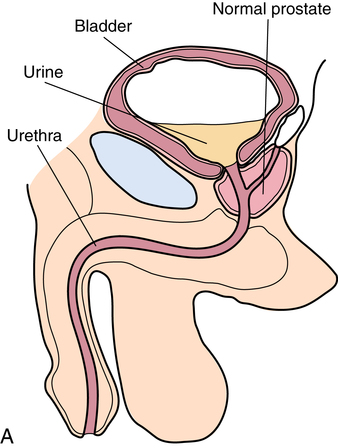
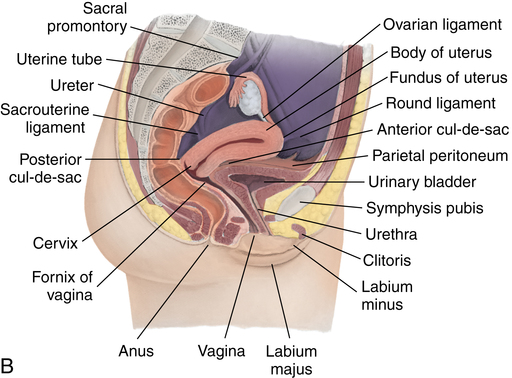
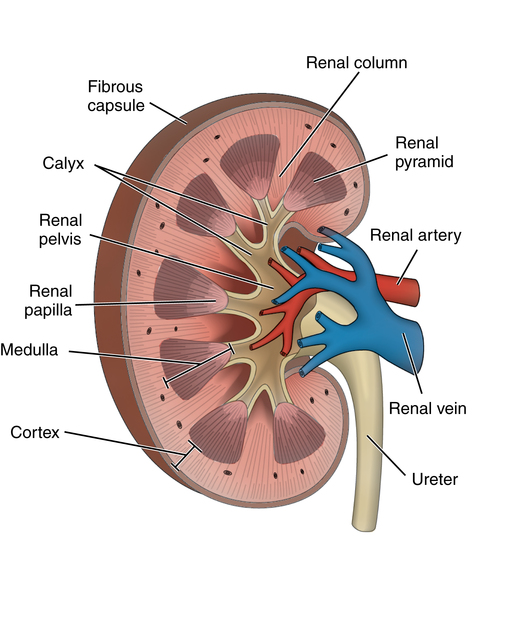
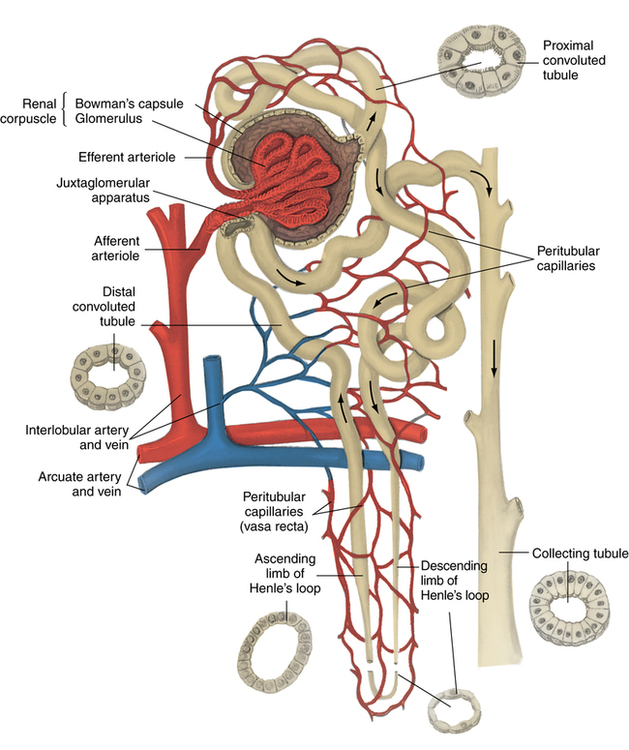
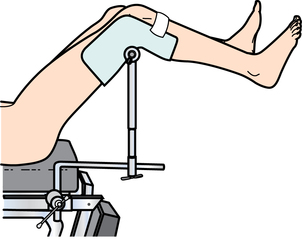
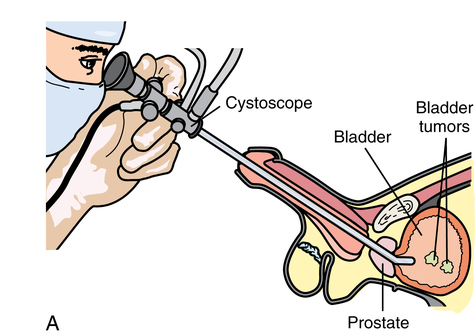
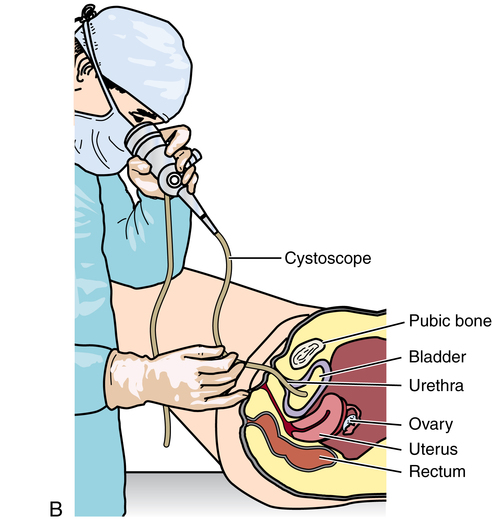
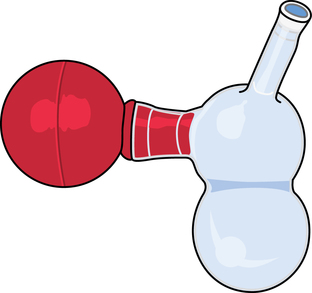
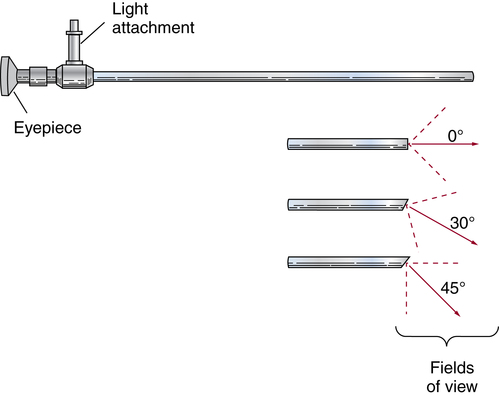
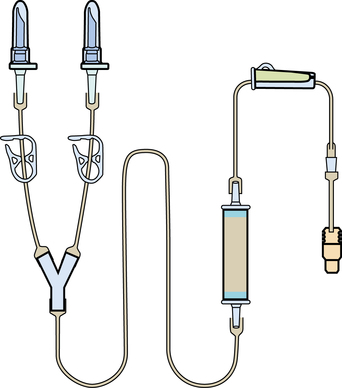
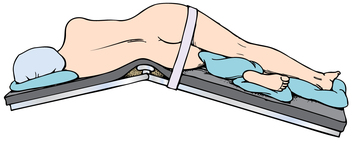
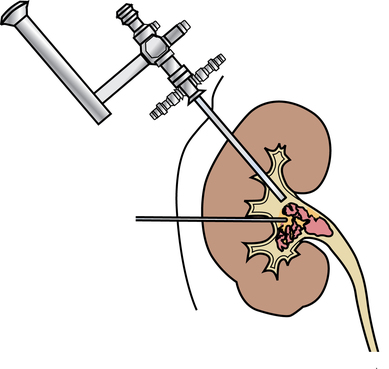
Chapter 35 After studying this chapter, the learner will be able to: • Identify the organs of the male genitourinary system. • Compare the differences between male and female urinary systems. • Describe the procedures performed for urinary incontinence. The urinary system provides the vital life-sustaining functions of extracting waste products from the bloodstream and excreting them from the body. Organs of this system include bilateral kidneys and ureters, the bladder, and the urethra (Fig. 35-1). An obstruction to blood flow in the renal arteries or any part of the urinary system can cause renal damage, which ultimately results in uremia (a biochemical imbalance) or renal failure if left undiagnosed and untreated. Vascular hypertension, tumors, infection, trauma, and other systemic or neurogenic disorders are of major concern to a urologist.9,16 Each kidney is enclosed in a thin, fibrous capsule referred to as Gerota’s fascia. The renal parenchyma, the substance of the kidney within the capsule, is composed of an external cortex and internal medulla. The medulla consists of conical segments called renal pyramids. Each pyramid and its surrounding cortex form a lobe. Within these pyramids are the essential components of renal function: the nephrons (Fig. 35-2). As the urine forms, it drips from the papillae at the tips of the pyramidal structures. • To remove metabolic waste, excess substances, and toxic substances from the blood • To regulate and maintain body fluid and pH balance • To help regulate and respond to blood pressure by producing the hormone renin • To produce the hormone erythropoietin to control the rate red blood cells are produced by the bone marrow • To assist in the production of water-soluble vitamin D, which is important for the metabolism of calcium Each nephron consists of a glomerulus, glomerular capsule, and tubules. A glomerulus is an aggregation of capillaries formed by an afferent branch of the renal artery. These capillaries unite to form an efferent vessel. This capillary network is enclosed in a glomerular capsule (capsule of Bowman), which is the dilated beginning of the renal tubule. From the capsule, the tubule becomes tortuous and forms the proximal convoluted tubule. The distal portion forms the descending and ascending limbs of the medullary loop (loop of Henle) (Fig. 35-3). The