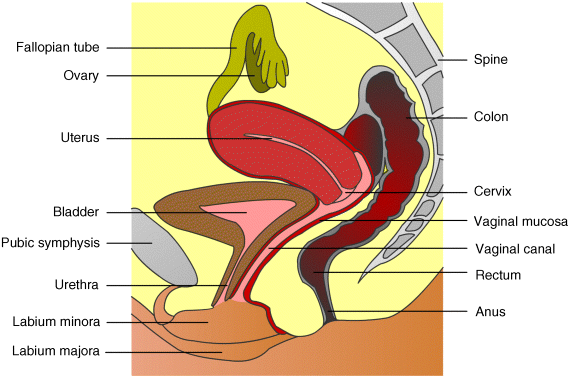
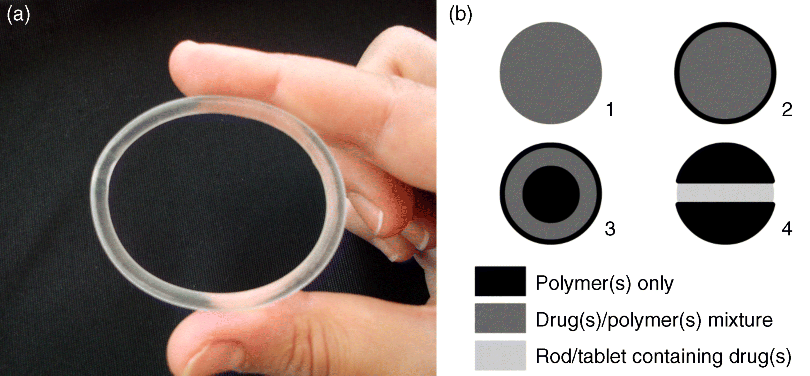
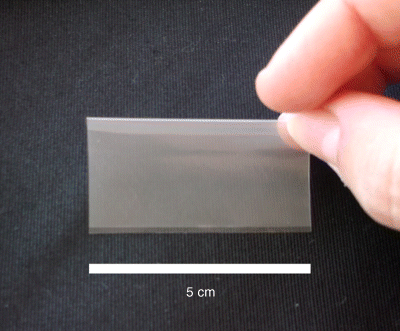
5 José das Neves1,2, Rita Palmeira-de-Oliveira3,4, Ana Palmeira-de-Oliveira3, Francisca Rodrigues5 and Bruno Sarmento1,2 1IINFACTS – Department of Pharmaceutical Sciences Instituto Superior de Ciências da Saúde – Norte CESPU Portugal 2INEB – Institute of Biomedical Engineering University of Porto Portugal 3Health Sciences Research Centre University of Beira Interior Portugal 4Pharmacy Department Hospital Center of Cova da Beira Portugal 5Requimte – Department of Chemical Sciences, Faculty of Pharmacy University of Porto Portugal Some of the first records of the vaginal administration of medicinal preparations date back to the Ancient Egypt, nearly 4000 years ago [1]. In modern days, this practice is well established for the management of local conditions or even for achieving systemic effects. Several advantages have been claimed for vaginal drug delivery [2,3]. In the case of local disease or disorder, using a vaginal product frequently avoids the delivery of significant amounts of drug(s) to the circulatory system and thus prevents side effects. It also allows self-administration and rarely requires the intervention of a health-care provider. Absorption in systemic relevant levels can also be achieved for different drugs, particularly those presenting hydrophobic properties and low molecular weight [4]. The vaginal route may be of particular importance in the case of drugs undergoing extensive hepatic metabolism, since it avoids the hepatic first-pass effect and allows the amount of administered drugs to be reduced (e.g. oestrogens [5]). However, important disadvantages limit the scope and utility of this route. The most obvious and relevant one is its gender specificity. Additionally, cultural issues and myths concerning the vaginal administration of drugs can strongly impact acceptability [6]. Inconsistent drug absorption behaviour may also be a concern due to the physiological variability observed during different stages of women’s development and hormonal status (e.g. childhood, pre- or post-menopausal, pregnancy). Sexual intercourse and the possibility of local irritation and other deleterious effects associated with topical application may impact on vaginal drug therapy. In this chapter basic concepts of vaginal drug delivery are reviewed alongside the latest developments and future perspectives in the field. Particular focus is set in essential aspects related to vaginal mucoadhesive materials and drug delivery systems. Also, special attention is paid to microbicides, which have been one of the main driving forces for research in the field of vaginal drug delivery in recent years. The vagina is an S-shaped fibromuscular, collapsed canal connecting the cervix to the vestibule (includes the labia minorum and labia majorum) [7]. Its anatomy and positioning in the female genital tract is depicted in Figure 5.1. The vaginal canal extends for around 7–15 cm [8] and its main functions are to accommodate the penis during sexual intercourse and allow the passage of menstrual fluids and the new born during natural childbirth. The width of the vagina is higher at the cervical level, decreasing towards the vaginal introitus [8]. At its distal third (from the introitus to the cervix), the vagina is almost in a horizontal plane which contributes to the retention of deeply inserted objects, such as drug dosage forms and devices [9]. Also, such objects are most likely to be unperceived due to the low sensory innervations at the upper two thirds of the vagina. The vaginal wall presents a series of transverse folds (rugae) that allow the vagina to extend considerably during penile penetration or child delivery. The total surface area of the vagina is an