, 2002; Winkler et al., 1994). Dehydroascorbic acid can be reduced chemically by glutathione (GSH) or enzymatically by the GSH-dependent enzymes: glutaredoxin, protein disulfide isomerase, and dehydroascorbate [sic] reductase. Glutathione disulfide (GSSG) produced in these reactions is reduced back to GSH by the NADPH-dependent enzyme, glutathione disulfide reductase (Figure 27-3). In addition, dehydroascorbic acid can be reduced by the NADPH-dependent enzymes, 3α-hydroxysteroid dehydrogenase and thioredoxin reductase. Because depleting intracellular GSH leads to deficits in dehydroascorbic acid reduction capacity (May et al., 2001b; May et al., 1996), GSH-dependent mechanisms for ascorbate recycling appear to predominate in cells. Nevertheless, recycling of ascorbate by reduction of dehydroascorbic acid and the ascorbyl radical in humans appears to be inefficient, because total body ascorbate is depleted over time on a vitamin C–free diet. Hence, nonsynthesizing species require vitamin C in the diet to make up for the deficit between recycling and destruction mechanisms. Currently there are no means to measure these rates separately in humans. What can be measured is ascorbate disappearance as a function of time and ascorbate dose, usually in plasma or blood cells. Such data show that when vitamin C ingestion ceases, healthy humans become vitamin C–deficient after approximately 30 days (Levine et al., 1996b; Levine et al., 2001). However, the time needed to reach a deficiency state depends on body stores when ingestion ceases and the rate of ascorbate oxidation, which can be influenced by many factors. Additionally, it is currently unknown if the rate of disappearance of vitamin C in plasma is indicative of the vitamin C status in all tissues. Some tissues, such as the brain, maintain higher vitamin C levels despite lower concentrations in other tissues. Ascorbate is synthesized in all green plants and is found in abundance in many fruits and vegetables. Fruits high in vitamin C include cantaloupe, kiwi, strawberries, lemons, and oranges. Good vegetable sources are broccoli, red pepper, cauliflower, spinach, tomatoes, Brussels sprouts, and asparagus. Fruit and vegetable juices that are good sources of the vitamin are orange, grapefruit, and tomato juices. Many foods, such as breakfast cereals and fruit drinks, are fortified with vitamin C. Vitamin C is present in many animal products, such as meat, poultry, and eggs, but is generally lost upon cooking or heating, making these foods unreliable sources of the vitamin. Unfortified grains and cereals and dairy products are generally low in vitamin C (U.S. Department of Agriculture, 2010). Both ascorbate and dehydroascorbic acid are present in foods. Ascorbate predominates, accounting for 80% to 90% of the total vitamin C content (Vanderslice and Higgs, 1991). There is limited information concerning the relationship between food aging and progressive ascorbate oxidation and dehydroascorbic acid formation, but increased oxidation is expected during prolonged storage, which may be slowed by cooling or freezing (Vanderslice and Higgs, 1991). Food preparation also can have a significant effect on vitamin C content. Heating destroys vitamin C in foods, which is accelerated by the presence of metals like copper or iron, which may be released from the cooked food or from cooking receptacles. Foods cooked quickly will retain some vitamin C, and quick boiling or microwave steaming is usually recommended for preserving vitamin C in cooked vegetables (Miglio et al., 2008; Vanderslice and Higgs, 1991). Foods treated with heat before or during preservation (e.g., pasteurization) contain less vitamin C than the fresh food. Although vitamin C is relatively stable for extended periods of time in neutral or basic solutions, it is best preserved at acidic pH. Oxygen and light exposure also affect the vitamin C content of food. Vitamin C is added to many food products to preserve freshness and enhance shelf life. Usually the amount of vitamin C added is minimal, and it may be oxidized during storage. The ascorbate epimer, erythorbic acid, also called D-isoascorbic acid and D-araboascorbic acid, is an antioxidant food additive used to preserve smoked or cured meats and some beverages. Although erythorbic acid has no vitamin C–like (antiscorbutic) activity, consumption of large amounts may result in false indications of ascorbate concentrations in blood or urine samples (Sauberlich et al., 1996). Studies in guinea pigs have shown