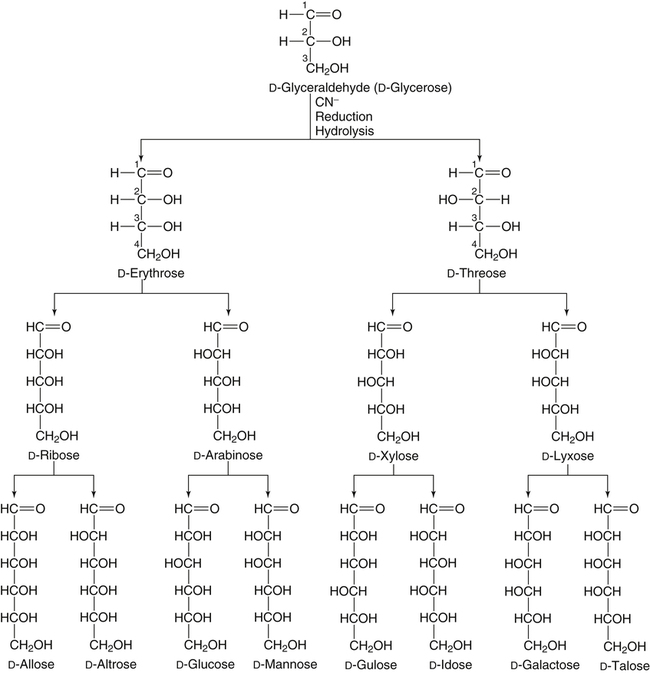
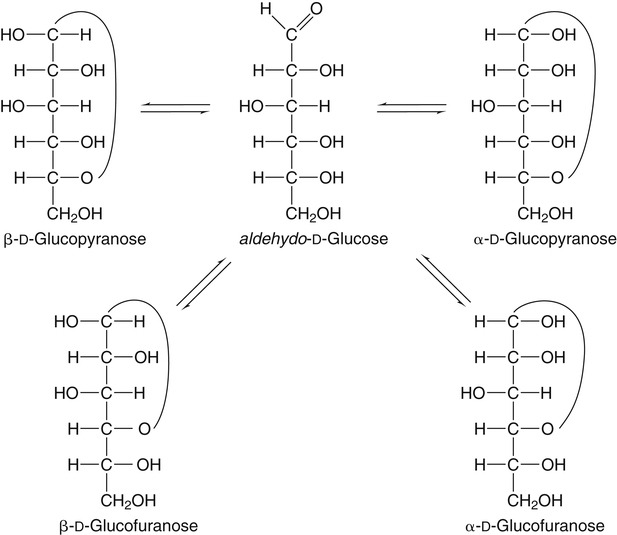
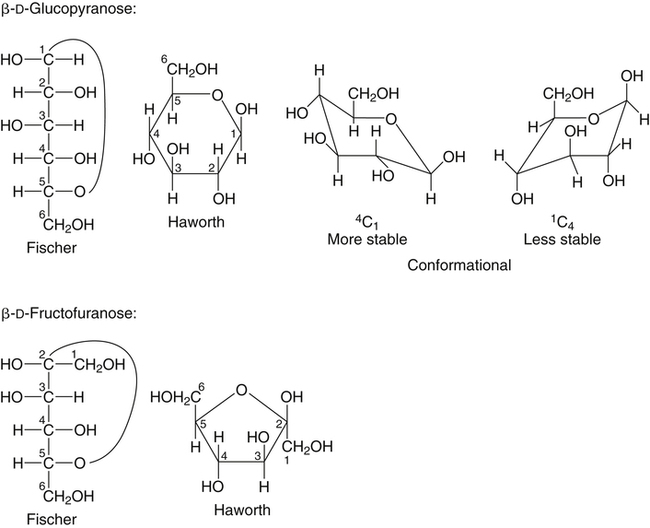
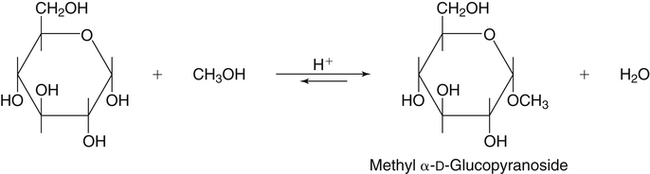
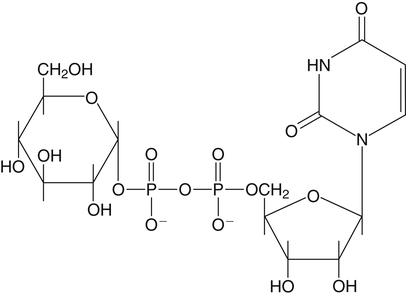
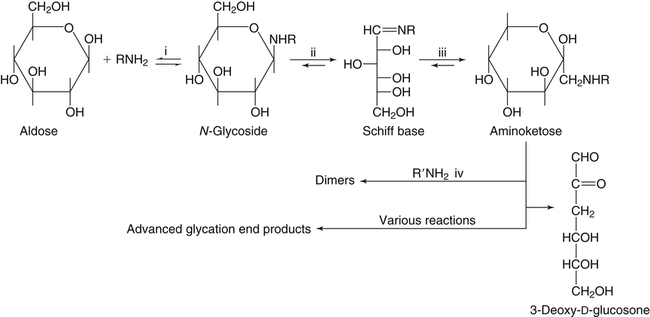
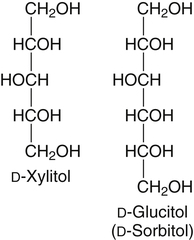
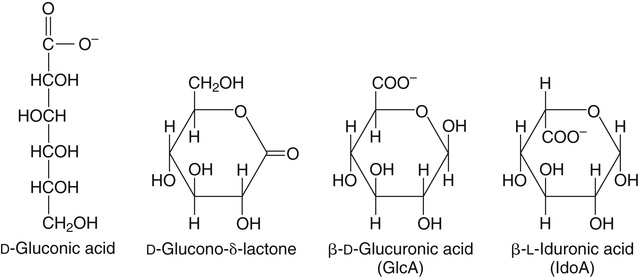
All D-aldoses are related to D-glyceraldehyde, and L-aldoses are similarly related to L-glyceraldehyde. The relation of D-aldoses to D-glyceraldehyde can be seen clearly by looking at a scheme for the chemical synthesis of the series of D-aldoses from D-glyceraldehyde. In the synthetic scheme shown in Figure 4-1, a nucleophilic cyanide ion (:CN) adds to the carbonyl carbon (C1) of glyceraldehyde, giving two cyanohydrin products. Selective reduction and hydrolysis of the CN group to an aldehyde completes the conversion of the triose D-glyceraldehyde to the pair of aldotetroses having a new chiral carbon at C2 (i.e., C1 of the glyceraldehyde precursor). Accordingly, this reaction scheme lengthens the carbon chain from the carbonyl end and gives two new aldoses, the last three carbons of which derive from glyceraldehyde. Therefore the configuration of the hydroxyl on the highest numbered chiral carbon of an aldose or ketose determines its D or L status. For a D-sugar, this hydroxyl is shown drawn to the right of the carbon chain in the Fischer formula. Each cycle of the synthesis creates a new chiral center (i.e., at C2 of the successive aldoses) and a pair of stereoisomers. Accordingly, there are two tetroses, four pentoses, and eight hexoses in the D– (see Figure 4-1) and also in the L-series; not all of these occur commonly in nature. The nutritionally important glucose is one of the eight possible D-aldohexose monosaccharides. The L-sugars are mirror images (enantiomers) of the D-sugars, with the configuration of all chiral carbons reversed (e.g., D-glucose and L-glucose). The names of each of the two aldotetroses, four aldopentoses and eight aldohexoses are shown in Figure 4-1. The carbonyl group in aldoses is always C1, but carbonyl groups may occur at any internal carbon atom in ketoses. In the common ketoses (Figure 4-2), the carbonyl group is usually at C2. A few ketoses, such as fructose, are known by their trivial names, but ketoses also are named systematically with the suffix “-ulose” denoting a ketose sugar. In this nomenclature, a group of up to four consecutive chiral carbons is named after the corresponding aldose sugar (e.g., triose, tetrose, pentose, or hexose) that possesses the same chiral group, and the number of carbon atoms is designated also. D-Fructose is the most common ketose, and its systematic name is D–arabino-hexulose, showing that the three chiral carbons in D-fructose have the same configuration as the three chiral carbons in D-arabinose. Frequently, the names ribulose and xylulose are used for the two ketopentoses. Their correct systematic names, however, are D–erythro-pentulose and D–threo-pentulose, respectively, showing that they have only two chiral carbons and indicating the relationship of these chiral carbons to those of erythrose and threose (see Figure 4-1). Although the common monosaccharides are pentoses and hexoses, sugars with more than six carbon atoms also occur naturally. Some have trivial names, but most sugars having more than six carbons and possessing four or more chiral