36
Viral Vaccines
CHAPTER CONTENTS
INTRODUCTION
Because few drugs are useful against viral infections, prevention of infection by the use of vaccines is very important. Prevention of viral diseases can be achieved by the use of vaccines that induce active immunity or by the administration of preformed antibody that provides passive immunity.
ACTIVE IMMUNITY
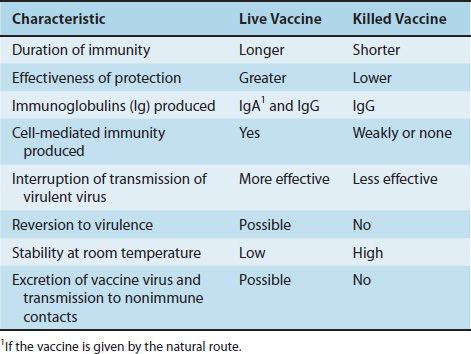
There are two types of vaccines that induce active immunity: those that contain live virus whose pathogenicity has been attenuated1 and those that contain killed virus. Some vaccines, such as the hepatitis B vaccine, contain purified viral proteins and are often called subunit vaccines. The features of subunit vaccines resemble those of killed vaccines because no viral replication occurs in these vaccines. The attributes of live and killed vaccines are listed in Table 36–1.
In general, live vaccines are preferred to vaccines containing killed virus because their protection is greater and longer-lasting. With live vaccines, the virus multiplies in the host, producing a prolonged antigenic stimulus, and IgA and IgG are elicited when the vaccine is administered by the natural route of infection (e.g., when polio vaccine is given orally). Killed vaccines, which are usually given intramuscularly, do not stimulate a major IgA response. Killed vaccines typically do not stimulate a cytotoxic T-cell response, because the virus in the vaccine does not replicate. In the absence of replication, no viral epitopes are presented in association with class I MHC proteins, and the cytotoxic T-cell response is not activated (see Chapter 58). Although live vaccines stimulate a long-lasting response, booster doses are now recommended with measles and polio vaccines.
One unique form of a live, attenuated viral vaccine is the influenza vaccine that contains a temperature-sensitive mutant of the virus as the immunogen. The temperature-sensitive mutant will replicate in the cooler air passages of the nose, where it induces IgA-based immunity, whereas it will not replicate in the warmer lung tissue and therefore will not cause disease.
There are three concerns about the use of live vaccines:
(1) They are composed of attenuated viral mutants, which can revert to virulence either during vaccine production or in the immunized person. Reversion to virulence during production can be detected by quality control testing, but there is no test to predict whether reversion will occur in the immunized individual. Of the commonly used live vaccines, only polio vaccine has had problems regarding revertants; measles, mumps, rubella, and varicella vaccines have not.
Even if the virus in the live vaccine does not revert, it can still cause disease because, although attenuated (weakened), it can still be pathogenic in a host with reduced immunity. For this reason, live viral vaccines should not be given to immunocompromised people or to pregnant women because the fetus may become infected.
(2) The live vaccine can be excreted by the immunized person. This is a double-edged sword. It is advantageous if the spread of the virus successfully immunizes others, as occurs with the live polio vaccine. However, it could be a problem if, for example, a virulent poliovirus revertant spreads to a susceptible person. Rare cases of paralytic polio occur in the United States each year by this route of infection.
(3) A second virus could contaminate the vaccine if it was present in the cell cultures used to prepare the vaccine. This concern exists for both live and killed vaccines, although, clearly, the live vaccine presents a greater problem, because the process that inactivates the virus in the killed vaccine could inactivate the contaminant as well. It is interesting, therefore, that the most striking incidence of contamination of a vaccine occurred with the killed polio vaccine. In 1960, it was reported that live simian vacuolating virus 40 (SV40 virus), an inapparent “passenger” virus in monkey kidney cells, had contaminated some lots of polio vaccine and was resistant to the formaldehyde used to inactivate the poliovirus. There was great concern when it was found that SV40 virus causes sarcomas in a variety of rodents. Fortunately, it has not caused cancer in the individuals inoculated with the contaminated polio vaccine.