important factor when considering drug absorption and, although variable, it has been estimated in the range of 50–600 cm2 [10], but more realistic median values have been calculated around 360 cm2 [11]. Further studies of the human vagina using polysiloxane casts found a mean surface area around 90 cm2 (range 65–107 cm2) [12]. However, this method may fail to account for the area increase provided by the distension of rugae, thus resulting in an underestimate of the total surface area. Overall, these results show substantial differences amongst different subjects, which should be taken into account when designing and formulating vaginal dosage forms. Figure 5.1 Schematic representation of the female genital tract (sagittal section) and related structures. Adapted from reference [55] with permission from Adis (© Springer International Publishing AG 2008. All rights reserved). The vaginal wall is covered by a nonkeratinized stratified squamous epithelium, the thickness of which depends on hormonal status [13]. Oestrogens are able to enhance metabolic activity of epithelial cells, with a particular increase in glycogen levels, and enhance the number of cell layers. Thus, women present a thickened epithelium during reproductive ages; conversely, the vaginal epithelium presents a progressive atrophy after menopause. Underneath the epithelium, the lamina propria is mainly composed of connective tissue, presenting multiple blood vessels, as well as lymphatic vessels, which drain chiefly into the internal iliac vein thus allowing the possibility for vaginally absorbed drugs to avoid the hepatic first-pass effect. Two additional layers do support the vaginal mucosa: the muscular layer, which confers the elongation ability, and the tunica adventitia, highly rich in blood and lymphatic supply [7]. Different immune cells are present in the vaginal mucosa, having important roles in response to infection and mucosal damage and widely implicated in HIV transmission [14]. Although the vaginal mucosa is deprived of secreting glands, the epithelium is coated with a thin layer of fluid. This fluid comprises a mixture of endometrial fluid, cervical mucus, tissue transudate, vestibular glands secretions, immune and epithelial cells, and residues of urine [15]. Its biochemical composition is complex but consists mainly of water and a small amount of mucin (1–2%) mainly derived from the cervical mucus, which is the chief responsible for structural, rheological and adhesive properties of the fluid. The vaginal fluid is acidic in nature in healthy women during their reproductive years (pH around 3.5–4.5) due to the presence of lactic acid, which is mainly produced from host glycogen by Lactobacilli metabolism [16]. The amount of available glycogen at the vaginal epithelium is influenced by oestrogen, as this hormone stimulates metabolic activity. Thus, low oestrogen status, as after menopause, results in loss of acidic conditions and the pH rises to around 6.0–7.5 [17,18]. Also, the amount of vaginal fluid is reduced by low oestrogen levels, which leads to typical vaginal dryness in pre-pubertal and post-menopausal women. On the other hand, the amount of fluid is highly increased upon sexual arousal in order to lubricate the vaginal mucosa and assist penile penetration. The basal amount of vaginal fluid in healthy women at any given time is estimated to be around 0.5–0.75 ml [15]. The increase in vaginal pH is also observed during bacterial and Trichomonas vaginitis, due to the depletion of Lactobacilli and consequent low production of lactic acid [19], or upon ejaculation because of the high buffering capacity of semen (mean pH around 7–8.5) [20]. Alterations in vaginal pH should always be taken into consideration when formulating for vaginal drug delivery, as this factor can substantially