CHAPTER 19
Valproic Acid
ELJIM P. TESORO, PharmD, BCPS
GRETCHEN M. BROPHY, PharmD, BCPS, FCCP, FCCM, FNCS
HENRY COHEN, MS, PharmD, FCCM, BCPP, CGP
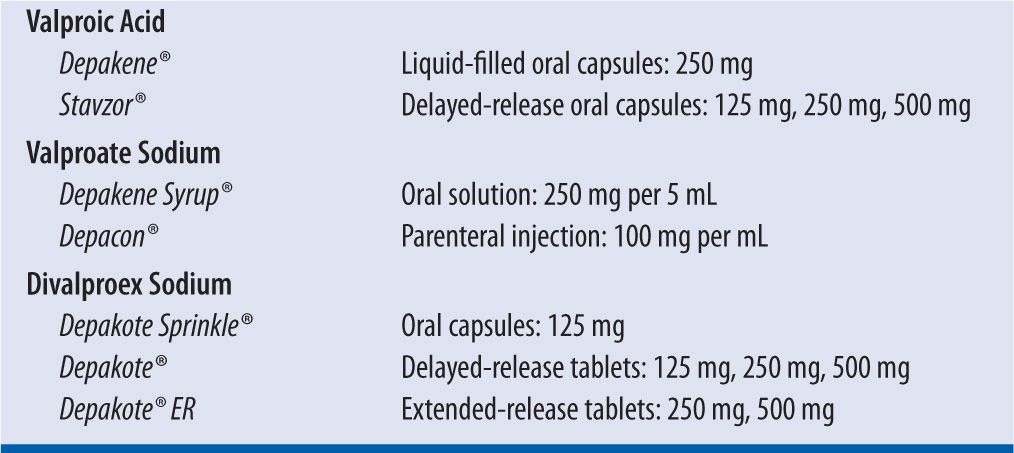
Valproic acid (VPA) is a broad-spectrum, carboxylic acid-derived anticonvulsant that has been used in the treatment of epilepsy, bipolar disease, schizophrenia, and migraine headache. Its main mechanism of action is not well understood, but it is thought that it increases the amount of the inhibitory neurotransmitter, gamma-aminobutyric acid (GABA), in the central nervous system (CNS). It comes in several different preparations and salt forms (Table 19-1). Divalproex sodium is a mixture of equal parts of the acid and sodium salts of valproic acid. The delayed-release (Depakote) and extended-release (Depakote ER) formulations are not bioequivalent.1 A 20 percent increase in the daily dose is recommended when switching from Depakote to Depakote ER to account for the differences in rate and extent of absorption.
| TABLE 19-1 | Valproic Acid Formulations |
DOSING
Dosing is done in terms of valproic acid content. For seizure disorders, initial oral dosing for patients 10 years of age and older is 10–15 mg/kg/day. Dosing intervals for the oral and parenteral preparations are typically every 8–12 hours (although dosing every 6 hours may be needed in some patients), with the exception of the extended-release formulation, which can be administered once or twice daily. The daily dose can be titrated weekly by 5–10 mg/kg/day to a maximum recommended dose of 60 mg/kg/day. Loading doses are not recommended for the oral VPA formulations due to intolerable gastrointestinal side effects; however, intravenous loading doses of valproate sodium 25 mg/kg are commonly given for patients in status epilepticus. Intravenous loading doses can be given at a rate of 1.5–3 mg/kg/min, but faster rates of up to 6 mg/kg/min appear to be safe.2,3,4 Therapeutic drug monitoring (TDM) is used in conjunction with the clinical exam to optimize seizure control and minimize toxicity. Measuring serum concentrations is routinely performed via immunoassay, and the therapeutic range is reported as 50–100 mcg/mL, although individual patients may have optimal responses outside these ranges. Concentrations higher than 150 mcg/mL are associated with a high incidence of CNS side effects. Free (unbound) concentrations are also available with a therapeutic range reported to be 6–22 mcg/mL with toxicity occurring above 50 mcg/mL. Trough levels (obtained prior to a dose) are preferred to assure that minimum therapeutic concentrations are maintained. Diurnal variations in the serum concentration of VPA have been reported, so it is important to be consistent when sampling in order to properly compare levels and adjust dosing regimens. Clearance tends to be higher in the evening compared to the morning times5 in both young adults and elderly subjects,6 so trough levels are recommended before the morning dose.