carbons are named systematically, as described in the preceding text for the aldoses and ketoses. Sedoheptulose (D–altro-heptulose), a seven-carbon sugar, and the other ketoses shown in Figure 4-2 are involved as phosphorylated intermediates in carbohydrate metabolism. N-Acetylneuraminic acid, a nine-carbon acidic ketose, is an important signaling epitope in glycoproteins. The abbreviations or symbols for the sugars usually consist of the first three letters of their names (Table 4-1). The abbreviations of glucose (Glc) and some ketoses are exceptions to this rule. TABLE 4-1 Abbreviated Names for Some Common Carbohydrates Abbreviations for di-, oligo-, and polysaccharides often add D– or L– to indicate the enantiomeric form, p or f to indicate the pyranose or furanose ring form, α- or β- to indicate the stereochemistry of the glycosidic linkage, and carbon numbers to indicate the carbon atoms that are O-linked by the glycosidic bond. The designation is often omitted for the more common D-enantiomers and p-ring form. Cyclization of an aldose occurs via an intramolecular reaction of the nucleophilic hydroxyl group on C4 or C5 with the C1 aldehyde, as shown for glucose in Figure 4-3. This spontaneous reaction transforms the carbonyl carbon (i.e., C1 of an aldose) into a chiral carbon (the anomeric carbon), giving the α- and β-anomers of both the furanose and pyranose ring forms. The same type of cyclization reaction occurs with the ketoses in which the C5 or C6 hydroxyl group reacts with the C2 ketose carbonyl. In the Fischer formula, the hemiacetal (anomeric) −OH of the α-anomer is drawn on the same side of the carbon chain as the D designator oxygen (e.g., C5−O− of a hexose). In aqueous solution, the acyclic and cyclic isomers are in equilibrium, with the most energetically stable isomers predominating. For most aldoses, the six-membered pyranose ring is more stable than the five-membered furanose ring. However, some sugars (e.g., arabinose, ribose, and fructose) frequently occur in the furanose ring form in disaccharides, oligosaccharides, and polymers. The equilibrium nature of the hemiacetal reaction dictates that all hydroxyls and the carbonyl group can undergo reactions; that is, the sugar can react in acyclic or in ring form. Sugar ring structures are depicted in several ways, as shown for glucose and fructose in Figure 4-4. The Fischer projection formula is a convention used to depict a stereoformula in two dimensions. The Haworth formula was introduced as a more realistic depiction of the bond lengths in the cyclic sugars. Hydroxyl groups on the right of the carbon chain in the Fischer projection are below the plane of the ring in the Haworth formula, and those on the left of the carbon chain in the Fischer projection are above the plane of the ring in the Haworth formula. The exocyclic group (e.g., the −CH2OH group attached to C5 of hexoses such as glucose and fructose shown in Figure 4-4) is placed above or below the plane in the Haworth formula, depending on the stereochemistry of the ring oxygen. If the hydroxyl group that contributes the ring oxygen is on the right of the carbon chain in the Fischer structure, then the exocyclic group is above the plane in the Haworth structure. If the ring connects from a hydroxyl group on the left of the carbon chain in the Fischer structure, then the exocyclic group is drawn below the plane in the