male urethra is approximately 25 to 30 cm long and has a diameter of 7 to 10 mm. It consists of two segments (proximal and distal) that are further subdivided into three segments referred to as the prostatic, the membranous, and the cavernous urethral segments. The proximal (posterior) prostatic urethra passes from the bladder orifice, through the prostate gland, and to the pelvic floor. The membranous urethra passes from the pelvic floor to the base of the penis. The distal (anterior) segment consists of parts of the membranous, bulbous, and anterior urethral segments that pass through the penis to the external urethral orifice at the meatus. The membranous and anterior urethras also serve as a passageway for secretions from the male reproductive system (see Fig. 35-1, A). The urethra is lined with urothelium that is continuous with the lining of the bladder. The female urethra is approximately 3 to 5 cm long and 6 to 8 mm in diameter. It is firmly embedded posterior to the clitoris and anterior to the vaginal opening. The Skene glands are situated bilaterally near the opening of the urethral meatus. Females are predisposed to urinary tract infections because the urethra is anatomically located near the vagina and the anus, both of which have resident flora that can cause infection if introduced into the urethra. Mechanical injury during coitus and the use of a pessary for vaginal support or diaphragm for contraception may be contributing factors to chronic urinary tract infection (see Fig. 35-1, B). A urologic bed differs from the standard operating bed in that it provides an x-ray–compatible base, a drainage system, and lithotomy knee supports with safety belts (Fig. 35-4). Some urologic beds incorporate electrosurgical units (ESUs). Urologic radiographic studies use conventional x-rays, fluoroscopy and image intensification, and tomography. The imaging system is an integral part of the urologic bed. Some beds have a built-in automatic x-ray film-handling system, others have a film cassette holder, and others adapt to a C-arm. The drainage system may have a drainage pan with tubing to drain port or single-use drainage bags. Under-knee/calf supports with gel pads and Velcro straps provide patient comfort in the lithotomy position (Fig. 35-5). Urologic beds are equipped with hydraulic or electric controls to adjust height and tilt. Some have tray attachments for the light source and auxiliary ESU, as well as hooks for irrigating solution bags. 1. Unless the procedure will be performed with the patient under general or regional anesthesia, the patient may be encouraged to drink fluids before coming to the cysto room. Fluids ensure rapid collection of a urine specimen from the kidneys. Some tests require the ability to void for bladder strength studies or while contrast medium passes through the urinary system. Electromyographic (EMG) data may be gathered during the process. 