BIOAVAILABILITY
All of the formulations of valproic acid have bioavailabilities near 100 percent (F = 1), reflecting the absence of a first-pass effect in the liver. The enteric-coated formulation has a bioavailability of approximately 90 percent (F = 0.9).7 Valproate sodium is rapidly converted to valproic acid in the stomach and then readily absorbed via the gastrointestinal tract. Peak concentrations are achieved within 0.5–2 hours after oral administration.8 The presence of food will delay the peak concentration but not the extent of absorption.9 The volume of distribution in adults is reported to range from 0.1 to 0.4 L/kg,10 indicating that VPA remains primarily within the intravascular and extracellular space. The volume of distribution tends to increase with higher doses due to saturable protein binding.11 VPA has been isolated from cerebrospinal fluid (10% of serum concentrations), breast milk (1–10% of serum concentrations), and saliva (1% of serum concentrations, although correlation is poor).12 It readily crosses the placenta13 and can increase the risk of neural tube defects if given in pregnant women.
PLASMA PROTEIN BINDING
VPA is approximately 90–95 percent protein bound, primarily to serum albumin. The free fraction of VPA ranges from 6 to 10 percent, and its protein binding depends on serum concentration, serum albumin, age, and end-organ failure. The free fraction has been reported to be increased in elderly patients (10.7%) versus younger adults (6.4%).6 At concentrations above 70 mcg/mL, VPA displays a higher free fraction with minimal changes in total concentration. Renal failure may increase the free fraction to 18 percent while cirrhosis can increase it to 29 percent. Caution must be used when interpreting total VPA serum levels in these patients because they can be falsely low while their free levels may be therapeutic or higher.14 In addition, VPA may displace other highly protein-bound drugs, such as phenytoin; therefore, free phenytoin levels should be monitored when these drugs are given concomitantly.15
METABOLISM
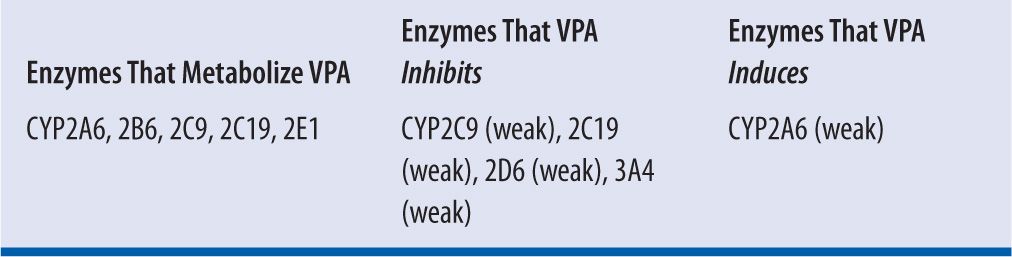
VPA is primarily metabolized in the liver via glucuronidation (40%), β-oxidation, and ω-oxidation (20%) and then eliminated via the kidneys, with less than 3 percent of the drug excreted in the urine unchanged. VPA is characterized as a low-extraction drug with its clearance being independent of hepatic blood flow. Many of the metabolites are thought to be responsible for anticonvulsant activity as well as toxicity.16 VPA has an average elimination half-life of 11 hours and follows first-order kinetics. The mean plasma clearance for total VPA is reported to be approximately 6–7 mL/hr/kg in adults6 (range 5–10 mL/hr/kg),8 which may be decreased in patients with renal or hepatic failure, and increased in patients taking concomitant hepatic enzyme-inducing agents. Total clearance increases with higher doses due to saturable protein binding, which leads to a higher free fraction and more unbound drug available for metabolism (assuming normal hepatic function).10 VPA acts as a substrate and inhibitor of various cytochrome P450 enzymes, reflecting a high potential for drug interactions (Table 19-2).
| TABLE 19-2 | Cytochrome P450 Enzymes Associated with Valproic Acid |
CASE STUDIES
CASE 1: VALPROIC ACID LOADING DOSE/MAINTENANCE DOSE
A 43-year-old female is admitted with status epilepticus. She has a history of coronary artery disease, hyperlipidemia, and asthma. Her medications prior to admission include aspirin 81 mg PO daily, amlodipine 10 mg PO daily, simvastatin 20 mg PO qhs, and Advair 250/50 ii puffs BID. She has no known drug allergies. Her vital signs are:
HR: 107
BP: 100/56
RR: 20
T: 38.8°C
Height = 63 inches
Weight = 80 kg
QUESTION
What is the best approach for loading and maintenance doses for this patient?