that ascorbic acid and dehydroascorbic acid are absorbed primarily in the jejunum of the small intestine (Rose, 1988). Ascorbate absorption is a sodium-dependent process, whereas absorption of dehydroascorbic acid is sodium-independent. Once absorbed, dehydroascorbic acid is reduced to ascorbate in enterocytes. Thus ingestion of either form of vitamin C results in ascorbate accumulation in the blood. In animals that are unable to synthesize vitamin C, ingested dehydroascorbic acid can prevent scurvy; but because there is extensive degradation of dehydroascorbic acid, much higher doses are needed compared to ascorbate (Ogiri et al., 2002). Dietary supplementation with dehydroascorbic acid should be avoided because of possible negative metabolic consequences of high intakes of dehydroascorbic acid (Arrigoni and De Tullio, 2002). The bioavailability and pharmacokinetics (or more appropriately, “micronutrient kinetics”) of vitamin C have been determined in young, healthy individuals. These studies indicate that greater than 80% of a single vitamin C dose between 15 and 100 mg is absorbed (Graumlich et al., 1997; Levine et al., 1996b). Bioavailability declines for higher doses and is slightly less than 50% for a dose of 1,250 mg. Pharmacokinetic data for plasma and leukocyte vitamin C concentrations obtained from seven healthy young men previously depleted of vitamin C (Levine et al., 1996b) are shown in Figures 27-4 and 27-5, respectively. Similar results were obtained in women (Levine et al., 2001). At low doses of vitamin C (30 to 100 mg/day), there was a steep dose-dependent increase of plasma and cell ascorbate levels. Plasma ascorbate levels reached near-saturation at daily doses of 200 to 400 mg of vitamin C (see Figure 27-4), whereas cell ascorbate levels reached near-saturation at doses between 100 and 200 mg/day (see Figure 27-5). The shape of these curves for ascorbic acid bioavailability and pharmacokinetics is determined by the regulation of vitamin C transport mechanisms. Ascorbate concentrations in plasma (usually 30 to 80 μmol/L) are lower than in tissues, most of which accumulate vitamin C in millimolar concentrations (Figure 27-6). Higher levels of ascorbate accumulate in extracellular fluids other than plasma, including air surface liquid of the respiratory tract (van der Vliet et al., 1999), gastric juices (Waring et al., 1996), cerebrospinal fluid (Bowman et al., 2010), seminal fluid (Fraga et al., 1991), and the aqueous humor of the eye (Taylor et al., 1991). It should be emphasized that the tissue concentrations of ascorbate shown in Figure 27-6 were obtained from limited literature sources, which are mostly compiled from older historical data (Hornig, 1975). The reported concentrations of ascorbate in most human tissues were not determined using modern techniques for preservation and analysis. More recent data, although limited, show that vitamin C levels in human tissues are higher than previously reported (Atanasova et al., 2005; Brubaker et al., 2000; Kathir et al., 2010; Kuiper et al., 2010; Waring et al., 1996) and similar to those measured in guinea pigs. Thus it is generally believed that adrenals, corneal epithelium, and pituitary glands have the highest concentrations of ascorbate in the human body. Other parts of the brain and eye, and spleen, pancreas, liver, and kidney also have high ascorbate levels, whereas most other tissues maintain relatively low millimolar levels ranging from 1 mg to 15 mg per 100 g of tissue (see Figure 27-6). In the blood, leukocytes maintain millimolar concentrations of ascorbate (Levine et al., 2001), whereas erythrocytes have the lowest cellular level because they do not concentrate vitamin C from the plasma (Evans et al., 1982). It is often assumed that the relationship between vitamin C levels in plasma and tissues is the same regardless of the organ studied. However, data from vitamin C depletion studies in experimental animals suggest otherwise. Ascorbate levels do not decline at the same rate in various tissues when plasma levels decrease. In particular, the brain and eye are less rapidly depleted than other organs of the body (Harrison et al., 2010b; Hill et al., 2003; Parsons et al., 2006; Tanaka et al., 1997). Upon Vitamin C-repletion, it is believed that ascorbate levels saturate in the brain before any other tissues, but the exact relationship with dietary vitamin C dose or plasma concentration is unknown. The organ-specific variation in vitamin C accumulation rates is considered an effect