2. Intraurethral procedures are often performed with topical agents or local infiltration anesthetics. The patient should be reassured that the procedure usually can be performed with only mild discomfort. Respect the patient’s modesty by providing appropriate drapes and keeping the cysto room door closed. 3. The patient is assisted into the lithotomy position with the knees resting in padded knee supports or stirrups. Gel pads behind the legs and knees help avoid undue pressure in popliteal spaces. Velcro straps are used to secure the patient’s legs. Some cystoscopic procedures are performed with the male patient in the supine position. 4. The drainage pan is pulled out of the lower break of the urologic bed after the patient’s legs have been positioned on knee supports and the foot of the bed has been lowered. 5. The pubic region, external genitalia, and perineum are mechanically and chemically cleansed with an antiseptic agent according to routine skin preparation procedure. Scrub soaps can be diluted with warmed sterile saline or water for the comfort of the patient. 6. Topical anesthetic agents are instilled into the urethra at the end of the prep procedure. A viscous liquid or jelly preparation of lidocaine hydrochloride, 1% or 2%, may be used. This medium remains in the urethra rather than flowing into the bladder. a. For the female: The female urethra is most sensitive at the meatus. A small, sterile cotton-tipped applicator dipped into the anesthetic gel and placed in the meatus is sufficient anesthesia for local urethral procedures. The applicator is removed when the urologist is ready to introduce an instrument. Cone-shaped syringes are commercially available for intraurethral instillation of local lidocaine 2% jelly. b. For the male: A disposable cylinder with an acorn tip can be used for intraurethral insertion. The agent is injected into the urethra, and the penis is compressed with a noncrushing spring-loaded penile clamp for a few minutes to retain the drug. 7. A sterile stainless steel filter screen is placed over the drainage pan. The patient is draped as for other perineal procedures in the lithotomy position. The urologist may need to have access to the rectum. The perineal sheet has two fenestrations: one exposes the genitalia, and the other fits over the screen on the drainage pan. A gauze filter is incorporated into this latter fenestration to capture resected tissue. Each type of procedure requires specific endoscopic equipment. All rigid urologic endoscopes have the same basic components, which are discussed in the following sections (Fig. 35-6). The hollow sheath, through which the urologic endoscope passes, may be concave, convex, or straight in configuration at the distal end (the end inserted into the urethra) (Fig. 35-7). The other end has a stopcock attachment for irrigation. Sheath sizes range from 11 Fr for infants to 30 Fr for adults. Space is provided within the sheath to accommodate instruments for work in the bladder or urethra. Other instruments and catheters can be inserted through the sheath into the ureters and/or kidneys for diagnostic or therapeutic procedures. The optical systems provide several angles of vision (Fig. 35-8): • Direct forward or 0-degree vision is useful for viewing the urethra and for use with the optical urethrotome (an instrument for sharp resection of tissue in the urethra). • Right-angle or 30-degree vision is most suitable for viewing the entire bladder and insertion of ureteral catheters. • Lateral, which deviates 70 degrees but includes an additional visual field at a right angle in the line of vision, is used for wide-angle viewing within the bladder. • Foroblique, which is a forward vision with an oblique view somewhat in front of a right angle, is used to examine the urethra and for transurethral surgical procedures. • Retrospective, which provides an approximate 55-degree angle of retrograde vision, is used to inspect the bladder neck. The resectoscope uses electric current to excise tissue from the bladder, urethra, or prostate (Fig. 35-9). Components of this instrument include the sheath, obturator, telescope, working element, and cutting electrode. The sheath, usually 24 to 28 Fr, is made of Bakelite or fiberglass to prevent a short circuit of the electric current. If the short beak post sheath is used with a wide-angle telescope, a Timberlake obturator is used to introduce the sheath into the urethra. • The Iglesias resectoscope uses a thumb control on a leaf spring–lock mechanism. The working element can be adapted for simultaneous irrigation and suction to control hydraulic pressure in the bladder. • The Nesbit resectoscope uses a thumb control on a spring. • The Baumrucker resectoscope uses finger control on a sliding mechanism. • The Stern-McCarthy resectoscope uses a rack and pinion to move the loop forward and back. This requires two hands. A spark-gap generator is commonly used for transurethral resection and fulguration. A spark-gap ESU requires high-voltage arcing, which is described as spray coagulation. Spark-gap generator use requires the patient to be grounded with a grounding pad. The power control settings on the ESU should be as low as possible. Recommended practices as suggested by the Association of periOperative Registered Nurses (AORN) for the use and care of electrosurgical equipment (e.g., return electrodes) apply also to urologic procedures. Additional information about electrosurgical units can be found in Chapter 20. Continuous irrigation of the bladder is necessary during cystoscopy (cysto) to do the