Haworth structure. Aldoses are reducing agents, and the carbonyl group of an aldose is simultaneously oxidized to a carboxyl group when aldoses act as reducing agents. Ketoses are not good reducing agents because simultaneous oxidation of the carbonyl would require carbon chain cleavage. However, ketoses can isomerize to aldoses in an alkaline reducing sugar test and therefore result in a positive reducing sugar test even though they are nonreducing sugars. Formerly, glucose in urine was analyzed by an assay for reducing sugars, but more specific methods are now available. In vivo, oxidation of the aldehyde group of glucose is catalyzed enzymatically by a dehydrogenase, and this reaction yields the lactone (an intramolecular ester of the newly formed carboxylic acid) as the product. An example of this type of reaction is the conversion of glucose 6-phosphate to 6-phosphoglucono-δ-lactone in the pentose phosphate pathway of metabolism (see Chapter 12, Figure 12-20). The carbon proton adjacent to the carbonyl (i.e., the C2 or α-carbon proton) in aldoses is acidic and easily abstracted in basic solution, leading to epimerization of the aldoses at C2 as well as their isomerization to ketoses. Thus glucose is epimerized to mannose and isomerized to fructose. Similar reactions occur in carbohydrate metabolism, as seen in the phosphoglucose isomerase–catalyzed conversion of glucose 6-phosphate to fructose 6-phosphate and in the phosphomannose isomerase-catalyzed conversion of mannose 6-phosphate to fructose 6-phosphate (see Chapter 12, Figures 12-4 and 12-5). Similar reactions occur with ketoses. The general term glycoside is used to describe any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. As an example, the acid-catalyzed reaction of a sugar (glucose) with an alcohol (methanol) to form methyl D-glucopyranoside, which is an example of an O-glycoside, is shown in Figure 4-5. Although only the α-glycoside is shown, both α- and β-glycosides form in nonenzymatic reactions. When properly activated, sugars react with each other to form specific oligosaccharides and polysaccharides in which a sugar residue is linked by a new bond between its anomeric carbon and one of the hydroxyl groups of the second sugar residue. Thus the sugar residues in oligosaccharides and polysaccharides are linked by O-glycosidic bonds. The terms glycone and aglycone are commonly used to refer to the two molecules linked by a glycosidic bond, particularly when the compound being described is not a chain of sugar residues (i.e., not an oligosaccharide or polysaccharide). The sugar group is the glycone and the nonsugar group is the aglycone part of the glycoside; for example, in Figure 4-5 glucose is the glycone and methanol is the aglycone portion of methyl D-glucopyranoside. The glycone can consist of a single sugar group (monosaccharide) or several sugar groups (oligosaccharide). Glycosides may be classified by the sugar group. If the sugar group is glucose, the molecule is a glucoside; if it is glucuronic acid (glucuronate), the molecule is a glucuronide. Various hydroxylated compounds are glycosylated in the liver by linkage to glucuronic acid, which increases their water solubility; formation of β-D-glucuronides is a major means of detoxification and excretion of both endogenous (e.g., bilirubin) and exogenous (e.g., acetaminophen) compounds. Sugars also react with amines