of the various vitamin C transporters in the body. Ascorbate is transported into cells by sodium-dependent vitamin C transporters (SVCTs) that exist in two different isoforms, SVCT1 and SVCT2 (Tsukaguchi et al., 1999). These two transporters belong to the nucleobase transporter superfamily (Hogue and Ling, 1999) but are otherwise dissimilar to other sodium-dependent transporters. Both transporters are highly specific for ascorbate and do not transport dehydroascorbic acid, glucose, or many other structurally related compounds (Rumsey et al., 1997; Tsukaguchi et al., 1999). However, some ascorbic acid derivatives with small structural changes are recognized by SVCTs (Corpe et al., 2005; Rumsey et al., 1999). SVCT1 and SVCT2 are also specific for transport of the sodium ion, cotransporting one or two sodium ions with each molecule of ascorbate into cells, although SVCT2 also requires calcium and magnesium for activity (Godoy et al., 2007). Removal of sodium from the extracellular fluid or inhibition of the Na+/K+-ATPase effectively halts all SVCT ascorbate-transport activity (Luo et al., 2008). Primarily located on the apical side of polarized epithelial cells, SVCT1 is expressed in intestine, liver, kidney, lung, epididymis, ovary, prostate, placenta, pancreas, and skin (see Figure 27-6). SVCT1 has a Km of approximately 60 to 200 μmol/L (Savini et al., 2008) and achieves a velocity approaching Vmax at a concentration of about 1 mmol/L (Varma et al., 2008). SVCT1 is responsible for intestinal absorption of ascorbate by the enterocyte and renal reabsorption of ascorbate in the proximal tubules of the kidneys. SVCT1 knockout mice continuously excrete vitamin C in the urine and, as a result, have very low plasma vitamin C concentrations (Corpe et al., 2010). SVCT2 is widely distributed in the body and expressed in every cell type except erythrocytes (May et al., 2007). SVCT2 has a reported Km of approximately 5 to 60 μmol/L with a velocity approaching Vmax at a concentration of about 60 to 100 μmol/L (Savini et al., 2008). Hence, compared to SVCT1, which is responsible for bulk vitamin C transport, SVCT2 is a high-affinity, low-capacity vitamin C transporter. In epithelial cells that express both SVCTs, SVCT1 is found on the apical side and SVCT2 on the basolateral side (Boyer et al., 2005; Maulen et al., 2003; Subramanian et al., 2004). Thus, SVCT2 is responsible for the exit of ascorbate from enterocytes or renal proximal tubules so that it can enter the circulation. SVCT2 also is responsible for uptake of ascorbic acid into tissues from the extracellular fluid, and genetic deficiency of SVCT2 is associated with near-depletion of vitamin C in every tissue examined (Sotiriou et al., 2002). SVCT2 is required for the accumulation of vitamin C in tissues that display the highest concentrations (10 to 25 mmol/L) of the vitamin, such as brain and adrenals. SVCT2 knockout mice do not survive past birth, because they quickly develop cerebral hemorrhage and are unable to breathe (Sotiriou et al., 2002), underscoring the need for vitamin C in normal prenatal development. The regulatory mechanisms of SVCT gene expression and protein synthesis and activity are incompletely understood. In cell culture systems, SVCT2 messenger RNA (mRNA) levels increase in response to oxidative stress (Chi and May, 2009; May et al., 2010; Qiao et al., 2009; Savini et al., 2007) and factors that stimulate cell division (Fujita et al., 2001; Liang et al., 2002; Qiao and May, 2009; Wu et al., 2007). In general, these observations suggest that SVCT2 is a redox-sensitive vitamin C transporter. SVCT1 gene expression is regulated by the transcription factor, hepatocyte nuclear factor 1 (HNF1) (Michels and Hagen, 2009), but the exact mechanisms of this regulation are still under investigation. Both SVCT proteins may undergo posttranslational modification, influencing their activity and presentation on the surface of the plasma membrane (Savini et al., 2008). As indicated earlier, SVCTs do not transport dehydroascorbic acid (Corpe et al., 2005; Daruwala et al., 1999; Takanaga et al., 2004). However, dehydroascorbic acid may be transported into cells by facilitated glucose transporters (Rumsey et al., 1997; Vera et al., 1993; Washko et al., 1993). Glucose transporters (GLUT) 1, 3, and 4 transport dehydroascorbic acid with high affinity, equal to or higher than that for glucose, which is due to the structural similarities of the two molecules (Rumsey et al., 1997, 2000). Dehydroascorbic acid is