Answer:
Intravenous (IV) loading doses will ensure rapid achievement of therapeutic serum levels and increase the likelihood of seizure control in emergent cases of status epilepticus. Valproate sodium (Depacon) can be given as an IV load in cases of status epilepticus, especially when concern for hemodynamic or pulmonary compromise is present. One study reported a series of patients receiving 21–28 mg/kg of IV valproate sodium at a rate of 3–6 mg/kg/min (the recommended rate for nonemergent administration is listed at 20 mg/min).17 Peak serum levels 20 minutes after the end of the infusion were 105–204 mcg/mL, with no reports of hemodynamic or CNS side effects. This loading dose would provide therapeutic serum levels until a maintenance dosing regimen could be initiated 8–12 hours later.
For this patient, a dose of 30 mg/kg (~2,400 mg) given at 400 mg/min would be reasonable to attain rapid therapeutic levels. Maintenance doses would be calculated the traditional way: 10–15 mg/kg/day or 800–1,200 mg/day, or valproate sodium 500 mg PO q12h with the first dose starting 12 hours after the IV load. A postload serum level may be collected to document achievement of the therapeutic range, but clinical cessation of seizure activity should take precedence. Postload levels should be obtained no sooner than one hour after the loading dose to assure complete distribution and an accurate reading.
Another way to calculate the maintenance dose is by using population-based pharmacokinetic parameters. Using the following equation:
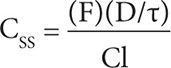
where CSS is the steady-state concentration, F is bioavailability (1 for immediate-release VPA; 0.9 for sustained-release VPA), D is dose in milligrams, τ is dosing interval in hours, and Cl is clearance (5–10 mL/hr/kg). A steady-state concentration of 50 mcg/mL is a reasonable target, and choosing a clearance of 10 mL/hr/kg would give us a conservative recommendation. So for this patient, the dose of extended-release VPA given every 12 hours that would achieve a CSS of 50 mcg/mL (or 0.05 mg/mL) would be:
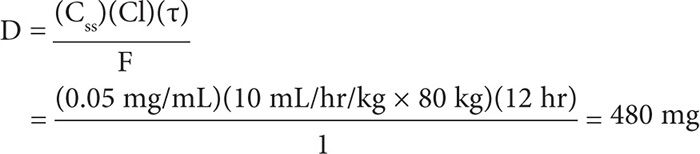
This dose can be rounded up to 500 mg every 12 hours. The timing of a serum level can be determined by calculating the half-life:
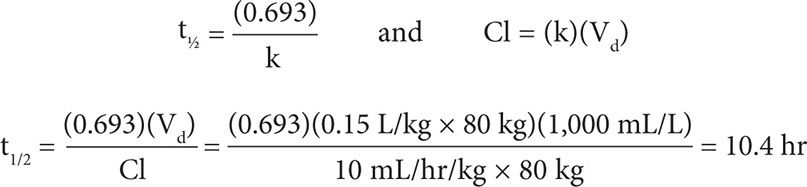
To obtain a true steady-state concentration, a VPA level should be checked in 3–5 half-lives, so a level can be checked after 52 hours (10.4 × 5 = 52 hr).
CASE 2: VPA DRUG INTERACTION THAT DECREASES LEVELS
A 42-year-old man is seen in the infectious diseases clinic for a two-month follow-up for his treatment of tuberculosis. He has a history of seizures for which he takes Depakote 500 mg PO BID and hypertension for which he takes metoprolol XL 100 mg PO daily. He takes Rifater® (rifampin, isoniazid/pyrazinamide) 6 tablets PO daily for his infection. He reports an increase in seizure activity in the last few weeks. A serum level is collected and is reported to be 20 mcg/mL (his serum level 2 months ago was 60 mcg/mL).
| TABLE 19-3 | Valproic Acid Drug Interactions |
Height = 70 inches
Weight = 92 kg
QUESTION
What approach is needed to address the patient’s increase in seizure activity?