or thiols to give N– or S-glycosides, respectively. For example, β-D-ribose and 2-deoxy-β-D-ribose in nucleic acids are bonded to purines and pyrimidines by N-glycosidic bonds. In uridine diphosphate (UDP)–glucose, the β-D-ribose is linked to uracil by an N-glycosidic bond, and the α-D-glucose and β-D-ribose units are each ester-linked to phosphate, as shown in Figure 4-6. The sugar nucleotides are used extensively in vivo for enzymatic synthesis of carbohydrates, including lactose and glycogen. In glycoproteins, oligosaccharide chains are linked to the β-carboxamide nitrogen of asparagine (an N-glycosidic bond) or to the hydroxyl of serine/threonine (an O-glycosidic bond). Plants use the glycosidic bond extensively in synthesizing different glycosides, many of which are physiologically active. Glycosides are more stable than aldoses and ketoses in several respects. The carbonyl/hemiacetal carbon in the glycoside is protected from base-catalyzed reactions and from reduction and oxidation. The pyranose and furanose ring structures and the anomeric configuration are also stabilized and do not undergo the interconversions shown in Figure 4-3. However, the glycosidic bonds can be hydrolyzed by acid or enzyme catalysis releasing the free sugar and the alcohol (with the alcohol often being another sugar molecule). Glycosidases, which catalyze hydrolysis of glycosides, typically have high specificity for the sugar or glycone portion and the stereochemistry of the anomeric linkage (α or β) but lower specificity for the aglycone or sugar that donates the hydroxyl group for the glycosidic bond. Such specificity has important implications for the enzymatic digestion of carbohydrates, as is discussed in Chapter 8. Aldoses and ketoses react with aliphatic primary and secondary amines, including amino acids and proteins, to form N-glycosides, which readily dehydrate to the respective Schiff base by the Maillard reaction, as shown in Figure 4-7 (reactions i and ii). The aldose Schiff base spontaneously undergoes an Amadori rearrangement at C1 and C2, giving a substituted 1-amino-1-deoxyketose (reaction iii). A ketose Schiff base will rearrange to a substituted 2-amino-2-deoxyaldose (not shown). These sugar amines undergo additional very complex reactions, leading to formation of highly reactive dicarbonyls (such as 3-deoxy-D-glucosone), cross-linking of proteins (as in reaction iv), formation of fluorescent compounds and brown pigments, and formation of low-molecular-weight compounds, some of which are useful flavoring agents. Although the Maillard complex of reactions has been studied extensively, the reactions are understood only in part. Lysine residues in proteins often react with sugars by the Maillard reaction. The reaction of glucose with hemoglobin was discovered first. Blood glucose reacts with hemoglobins via the Maillard reaction, and the modified protein, detected by gel electrophoresis, is an indicator of plasma glucose levels in diabetics over the lifespan of the erythrocytes. The term glycated protein is used to distinguish these Maillard-derived, carbohydrate-modified proteins from true glycosylated proteins (glycoproteins). The Maillard and subsequent reactions also occur in food products exposed to heat, such as in powdered or evaporated milk during processing or storage, giving an off-white color to the product and decreasing the nutritive