rapidly reduced to ascorbate in the cell, as described previously. The dominant pathways for vitamin C transport in the body seem to be the SVCTs and not the GLUTs, as evidenced most clearly by SVCT2 knockout mice. Despite the presence of functional GLUTs and endogenous ascorbic acid synthesis, these mice display severe ascorbate deficiency and die at birth (Sotiriou et al., 2002). Dehydroascorbic acid uptake by GLUTs may be considered a rapid “scavenger system,” allowing the oxidized form of vitamin C in the extracellular milieu to be recycled intracellularly. It has been proposed that erythrocytes, which contain GLUTs but no SVCTs in their plasma membrane, take up dehydroascorbic acid formed in plasma and reduce it intracellularly to ascorbate (May et al., 2001b). Heightened production and release of reactive oxygen species by activated phagocytes may result in oxidation of extracellular ascorbate to dehydroascorbic acid, followed by rapid, GLUT-mediated uptake of dehydroascorbic acid and intracellular ascorbate recycling (see Figure 27-3). This may occur by either the phagocytes themselves or other nearby cells, a process called the “bystander effect” (Nualart et al., 2003). Because of rapid scavenging mechanisms and the high rate of spontaneous decomposition (Retsky et al., 1993), dehydroascorbic acid cannot be reliably detected in plasma or tissues. Cellular organelles accumulate vitamin C, but neither the exact mechanism of ascorbate transport or steady-state levels of ascorbate in various cellular compartments have been determined. GLUTs have been implicated in the transport of dehydroascorbic acid across the membrane of the ER, where ascorbate transport is lacking (Banhegyi et al., 1998). Though not an ascorbate transport mechanism per se, single electrons from cytosolic ascorbate may be transferred via membrane-bound cytochrome b561 to the ER lumen, where they may be used to reduce ascorbyl radicals to ascorbate (Dhariwal et al., 1991; Fleming and Kent, 1991; Szarka et al., 2002). This process also provides reducing equivalents for enzymes that reside within the ER/Golgi/vesicular system such as dopamine β-hydroxylase (see later). The cytosolic ascorbyl radical produced by transfer of an electron from ascorbate to cytochrome b561 may then dismutate to form ascorbate and dehydroascorbic acid. By proximity, this dehydroascorbic acid may be transported by the GLUT transporters across the ER membrane. In the ER lumen, it can be recycled back to ascorbate thereby adding to its vitamin C content. A similar system has been described for electron transfer across the plasma membrane and is thought to occur primarily in erythrocytes (Lane and Lawen, 2009). Efflux of ascorbic acid from cells and tissues is not well understood but has been measured in several organs (Lane and Lawen, 2009). It has been proposed that general volume-sensitive organic anion channels may release ascorbate from the cytoplasm in response to osmotic stress. In addition, ascorbate can be released through exocytosis of ascorbate-containing vesicles. Vesicles formed by ER and Golgi complex contain high levels of ascorbate, which, upon fusion with the plasma membrane, release vitamin C into the extracellular milieu. This process has been observed in cells from the adrenal and salivary glands (Lane and Lawen, 2009). Although it is unclear if exocytosis of ascorbate occurs in other cells, exocytosis is likely the route of efflux for ascorbate synthesized de novo, because L-gulonolactone oxidase activity is only found in the interior of the ER of liver. Vitamin C is known to participate as an electron donor required for maximum activity of several biosynthetic enzymes (Englard and Seifter, 1986; Levine, 1986). Three enzymes are found in fungi and are involved in reutilization pathways for pyrimidines or the deoxyribose moiety of deoxynucleosides. Because these enzymes are not known to be involved in reactions in mammals, they will not be discussed further. The known vitamin C–dependent mammalian enzymes participate in hydroxylation of procollagen and other proteins, amidation of peptide hormones tyrosine metabolism, and carnitine and norepinephrine biosynthesis. Additional enzymes using vitamin C as a reducing agent may exist, but the specificity of these enzyme reactions for ascorbate has not been experimentally established. Enzymes that require ascorbate have either monooxygenase or dioxygenase activity. The monooxygenases, dopamine β-hydroxylase and peptidylglycine α-monooxygenase, incorporate