Certain viral vaccines, namely, influenza, measles, mumps, and yellow fever vaccines, are grown in chick embryos. These vaccines should not be given to those who have had an anaphylactic reaction to eggs. People with allergies to chicken feathers can be immunized.
In addition to the disadvantages of the killed vaccines already mentioned—namely, that they induce a shorter duration of protection, are less protective, and induce fewer IgA antibodies—there is the potential problem that the inactivation process might be inadequate. Although this is rare, it happened in the early days of the manufacture of the killed polio vaccine. However, killed vaccines do have two advantages: They cannot revert to virulence, and they are more heat-stable. Therefore, they can be used more easily in tropical climates.
Most viral vaccines are usually given before a known exposure (i.e., they are administered preexposure). However, there are two vaccines, the vaccines against rabies and hepatitis B, that are also effective when given postexposure because the incubation period of these diseases is long enough that the vaccine-induced immunity can prevent the disease. Thus the rabies vaccine is most often used in people after they have received a bite from a potentially rabid animal, and the hepatitis B vaccine is used in people who have sustained a needle-stick injury.
The prospect for the future is that some of the disadvantages of current vaccines will be bypassed by the use of purified viral antigens produced from genes cloned in either bacteria or yeasts. The advantages of antigens produced by the cloning process are that they contain no viral nucleic acid and so cannot replicate or revert to virulence, they have no contaminating viruses from cell culture, and they can be produced in large amounts. A disadvantage of these cloned vaccines is that they are unlikely to stimulate a cytotoxic T-cell response because no viral replication occurs.
Another prospect for the future is the use of “DNA vaccines.” These vaccines contain purified DNA encoding the appropriate viral proteins genetically engineered into a viral vector or plasmid. Immunization with this composite DNA elicits both antibody and cytotoxic T cells and protects against disease in experimental animals.
Certain live viral vaccines, such as the vaccines containing vaccinia virus, adenovirus, and poliovirus, are being used experimentally to immunize against other viruses such as HIV. This is done by splicing the HIV gene into the live viral genome and then infecting the experimental animal with the constructed virus. The advantage of this procedure is that a cytotoxic T-cell response is elicited (because the virus is replicating), whereas if the purified antigen alone were used to immunize the animal, an antibody response but not a cytotoxic T-cell response would be elicited.
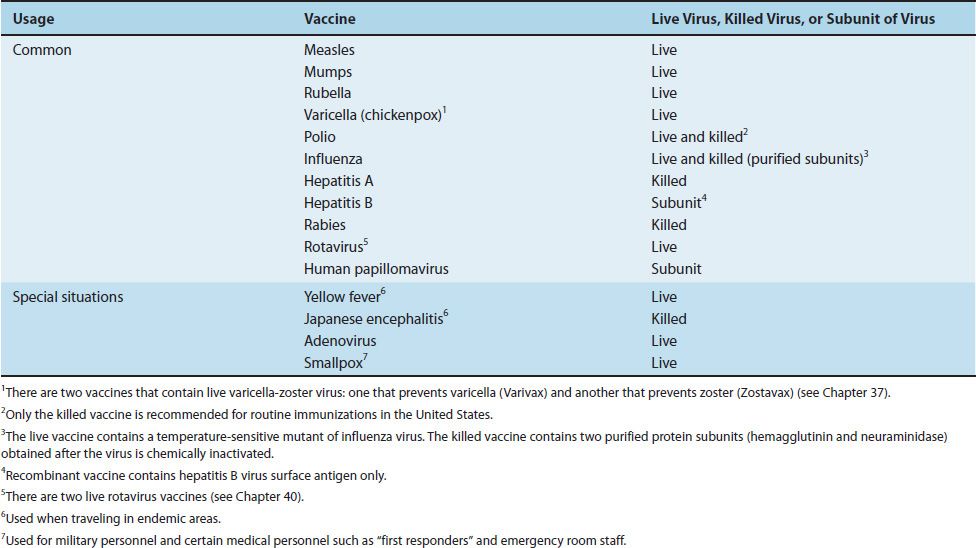
The viral vaccines currently in use are described in Table 36–2. The vaccines, both viral and bacterial, recommended for children from 0 to 6 years of age are listed in Table 36–3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree