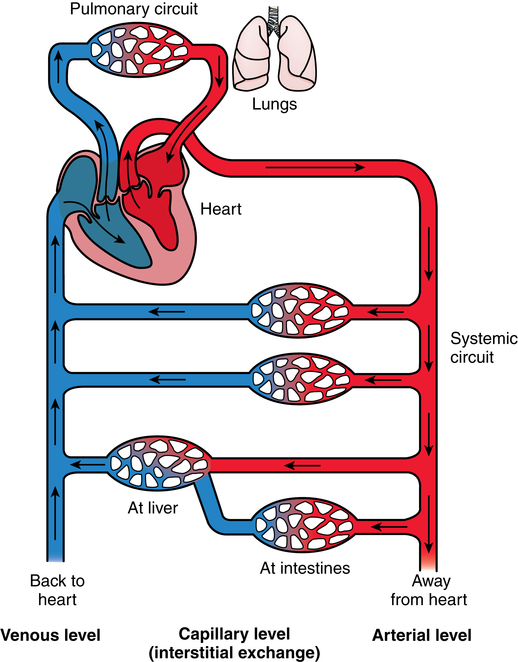
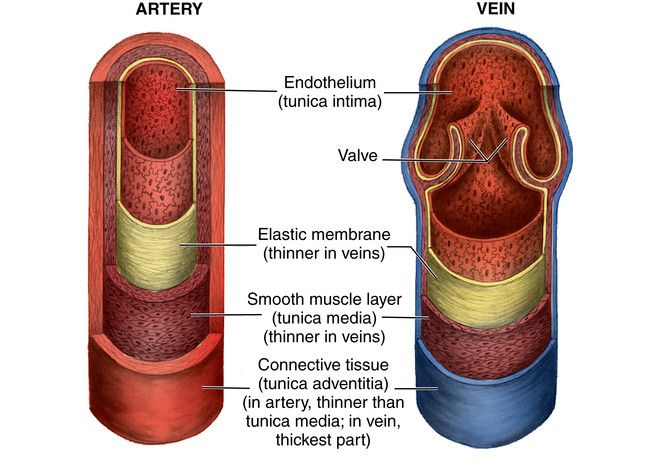
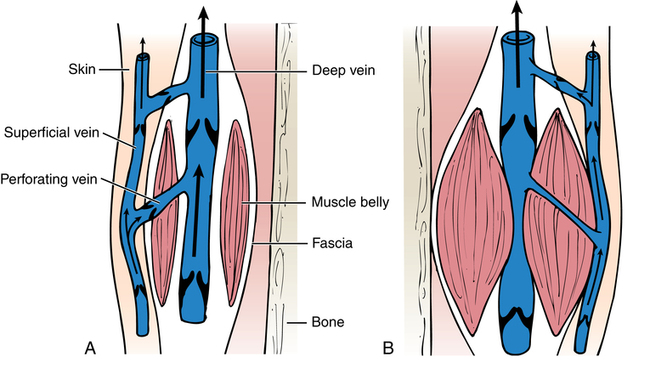
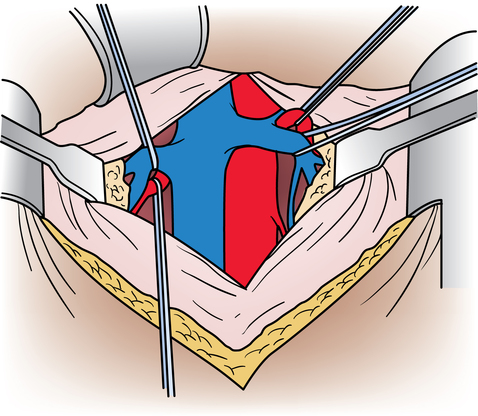
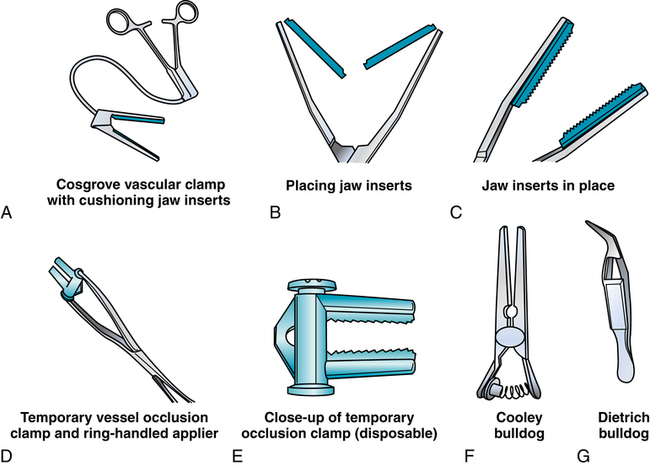
Chapter 44 After studying this chapter, the learner will be able to: • Identify the pertinent anatomy of the peripheral vascular system. • Describe the potential complications associated with peripheral vascular disease. • Describe the care of the patient with peripheral vascular disease. • List the differences in aneurysms and their treatment. An abnormal outpouching or bulging of an artery. Progressive dilation of an artery. False aneurysm (pseudoaneurysm) Injury to all three layers of the arterial wall that permits blood to accumulate in the connective tissue. The intima separates from the media. A false passage fills with blood, causing further separation of intima and media. Bacterial vegetation embolizes from the valve of the heart to the arteries, where it implants and grows, causing the weakened artery to distend and rupture. Alteration in the cellular activity associated with clotting. Material (i.e., fat, plaque, vegetation, or clot) in a blood vessel becomes bloodborne with the potential for lodging in smaller vascular tributaries. Condition inadvertently caused by the medical or surgical treatment performed by a physician. Spaces in a woven surface or between the strands of a braided suture. Blood within a vascular structure clots and occludes the lumen. Vessels in the thorax, abdomen, extremities, and extracranial cerebrovascular area constitute the circulatory system (Fig. 44-1). The ascending aorta, which originates from the left ventricle, carries oxygenated blood from the heart to the arteries. The major arteries leading to the head and upper extremities—the brachiocephalic trunk, left common carotid, and subclavian arteries—branch off from the aortic arch in the middle mediastinum above the heart. The thoracic aorta then descends through the posterior mediastinum at the left side of the vertebral column. Oxygenated blood flows from the arterial system through the capillary network and returns deoxygenated to the heart via the venous system (Fig. 44-2). The venae cavae enter the right atrium. The superior vena cava, formed by the union of the two brachiocephalic veins, returns deoxygenated venous blood from the head, neck, upper extremities, and chest. The inferior vena cava, which begins at the level of the fifth lumbar vertebra, returns blood from the lower extremities, pelvis, and abdominal organs. There are two exceptions to this oxygenation/deoxygenation pattern: 1. The pulmonary arteries carry deoxygenated blood to the lungs, and the pulmonary veins carry oxygenated blood to the heart. 2. In fetal umbilical circulation, one vein carries oxygenated blood and two arteries carry mixed blood. Arteries are elastic and constrict in response to hemorrhage. The arterial walls are made up of three layers that are separated by internal and external elastic membranes (Fig. 44-3). The innermost layer is the intima (tunica intima), a single layer of endothelial cells on a thin matrix of hyaluronic acid, collagen, and elastic fibers. The middle layer is the media (tunica media, or yellow fibrous). It is the thickest of the layers and is composed of a combination of smooth muscle fibers, yellow collagen, and some elastic fibers. The medial layer gives the arterial wall the flexibility and strength to withstand higher internal pressure. Venous walls are structured in three layers. The innermost layer is the intima and is composed of endothelial cells that produce coagulation factors. The middle layer, or media, is much thinner in the deep venous system and may even be hard to identify microscopically. The superficial veins have a thicker medial layer composed mainly of smooth muscle fibers. This provides some resistance and response to intraluminal pressure changes. The external layer, or the venous adventitia, is composed of loose connective tissue (see Fig. 44-2). Because of lower flow pressure, larger veins have internal semilunar valves to maintain the direction of the blood flow. Venous anatomy has three structural components: superficial veins, deep veins, and perforators (also known as communicating veins) (Fig. 44-4). The venous structure and flow of the lower extremities are examples of this system. At the tissue level, the origin of the venous system at the capillary bed is referred to as venules. The venous drainage of deoxygenated blood from the superficial veins returns through the perforators to the deep veins during muscular contraction. The deoxygenated blood then passes from the deep veins to the inferior vena cava. Atherosclerosis is the principal factor in transient ischemic attacks (TIAs, or “mini strokes”), cerebrovascular accidents (strokes, or “brain attacks”), myocardial infarctions (heart attacks), and aortic stenosis. Risk factors include familial history, a high level of serum cholesterol, smoking, and hypertension (Box 44-1). Relief of the obstruction by conservative medical management is not always successful. SVC syndrome can be a surgical emergency. Endovascular treatment is usually the best choice.7,14 In select patients a surgical bypass may be necessary.7 Table 44-1 compares the assessment factors of peripheral arterial and venous obstructive diseases in an extremity. Peripheral vascular laboratories, which are similar to cardiac cath labs, have been established in many health care facilities to perform noninvasive and invasive studies before and after surgical intervention. Interventional radiologists perform many of the diagnostic tests. X-ray studies, scans, imaging, ultrasound, and Doppler assessment reveal most pathologic conditions.1 TABLE 44-1 Comparison of Peripheral Arterial and Venous Obstructive Diseases in an Extremity Intraoperative assessment of shunt performance or vessel patency or stenosis, as well as identification of an arteriovenous fistula (AVF), is easily performed with a sterile Doppler probe (Fig. 44-5). An audible signal is transduced and is similar to the sounds transmitted by a Geiger counter. Selective angiography permits the x-ray study of a particular segment of the vascular system.1,4 Aortography visualizes the aorta (Fig. 44-6). Arteriography shows the patency of an artery or a branch of the aorta and its collateral circulation. • A thorough understanding of the principles of general surgery should be combined with special training in vascular surgical techniques. Speed and accuracy are imperative. • Local or monitored anesthesia care (MAC) is usually preferred for most conservative interventional procedures. General anesthesia or regional block is used for longer or more extensive procedures. • Skin preparation is performed very gently. Vascular pathology (i.e., carotid stenosis, aneurysms, or venous thrombosis) should never be rubbed or pressed during the prep because plaque or clots could embolize, causing serious limb or brain damage. Aneurysms could rupture. • Temperature regulation may be a problem during long procedures or when multiple blood transfusions are given. Warmed fluids can be administered via rapid infusing pumps. Forced-air warming blankets provide a normothermic temperature. Intentional hypothermia can be used to preserve the spinal cord during aortic surgery.13 The low temperatures decrease the metabolic needs of the cord by minimizing the effects of temporary ischemia during cross-clamping of the aorta. • Meticulous care is exercised during anastomosis of vessels to avoid the danger of postoperative thrombosis and stenosis. To prevent undue trauma to vessels, an assortment of curved and angled scissors, noncrushing vascular clamps, and forceps specifically designed for vascular surgery is included in the instrument setup (Fig. 44-7). Umbilical tape (also called hernia tape) or synthetic vessel loops are used for retraction and vessel control (Fig. 44-8). An operating microscope may be used for anastomosis of vessels. Appropriate instrumentation for microsurgery is made available. Synthetic nonabsorbable monofilament suture materials are preferred because they are strong and pass through vessel walls and grafts easily with minimal trauma and tissue reaction. Swaged needles also minimize trauma. A larger swaged suture-to-needle ratio is advantageous to avoid leakage. Holes made in graft materials by the needle are occluded by the larger suture. Double-armed needle sutures are often used for vessel anastomosis. Structurally the surgeon may use a triangulating technique in which the vessel edges are gently straightened to create a straight sutured angle instead of suturing a circular stitch. • Heparinized solution is available for use as an anticoagulant irrigation. Preoperatively, heparin (5000 units) may be given subcutaneously for DVT prophylaxis 1 hour before the surgical procedure.6 Intraoperatively, the optimal dose is 70 to 100 units/kg of body weight if given intravenously for immediate systemic effect. Thromboelastography may be used intraoperatively to monitor the effects of heparin administration. Sensitivity to bovine sources of heparin can result in heparin-induced thrombocytopenia (HIT), also known as white clot syndrome. Clots composed of fibrin and platelets form after 