a single oxygen atom into a substrate, either dopamine or a peptide with a terminal glycine (Figure 27-7). Both monooxygenase enzymes require two copper atoms in their active site to be reduced by ascorbate, receiving one electron from each of two ascorbate molecules (Fleming and Kent, 1991; Stewart and Klinman, 1988). The ascorbyl radicals formed during this reaction dismutate, resulting in the full oxidation of one molecule of ascorbate per enzyme cycle. The remaining enzymes are dioxygenases, which incorporate molecular oxygen (O2), with each oxygen atom incorporated in a different way. Most of these dioxygenases require α-ketoglutarate as a cosubstrate and incorporate one oxygen atom into α-ketoglutarate (with decarboxylation and formation of succinate) and the other oxygen atom into the enzyme-specific substrate (Figure 27-8). One exception is 4-hydroxyphenylpyruvate dioxygenase, which incorporates both oxygen atoms into different locations of the same substrate (4-hydroxyphenylpyruvate). These dioxygenase enzymes can proceed along their normal biological reaction sequence in the absence of ascorbate as long as sufficient substrate is present, incorporating electrons from the α-keto acid cosubstrate into the specific substrate. If the substrate concentration becomes limiting, however, the enzyme performs an uncoupled reaction oxidizing its iron center so that the enzymatic reaction can no longer proceed. In this case, ascorbate donates its electrons to the resulting Fe(IV)=O/Fe(III)-O•− complex, restoring the enzyme to an active state (see Figure 27-8). In this manner, ascorbate acts as a “pseudo-cofactor” for the reaction. However, uncoupled enzyme activity and formation of an oxidized iron complex have not been demonstrated unequivocally in vivo, leaving the exact role of ascorbate in dioxygenase function subject to further debate. Dopamine β-hydroxylase is necessary for hydroxylation of dopamine during the synthesis of the catecholamine, norepinephrine, in peripheral neurons, central neurons, and the adrenal medulla. Because of its abundance, the enzyme from the adrenal medulla has been characterized in detail. The enzyme is a tetrameric glycoprotein, with subunits arranged as pairs of disulfide-linked monomeric species. There are both membrane-bound and soluble enzyme forms, which probably differ in subunit composition (Fleming and Kent, 1991). The enzyme contains two copper atoms per subunit. Dopamine β-hydroxylase is localized in neurosecretory vesicles of neurons and in secretory vesicles (chromaffin granules) of the adrenal medulla (Levine et al., 1991). The enzyme uses molecular oxygen and dopamine as substrates and ascorbate as the preferred electron donor, as shown in Figure 27-7. The kinetics for ascorbate in norepinephrine biosynthesis were determined in situ, meaning in intact animal tissue (Levine et al., 1991). The Km of dopamine β-hydroxylase for intravesicular ascorbate was found to be approximately 0.5 mmol/L, which is similar to that of the isolated enzyme (Kaufman, 1974). Intact secretory vesicles of the brain and adrenals contain 10 to 15 mmol/L of ascorbate, which is sufficient to saturate dopamine β-hydroxylase with ascorbate. Many biologically active peptides are synthesized from inactive precursors by posttranslational modification. To confer activity to many bioactive peptides, a carboxy-terminal α-amide group must be added by a process called α-amidation. α-Amidation is mediated by peptidylglycine α-amidating monooxygenase (PAM) (Eipper et al., 1993; Prigge et al., 2000). Amidated peptide hormones include thyrotropin-releasing hormone, gonadotropin-releasing hormone, oxytocin, vasopressin, cholecystokinin, gastrin, calcitonin, substance P, and neuropeptide Y. PAM is a bifunctional enzyme that contains two distinct monofunctional domains connected by a linker region (Eipper et al., 1993; Prigge et al., 2000). The two domains are peptidylglycine α-hydroxylating monooxygenase (PHM) and peptidyl-α-hydroxyglycine α-amidating lyase (PAL). PAM is localized in secretory vesicles, where it is anchored to the vesicle membrane (Eipper et al., 1993; Prigge et al., 2000). Amidation is a two-step reaction, with each reaction mediated by one of the PAM domains. The initial, rate-limiting reaction is mediated by PHM and results in hydroxylation of the C-terminal glycine of the substrate peptide with formation of peptidylhydroxyglycine. In the second step, which is mediated by PAL, the peptidylhydroxyglycine moiety is cleaved into