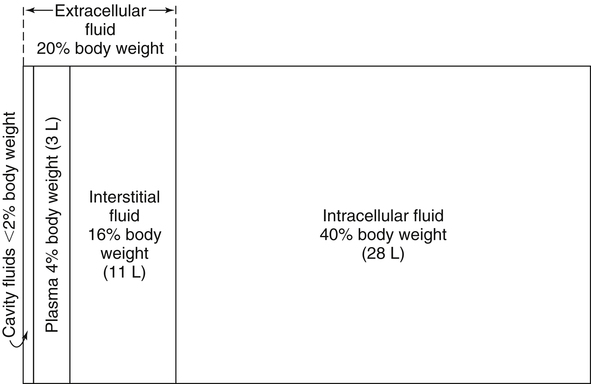
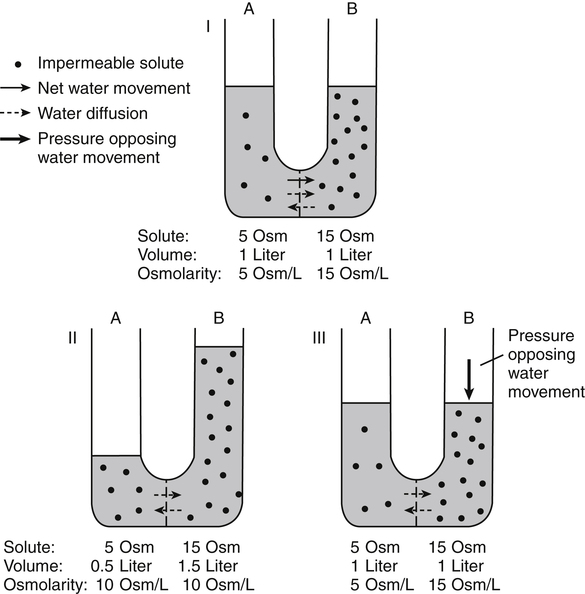
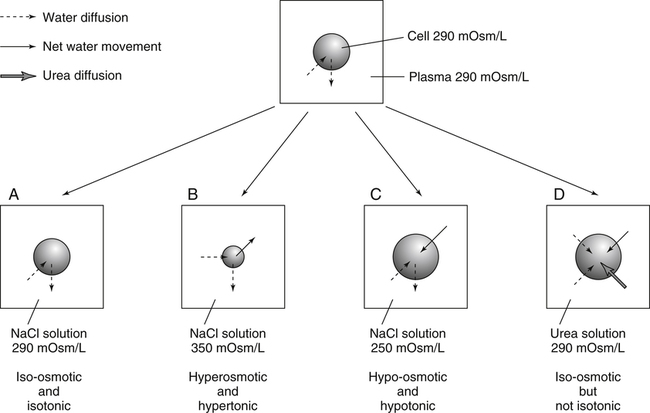
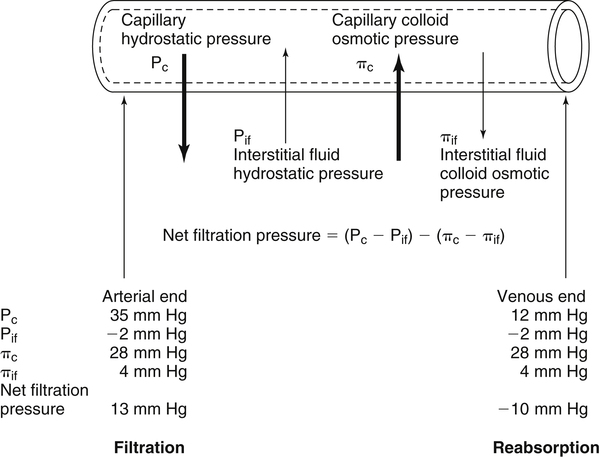
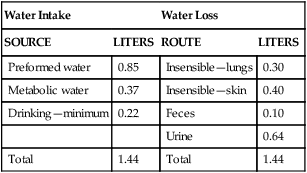
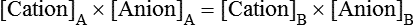Water is an essential nutrient vital to the existence of both animals and plants. In the body, water is present inside and around the cells and within all blood vessels. It lubricates joints and moistens tissues such as those in the eyes, nose, and mouth. The volume of the intracellular fluid provides turgor to the tissues, which is important for the tissue or organ form and ultimately the body form. Water performs several functions that are essential to life. It is the principal fluid medium in which nutrients, minerals, gases, and enzymes are dissolved. The extracellular water bathing the cells serves as a medium for the transport of nutrients and oxygen to the cells and for removing wastes from the cells, which will be eliminated by the liver and kidneys. The intracellular water establishes the physicochemical medium that allows various metabolic processes to take place. Another important physiological function of water is its role in the regulation of body temperature. This is achieved by removing excess heat from the body by evaporative water loss from the skin. Water in the body is distributed throughout the various body fluid compartments. The simplest subdivision is into an intracellular and an extracellular compartment, with the two compartments separated by the cell membrane (Figure 35-1). On average, a 70-kg adult has 42 L of water, of which two thirds (28 L) is intracellular and one third (14 L) is extracellular. The extracellular fluid is made up of the interstitial fluid (11 L), which bathes the cells and includes the lymph, the plasma (3 L), and the cavity or transcellular fluids, of which the largest volume is secretory fluids in the lumen of the gastrointestinal tract. Osmotic forces across a semipermeable membrane (impermeable to solutes but permeable to water) separating two compartments govern the distribution and direction of water movement between these compartments. This concept is explained simply in Figure 35-2. The two compartments, A and B, which contain the same volume of fluid but different numbers of solute particles are separated by a semipermeable membrane. Water diffuses across the membrane in both directions, but more water molecules diffuse from compartment A, a region of higher water concentration (lower solute concentration), to compartment B, which is a region of lower water concentration (higher solute concentration). This process of net movement of water caused by a concentration difference of water or solutes is called osmosis. Osmosis of water results in the expansion of compartment B at the expense of compartment A. No further net diffusion of water occurs when the solute concentrations in both compartments are equal, that is, when osmotic equilibrium is established. To reach this state of equilibrium, the volume of compartment B has increased at the expense of compartment A. This situation can occur only when the two compartments are flexible volumetrically so that the net flow of water from one compartment to another does not create a pressure difference across the membrane. However, if the walls of compartment B do not expand, the increase in hydrostatic pressure in compartment B due to influx of water will oppose further inflow of water. The amount of pressure to be applied in order to prevent the inflow of water through the membrane into compartment B is called osmotic pressure (Rose and Post, 2001). The osmotic pressure of a solution therefore reflects the concentration of osmotically active particles in that solution. The terms iso-osmotic, hypo-osmotic, and hyperosmotic are used to describe the relation of osmolar concentrations between different solutions. When two solutions are of equal osmolarity, they are iso-osmotic. A solution is hypo-osmotic when its osmolarity is lower than that of the reference solution and hyperosmotic when its osmolarity is higher. When cells are suspended in a hypo-osmotic solution, water enters the cells, causing them to