Answer:
VPA is metabolized by glucuronidases in the liver and rifampin is a potent inducer of these enzymes. A 40 percent increase in clearance can result from concomitant use of rifampin or its derivatives, and its effect can be seen relatively quickly. To adjust for this interaction, a higher daily dose can be given (increase to 2 g/day or 20 mg/kg/day), and the dosing interval can be shortened to 6 hours (500 mg PO q6h). A serum trough level can be obtained in 3–4 days to ensure attainment of therapeutic concentrations if no seizures are noted beforehand. Once the rifampin therapy is complete, the VPA can be decreased to the previous regimen of 500 mg PO every 12 hours. The timing of this change may be difficult to predict. It is reasonable to wait until mild symptoms of toxicity occur (e.g., drowsiness), which signal a down-regulation of enzymes responsible for VPA metabolism with subsequent increase in serum levels. Another option would be obtaining a serum level in weekly intervals to document the increase in serum VPA concentrations before decreasing to the previous dose.
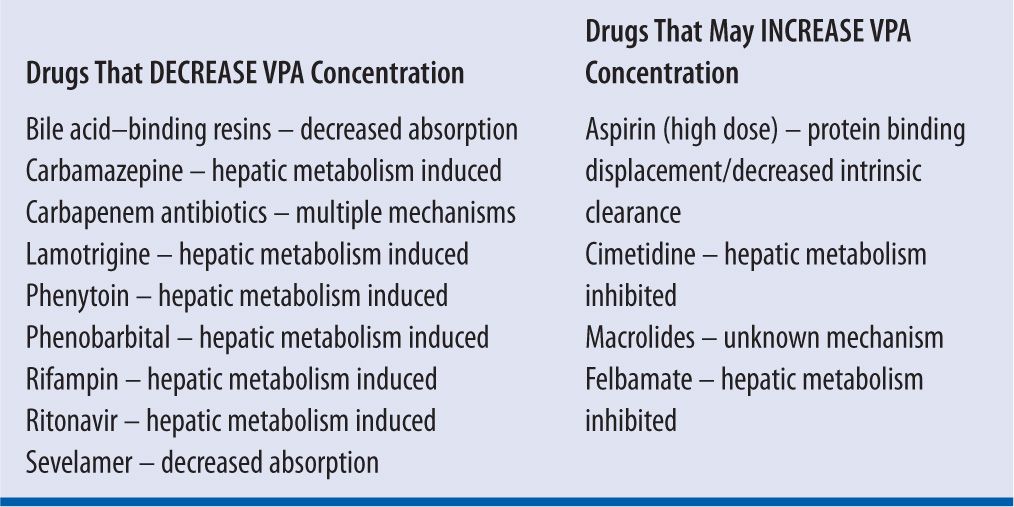
Other agents that can induce hepatic enzyme activity and thus decrease the steady-state serum concentrations of VPA include phenytoin,18,19,20 lamotrigine,21 and carbamazepine. As seizure control sometimes requires more than a single agent, anticonvulsants that induce or inhibit each other’s metabolism15,22,23 should be closely monitored for clinical effect as well as therapeutic serum concentrations.
CASE 3: DOSING IN RENAL DYSFUNCTION
JJ is a 54-year-old woman admitted with acute kidney injury from interstitial nephritis. Her baseline serum creatinine is 0.9 mg/dL, and currently it is 2.3 mg/dL. She has a history of seizures for which she takes Depakote 250 mg PO TID.
QUESTION
What is the best recommendation for dose adjustments in a patient with renal insufficiency?
Answer:
Only 3 percent of VPA is excreted unchanged by the kidneys. Dosing adjustment in renal impairment is unnecessary. However, patients with chronic renal dysfunction have altered protein binding, usually as a result of low albumin and retention of serum proteins that may displace VPA from its binding sites. The result may be increased free fraction of VPA with typically normal total VPA levels. Therapeutic drug monitoring using free VPA levels may be necessary to prevent unnecessary dosage increases and subsequent toxicities.
CASE 4: DOSING IN HEMODIALYSIS
SB is a 58-year-old man with end-stage renal disease on hemodialysis three times weekly. He has a new diagnosis of migraine headaches and was prescribed Depakote ER 500 mg PO daily.
QUESTION
What modifications, if any, are required to manage his VPA therapy?
Answer:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