glyoxylate and the amidated peptide. The overall reaction is shown in Figure 27-7. PHM is similar to dopamine β-hydroxylase in several respects. Both enzymes are monooxygenases. PHM and dopamine β-hydroxylase share significant amino acid sequence similarity, indicating that these enzymes are evolutionarily linked. The enzymes have 28% identity extending through a common catalytic domain of approximately 270 residues (Eipper et al., 1993; Prigge et al., 2000). Like dopamine β-hydroxylase, PHM requires ascorbate, oxygen, and copper. In addition, PAM uses intravesicular ascorbate maintained by transmembrane transfer, similar to dopamine β-hydroxylase (Eipper et al., 1993; Prigge et al., 2000). A cardinal symptom of vitamin C deficiency is poor wound healing, which provided an early clue that vitamin C may be involved in collagen biosynthesis (Lind, 1753; Peterkofsky, 1991). The well-characterized enzymatic role of vitamin C in collagen biosynthesis is its participation in prolyl and lysyl hydroxylase reactions. Hydroxylation of the proline residues greatly increases the stability of collagen under physiological conditions, whereas some of the hydroxylysine residues undergo further modifications that aid collagen’s function. Newly synthesized collagen precursor proteins, called procollagen, undergo modification in the ER before translocation to the Golgi apparatus (see Chapter 5). Hydroxylation of certain proline and lysine residues occurs along the length of procollagen polypeptides (Figure 27-9), which stimulates proper folding of the polypeptides and increases extracellular stability of mature collagen. Vitamin C acts with prolyl 3-hydroxylase, prolyl 4-hydroxylase, and lysyl hydroxylase for procollagen hydroxylation (Myllyharju, 2003; Peterkofsky, 1991). Much of what is known about dioxygenase reactions and the role of ascorbate has been derived from the study of prolyl and lysyl hydroxylases. These dioxygenases have specific binding sites for ascorbate near the iron center (Majamaa et al., 1986), but it is not clear whether ascorbate binds to these sites during the normal catalytic cycle. If this were the case, ascorbate might help prevent the active-site iron from becoming oxidized, without ascorbate being oxidized itself. However, the only experimentally verified reaction of the enzyme with ascorbate is the reduction by ascorbate of the enzyme’s oxidized iron center following an uncoupled reaction, as shown in Figure 27-8. Vitamin C also appears to affect procollagen gene transcription and mRNA stability and the release of mature collagen from cells, but the underlying mechanisms are currently poorly understood (Peterkofsky, 1991). Hydroxylation of proline and asparagine residues has been described as a regulatory mechanism for the transcription factor, hypoxia inducible factor 1α (HIF-1α). HIF-1α is synthesized constitutively in cells, but under normal cellular oxygen concentrations (normoxia) key proline and asparagine residues are hydroxylated (see Figure 27-9), leading to HIF-1α proteosomal degradation (Figure 27-10). Hydroxylation of HIF-1α does not occur under some conditions, in particular, low oxygen concentrations (hypoxia). Without hydroxylation, HIF-1α binds with HIF-1β to form a stable dimer, which induces hypoxia-responsive genes that play a role in angiogenesis, erythropoiesis, and glycolysis (Bardos and Ashcroft, 2005; Marin-Hernandez et al., 2009; Pugh and Ratcliffe, 2003). HIF-1α is critical to initiating apoptosis in neutrophils, a normal process critical for the resolution of inflammation (Vissers and Wilkie, 2007). Elevated HIF-1α levels have been found in many solid tumors and are linked to poor prognosis in cancer patients (Rankin and Giaccia, 2008). It is not clear if the increase in HIF-1α in tumors is due to the hypoxia that normally occurs in large, poorly vascularized tumors, or if its expression is enhanced during cancer progression as a consequence of gene dysregulation. In either case, HIF-1α is considered a promising target for cancer therapy. HIF-1α proline hydroxylation is accomplished by a distinct family of prolyl 4-hydroxylases (PH) termed PHD1, PHD2, and PHD3. In addition, a protein called factor inhibiting HIF (FIH) acts as the associated HIF-1α asparaginyl hydroxylase. These enzymes require ascorbate for full activity in a similar fashion to the collagen prolyl and lysyl hydroxylases (Bruick and McKnight, 2001; Myllyharju, 2003). Ascorbate deficiency is thought to lead to loss of hydroxylation and stabilization of