expand. Conversely, when cells are suspended in a hyperosmotic solution, water diffuses out of the cells, causing them to contract. One would expect that, when cells are suspended in an iso-osmotic solution, no net flux of water would occur and cell size would remain the same. This is true only if cells are suspended in an iso-osmotic solution in which no net movement of solutes occurs across cell membranes. If cells are suspended in an iso-osmotic solution containing a highly permeant solute, such as urea, it diffuses into cells along its concentration gradient, causing an osmotic flow of water into cells, and the cells expand (Figure 35-3). Thus another term, tonicity, is used to describe the physiological osmolar concentration of a solution. Starling first proposed the concept that the two opposing forces governing water movement across the capillary endothelium are created by the difference in hydrostatic pressure and the difference in colloid osmotic pressure across the capillary wall (Taylor, 1981). Therefore four variables determine the movement of fluid: hydrostatic pressures and protein concentrations in the plasma and the interstitial fluid. The following equations describe the forces responsible for net water movement across an idealized capillary endothelium: As blood flows along the capillary, the balance of these forces is a net pressure gradient favoring the movement of a small amount of fluid from the arterial end of the capillary into the interstitium. This causes hydrostatic pressure to decrease along the length of the capillary so that much of the fluid reenters the capillary at the venous end (Figure 35-4). The small amount of fluid that remains in the interstitium is returned to the circulation by the lymphatic vessels, which empty into the subclavian vein via the thoracic duct (Taylor, 1981). For an individual to maintain water balance, the amount of water consumed must equal the amount lost from the body. This is illustrated in Table 35-1 for a 65-kg man in a temperate environment who consumes a balanced diet that is adequate for his energy requirements. Even with the excretion of a maximally concentrated urine, water normally contained in the food (preformed water) and water produced by oxidation of food (metabolic water, or water of oxidation) are inadequate to compensate for losses of water from the respiratory tract, skin, gastrointestinal tract, and kidneys. Therefore an individual must ingest free water to maintain water balance. The body possesses several homeostatic regulatory mechanisms capable of maintaining balance of water over a wide range of water intakes so that health remains unimpaired. An inequality between intake and loss of water ultimately alters the composition and osmolarity of body fluids. TABLE 35-1 Daily Water Balance in a 65-kg Man Calculated to Illustrate Minimal Required Drinking Water Intake Water is lost continuously from the body by two passive evaporative routes: from the upper respiratory tract during respiration and from the skin. These passive evaporative losses are termed “insensible losses” or “insensible perspiration” because they occur continuously and without our awareness. The amount of evaporative water loss from the respiratory tract depends on the ventilatory volume and water pressure gradient. A water pressure gradient occurs because expired air is saturated with moisture to a vapor pressure of about 47 mm Hg, whereas the vapor pressure of inspired air is usually less than 47 mm Hg. An individual loses an average of between 0.14 and 0.47 L daily by this route; the amount of the loss depends on body size, the degree of physical activity, and ambient temperature and humidity. It is to be expected that evaporative water loss from the lungs is increased in physical activity and when the atmospheric vapor pressure decreases (i.e., in cold, dry climates). Insensible water loss from the skin, which occurs independent of sweating, averages between 0.3 and 0.5 L daily for an individual living in a temperate environment and doing minimal physical activity (Geigy Scientific Tables, 1981). On average, an individual will lose a total of 0.4 to 0.9 L of water daily from insensible loss through both the respiratory tract and skin. When body temperature rises higher than 39° C, evaporative loss from the respiratory tract increases because of a significant increase in the respiratory minute volume (Reithner, 1981). The increase in water loss by the respiratory route can be as much as an additional 0.1 L daily at elevated body temperatures. Dermal losses can increase much more due to sweating. Dermal losses due to sweating at high body temperatures can be substantial. Cutaneous water loss due mainly to sweating can increase by as much as 6 to 8 times the basal level when rectal temperature is above 39.5° C (Lamke et al., 1980). Normally the volume of sweat in a 65-kg adult doing light work at an ambient temperature of 29° C (84.2° F) amounts to about 2 to 3 L daily, but it can increase to a maximum of about 2 to 4 L per hour for a short time in an unacclimatized individual who is performing heavy physical activity in a hot and humid environment. This levels off to about 0.5 L per hour over a 24-hour period as the duration of perspiration increases (Geigy Scientific Tables, 1981). Even at maximal sweating, the rate of heat loss may not be rapid enough to dissipate the heat from the body. When the body temperature rises to a critical level, higher than 40.5° C (105° F), the individual is likely to develop heatstroke. However, after acclimatization to hot weather for a few weeks, an individual will have greater tolerance of the hot and humid environment and can as much as double his or her sweating rate. Evaporation of this large volume of sweat effectively removes the excess heat from the body. Acclimatization also involves a decrease in the concentration of sodium chloride in the sweat, allowing for better conservation of sodium chloride (Takamata et al., 2001). The loss of several liters of sweat a day in a hot climate results in serious losses of both sodium chloride and water, which need to be replaced. The volume of water loss in feces is small, about 0.1 L a day, and does not cause problems with water balance unless excessive loss occurs during diarrhea. The volume of fluid ingested varies among individuals but averages about 1.7 L daily. Added to this ingested volume, the small intestine receives an additional 7 L of secretory fluids, which are made up of salivary, gastric, biliary, pancreatic, and intestinal secretions. Normally approximately 90% to 95% of these fluids is absorbed by the small intestine, and the remainder by the colon, leaving only approximately 0.1 L of water to be excreted in the feces. Absorption of water in the intestine is passive, along an osmotic gradient created by the absorption of nutrients from the lumen of the intestine into the plasma. In diseases of the gastrointestinal tract, large volumes of water can be lost in the feces, causing diarrhea. This occurs in gastroenteritis due to bacterial or viral infection or in any situation in which absorption of nutrients is compromised. Certain bacterial toxins, such as cholera toxin, can increase the secretion of sodium chloride from the crypt cells of the small intestinal mucosa into the lumen of the small intestine. The lumen becomes hyperosmotic, and water diffuses from the plasma into the lumen, causing diarrhea. Several liters of fluid, up to 10 to 20 L, can be lost, resulting in dehydration (Hall, 2011b).
Body Fluids and Water Balance
Physiological Functions of Water
Body Water Compartments
Body Water Content
Distribution of Body Water
Fluid Distribution Between Extracellular and Intracellular Fluid Compartments
Osmosis and Osmotic Pressure
Iso-osmotic, Hypo-osmotic, and Hyperosmotic Solutions
Fluid Distribution Between Plasma and Interstitial Fluid Compartments
Movement of Fluid Across Capillary Endothelium
Starling’s Law
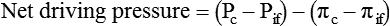
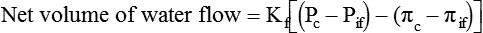
Water Balance
Water Intake
Water Loss
SOURCE
LITERS
ROUTE
LITERS
Preformed water
0.85
Insensible—lungs
0.30
Metabolic water
0.37
Insensible—skin
0.40
Drinking—minimum
0.22
Feces
0.10
Urine
0.64
Total
1.44
Total
1.44
Loss of Water
Water Loss through Respiratory Tract and Skin
Insensible Water Loss
Sweat
Water Loss by the Gastrointestinal Tract