following: • Distend bladder walls to create a working space so the urologist can visualize them. • Wash out blood, bits of resected tissue, or stone fragments to permit continuous visibility and collection of specimens. A sterile, disposable, and closed irrigating system is used because it prevents airborne contamination of the solution. The tubing is Y shaped (Fig. 35-10). Two or more liters of sterile irrigating solution may be needed for a single examination. Disposable tandem sets may be used to connect several containers together. Tandem sets allow for a continuous flow and replacement of containers without interruption of flow. • Ellik evacuator. The Ellik evacuator is a double bowl-shaped glass or firm disposable plastic receptacle (Fig. 35-11). It contains a trap for fragments so they cannot be washed back into the sheath of the endoscope during irrigation with pressure on the compression-bulb attachment. • Toomey evacuator. The Toomey evacuator is a syringe-type evacuator with a wide opening into the barrel (Fig. 35-12). It may be used with any endoscope sheath. A metal adapter permits its use with a catheter. 1. Sterilization is preferred, although high-level disinfection with 2% glutaraldehyde is commonly used. Endoscopes and reusable accessories must be free of debris and residue for sterilization and/or high-level disinfection techniques to be effective. a. Disassemble all parts, and open all outlets. b. Clean all parts in an appropriate liquid detergent solution. Clean the interior of sheaths and openings with a soft brush. c. Rinse in clean water, and dry thoroughly. 2. After cleaning, place endoscopes on a towel to drain. With air, blow-dry the lumens. Moisture in the channels can dilute chemical sterilants and, if ethylene oxide is used, will form ethylene glycol, which is toxic to tissues. 3. Check the function of all moving parts, the clarity of vision through telescopes, and the patency of channels through instruments and catheters. 4. Keep sets of sounds, bougies, and filiforms and followers together so the urologist has a complete range of sizes readily accessible. 5. Wrap items for sterilization after cleaning. Flexible instruments should be protected by a rigid container. 6. Clean and dry stone baskets before sterilizing by low-temperature sterilization methods, such as plasma vapor, peracetic acid, or ethylene oxide. Retractable stone baskets are sterilized in the open position. 7. The principles and methods of sterilization apply to urologic endoscopy equipment. 