4 to 15 days of heparin therapy. A severe decrease in the platelet count predisposes the patient to thrombosis and acute arterial occlusion. • Patients with preoperative anticoagulation with warfarin for known blood coagulation pathology are at risk for extreme blood loss. Temporary reversal of the therapeutic anticoagulation for the duration of an emergent surgical procedure is accomplished by the administration of fresh frozen platelets (FFP).6 Onset of FFP action is immediate and easily amenable to postoperative anticoagulation as necessary. The activity of FFP is short term. Less urgent surgical procedures for the anticoagulated patient can be performed with minimal blood loss after the administration of vitamin K. Studies have shown that 1 mg of intravenous vitamin K reversed the effects of therapeutic anticoagulation within 27 hours of administration.6 Subcutaneous vitamin K injections are absorbed at an unpredictable rate and are rarely used. Low-dose vitamin K does not interfere with postoperative therapeutic anticoagulation regimes. • Before closure at the end of the procedure, protamine sulfate, a heparin antagonist, is given to reverse the anticoagulant effect; 1 mg of protamine is given to counteract 100 units of heparin. Protamine is derived from fish semen and testicular tissue and may elicit a sensitivity reaction in patients who are allergic to fish. Patients with type 1 diabetes who take NPH insulin also may be predisposed to a sensitivity reaction to protamine. Some men who have had a vasectomy may exhibit allergic or hypersensitivity reactions. • Hemostatic agents can be used independently or in combination (Box 44-2). Care is taken not to permit hemostatic materials to be suctioned into the blood-salvage or cell-saving device. These products can be hazardous if permitted to enter the patient’s vascular system. Containers and delivery devices for thrombin should be carefully labeled with concentration and dosage to prevent accidental injection.
Vascular surgery
Anatomy and physiology of the vascular system
Arterial anatomy
Venous anatomy
Vascular pathology
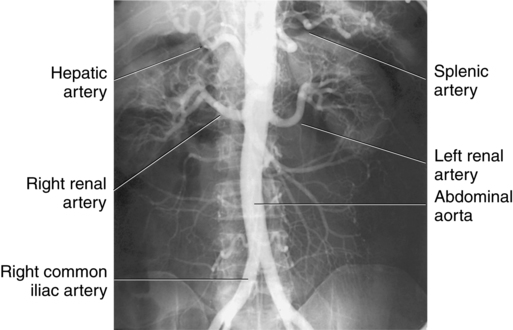
Diagnostic procedures
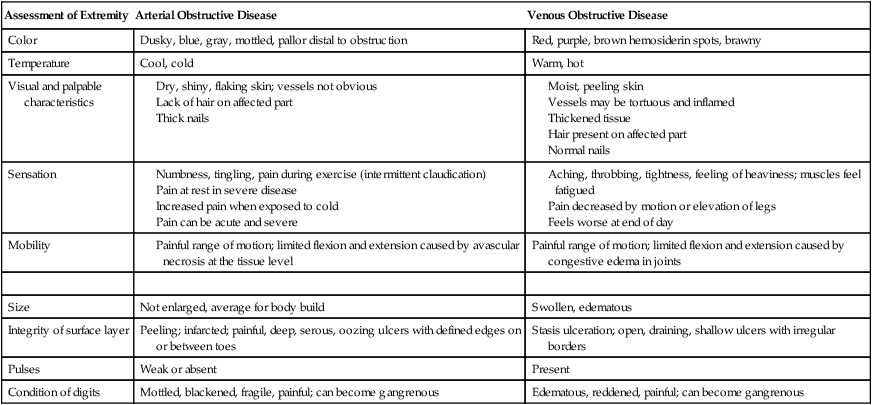
Assessment of Extremity
Arterial Obstructive Disease
Venous Obstructive Disease
Color
Dusky, blue, gray, mottled, pallor distal to obstruction
Red, purple, brown hemosiderin spots, brawny
Temperature
Cool, cold
Warm, hot
Visual and palpable characteristics
Sensation
Mobility
Painful range of motion; limited flexion and extension caused by congestive edema in joints
Size
Not enlarged, average for body build
Swollen, edematous
Integrity of surface layer
Peeling; infarcted; painful, deep, serous, oozing ulcers with defined edges on or between toes
Stasis ulceration; open, draining, shallow ulcers with irregular borders
Pulses
Weak or absent
Present
Condition of digits
Mottled, blackened, fragile, painful; can become gangrenous
Edematous, reddened, painful; can become gangrenous
Noninvasive procedures

Invasive procedures
Special features of vascular surgery
Vascular surgery

 Website
Website