HIF-1α under normoxic conditions (Bruick and McKnight, 2001; Myllyharju, 2003). Currently, the effects of low tissue ascorbate levels are being investigated in cancer, anemia, and ischemic heart disease for HIF-1α hydroxylase involvement (Chen et al., 2009; Rankin and Giaccia, 2008). One of the first clinical effects of vitamin C deprivation is fatigue (Levine et al., 1996b, 2001; Lind, 1753), a symptom that precedes scurvy (see later). L-Carnitine, a zwitterionic quaternary amino acid, is required to form acyl carnitine derivatives that transfer long-chain fatty acids into mitochondria for subsequent β-oxidation and ATP synthesis (see Chapter 16). Ascorbic acid participates in two hydroxylation reactions that are required for the synthesis of carnitine from its amino acid precursors, lysine and methionine. In mammals, certain proteins containing lysine are methylated using S-adenosylmethionine to form ε-N-trimethyllysine residues, which are subsequently released by proteolytic cleavage. The free trimethyllysine may then undergo a four-step reaction sequence to form carnitine (Figure 27-11). The first reaction is hydroxylation of ε-N-trimethyllysine to β-hydroxy-ε-N-trimethyllysine catalyzed by trimethyllysine hydroxylase. In the second reaction, glycine is released to form γ-trimethyla-minobutyraldehyde, which undergoes dehydrogenation in the third reaction to form γ-butyrobetaine. In the fourth reaction, γ-butyrobetaine is hydroxylated by γ-butyrobetaine hydroxylase, with carnitine as the product (Dunn et al., 1984; Englard and Seifter, 1986; Rebouche, 1991). The two hydroxylation reactions in the carnitine synthesis pathway are catalyzed by dioxygenases that require iron, α-ketoglutarate, and ascorbate (Dunn et al., 1984). Although it has not been extensively studied, the mechanism of ascorbate action is probably similar to that in the prolyl and lysyl hydroxylase reactions, where ascorbate maintains iron in a reduced state, while the reducing equivalents for the hydroxylation reaction are obtained via the oxidative decarboxylation of cosubstrate α-ketoglutarate (Englard and Seifter, 1986). Although these two enzymes for carnitine biosynthesis are most active with ascorbate as a reductant, carnitine biosynthesis is not strictly dependent on ascorbate. Vitamin C–deficient guinea pigs provided with excess substrate for these hydroxylation reactions, such as trimethyllysine or γ-butyrobetaine, showed nearly normal rates of carnitine biosynthesis, indicating that other reducing agents can replace ascorbate in vivo (Rebouche, 1995). Furthermore, recent data show that vitamin C–deficient gulonolactonase-knockout mice exhibit no changes in the capacity to synthesize carnitine (Furusawa et al., 2008), and lethargy in ascorbate-deficient SVCT1 knockout mice is not affected by carnitine supplementation (Corpe et al., 2010). Together, these findings suggest that carnitine biosynthesis is not strictly dependent on the availability of vitamin C, and conversely carnitine levels are not a sensitive indicator of vitamin C status. As part of tyrosine catabolism, 4-hydroxyphenylpyruvate dioxygenase catalyzes the conversion of 4-hydroxyphenylpyruvate to homogentisate (Moran, 2005). The enzyme uses molecular oxygen to catalyze coupled oxidations. It appears to be a true dioxygenase and is similar to other keto acid–dependent dioxygenases, such as the α-ketoglutarate-dependent dioxygenases that are involved in prolyl and lysyl hydroxylation and carnitine synthesis. However, in the hydroxyphenylpyruvate reaction, the α-keto acid required for electron donation is part of the same molecule that is hydroxylated (Figure 27-12) (Englard and Seifter, 1986).
Vitamin C
Vitamin C Nomenclature, Structure, and Chemical Properties
Ascorbate
Ascorbyl Radical
Dehydroascorbic Acid
Food Sources of Vitamin C
Vitamin C Transport
Vitamin C Absorption and Bioavailability
Transport of Ascorbate Across Cell Membranes
Relationship between Plasma and Tissue Ascorbate Levels
Accumulation of Ascorbate in Tissues
Cellular Uptake of Dehydroascorbic Acid
Vitamin C Uptake by Cellular Organelles
Efflux of Ascorbate From Cells
Enzymatic Functions of Vitamin C
Monooxygenases
Dopamine β-Hydroxylase
Peptidylglycine α-Amidating Monooxygenase
Dioxygenases
Prolyl 4-Hydroxylase, Prolyl 3-Hydroxylase, and Lysyl Hydroxylase
Hypoxia Inducible Factor Hydroxylases
Trimethyllysine Hydroxylase and γ-Butyrobetaine Hydroxylase
4-Hydroxyphenylpyruvate Dioxygenase
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Vitamin C