value of food proteins. Lysine residues in proteins are irreversibly modified by the Maillard reaction, and modification of lysine residues is partially responsible for the nonenzymatic browning and decrease in nutritive value. The alditols, or polyols, which occur naturally in plants and other organisms, are reduction products of aldoses and ketoses in which the carbonyl has been reduced to an alcohol. Two common alditols are shown in Figure 4-8, xylitol and sorbitol (glucitol). Reduction of ketoses gives an epimeric pair of alditols unless the reaction is enzyme-catalyzed and therefore stereospecific. The alditols, like the sugars, are soluble in water and vary in degree of sweetness. Xylitol, the sweetest, approaches the sweetness of sucrose. Because the alditols do not have a carbonyl group, they are considerably less reactive than their corresponding sugars. They do not undergo base-catalyzed reactions of epimerization and isomerization, the Maillard reaction, or the formation of glycosides (unless they are participating as the “alcohol” or aglycone component). Alditols share the same hydrophilic character as the sugars and are used in products as humectants to prevent excessive drying. Sorbitol and xylitol are not readily metabolized by oral bacteria and are used in chewing gums and candies for this noncariogenic characteristic. Both sorbitol and xylitol are passively absorbed in the small intestine and metabolized in the liver. Excessive amounts of alditols passing into the colon may induce diarrhea owing to their fermentation leading to increased luminal osmolarity (Grabitske and Slavin, 2009). Sugar acids are sugars in which the carbonyl group and/or the terminal −CH2OH group has been oxidized to a carboxyl group (−COOH). Uronic acids are formed when the terminal −CH2OH group of an aldose or ketose is oxidized to a terminal carboxyl group (−COOH). Two examples of uronic acids are shown in Figure 4-9. D-Glucuronic acid is an important constituent of glycosaminoglycans in mammalian systems, and its C5 epimer, L-iduronic acid, is present to a lesser extent. Glucuronic acid (and its 4-O-methyl ether), D-galacturonic acid, D-mannuronic acid, and the less common L-guluronic acid are constituents of the nondigestible polysaccharides of plants and algae, which contribute to dietary fiber.
Structure, Nomenclature, and Properties of Carbohydrates
Monosaccharides or Sugar Residues
Structures and Nomenclature of the Aldoses and Ketoses
Chirality of Monosaccharides
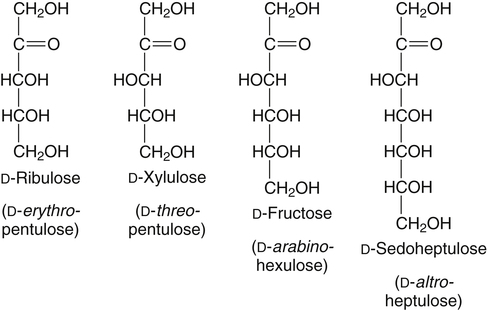
Systematic Naming of Aldoses and Ketoses
NAME
ABBREVIATION
Arabinose
Ara
Fructose
Fru
Fucose
Fuc
Galactose
Gal
Galacturonic acid
GalA
N-Acetylgalactosamine
GalNAc
Glucose
Glc
Glucuronic acid
GlcA
N-Acetylglucosamine
GlcNAc
Iduronic acid
IdoA
Mannose
Man
N-Acetylneuraminic acid
Neu5Ac
Rhamnose
Rha
Xylose
Xyl
Cyclic and Conformational Structures for Monosaccharides and Sugar Residues
Cyclization of Aldoses and Ketoses to Pyranose and Furanose Ring Structures
Drawing Sugar Ring Structures
Chemical Reactivity of the Monosaccharides and Sugar Residues
General Reactivity of Sugars
Formation of Glycosidic Linkages
Maillard Reaction of Reducing Sugars with Amines
Other Classes of Carbohydrate Units
Alditols
Glycuronic, Glyconic, and Glycaric Acids
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Structure, Nomenclature, and Properties of Carbohydrates