8. Endoscopic processors and sterilizers, such as the STERIS unit, are useful for sterilizing instrumentation between patient uses. The instrumentation is used immediately after processing and is not stored in the processing tray. 9. If sterilization between patient uses is not feasible, high-level disinfection may be the method of choice for processing urologic endoscopic instruments. Most patients commonly seen by urologists are in the preadolescent or older age groups. Congenital and common pediatric problems are discussed in Chapter 8. This chapter focuses on adult problems, most of which occur in men older than 50 years who have prostatic disease and women who have urinary incontinence. The kidney is usually approached posteriorly with the patient in a lateral position (Fig. 35-13). The kidney rest is raised, and the bed is flexed until the flank muscles become tense. After the patient is secured in position, the bed is tilted in Trendelenburg’s position until the flank is horizontal to the floor. A flank incision is made parallel to and just below or over the eleventh or twelfth rib; the twelfth rib may be removed. The retroperitoneum is opened to expose the kidney. An incision through the renal parenchyma or into the renal pelvis may be necessary to establish temporary or permanent drainage when an obstruction prevents the flow of urine from the kidney (Fig. 35-14). A tube placed in the kidney exits through the skin. A cutaneous nephrostomy tube may be used to drain a kidney postoperatively during healing after renal reconstruction or revascularization. Silastic tubes placed internally through a cystoscope via the ureter eliminate the need for an open surgical procedure for a temporary urinary diversion. Patients with chronic renal failure can tolerate extensive surgical procedures with minimal complications. Their management in the OR must include strict attention to maintaining a patent hemodialysis access shunt, fistula, or catheter; careful monitoring of fluid and electrolyte balance; and avoiding postoperative infections.4 After kidney transplantation, the patient may require postoperative dialysis; thus access must remain patent during and after the surgical procedure. In hemodialysis, waste products are removed from the blood through the semipermeable membrane of a dialyzer (artificial kidney machine). An arteriovenous (AV) shunt or fistula is created subcutaneously in either an arm or a leg to provide access to the patient’s circulation (Fig. 35-15).17
Urologic surgery
Anatomy and physiology of the urinary system
Kidneys
Urethra
Special features of urologic surgery
Urologic endoscopy
Urologic bed
Patient preparation for a cystoscopic examination
Urologic endoscopes
Sheath
Telescope
Types of urologic endoscopes
Resectoscope
Endoscopic accessories
Electrodes
Irrigating equipment
Evacuators
Care and preventive maintenance of urologic endoscopes
Surgical procedures of the genitourinary system
Kidney
Nephrostomy or pyelostomy
Dialysis
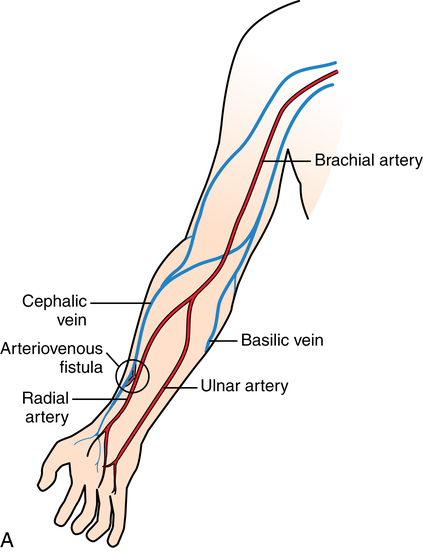
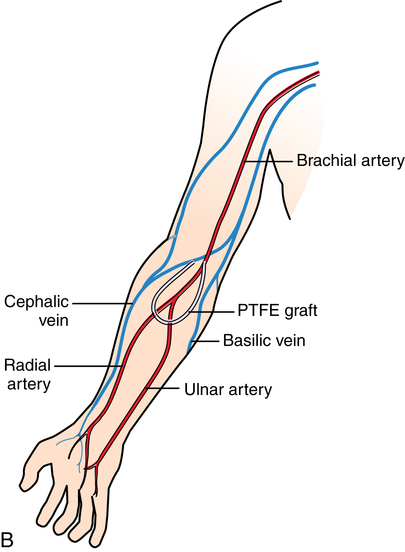
Hemodialysis.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 Website
Website