influence the performance of drug products [21]. Alongside the maintenance of the acidic pH, Lactobacilli are also able to produce important antimicrobial compounds (e.g. hydrogen peroxide, bacteriocins) that play an important role in the prevention of infection [22]. Another relevant characteristic of the vaginal milieu is its low enzymatic activity when compared to other drug delivery routes, namely the oral route. This feature may be advantageous when considering the administration of enzymatically-labile compounds. However, loss of activity may be observed, particularly when considering peptide and protein active molecules [23]. The vaginal absorption of different compounds at systemic relevant levels has been shown to be attainable [3,4]. Indeed, different compounds have been shown to permeate the human vaginal mucosa at higher rates than through the oral and/or intestinal mucosa [24–26]. Permeation can occur by passive diffusion (either through the transcellular or paracellular pathways) or, to a minor extent, by active transport-mediated mechanisms. Intrinsic drug factors (molecular weight, lipophilicity/hydrophilicity), dosage form features (drug release, in loco retention, presence of solubility or absorption enhancers, or drug stabilizers) and physiological conditions (pH, amount of fluid, epithelial thickness) can be highly influential in the fate of a drug when administered in the vagina [3,27]. Until 1918, when Macht [28] reported the absorption of different compounds through the vagina, the later was not considered to be a site suitable for systemic drug delivery. Since then, the vagina has gained relevance as a route for drug delivery in modern medicine [3]. Several drugs have been approved for vaginal administration, the majority to treat local conditions but a few others aimed at systemic effects. The vaginal route is now considered an option for several therapeutic strategies. Hormones and antibiotics have been largely included in vaginal dosage forms but recently other therapeutic purposes have also been explored, such as prevention of infection and immunization. Also, the possibility of systemically delivering molecules with high molecular weight, such as calcitonin and insulin, through the vaginal route has been explored [29,30]. The vaginal route is being increasingly used for hormone administration, as it exhibits the great advantage of preventing gastrointestinal side effects and the hepatic first-pass effect. This is clearly useful for molecules that undergo a high degree of hepatic metabolism, such as natural oestrogens. While vaginal administration of estriol is indicated for atrophic vaginitis treatment mainly in post-menopausal women, ethinyloestradiol and etonogestrel are included in a vaginal ring for combined contraception. Vaginal progesterone is very effective in adjunctive hormone replacement treatment (HRT) for post-menopausal women, or in in vitro fertilization and in supporting early and pre-term pregnancy [31,32]. However, HRT has been restricted in recent years mainly due to the associated increase in cancer and other diseases risk [33]. In pre-term labor prevention, indomethacin administered intravaginally has also been found useful and even advantageous when compared to its oral administration [34]. The vaginal route is also considered the best option when the opposite aim is intended: labor induction. Vaginal misoprostol or dinoprostone are widely used for this purpose [35,36]. Over the last few years, hormonal contraception has been achieved mainly by the oral or transdermic routes. However, the introduction of oral hormone pills into the vagina was shown to have good efficacy and acceptability rates, thus opening ways for vaginal hormonal contraception [37]. Nonetheless, it was the introduction of the vaginal ring which promoted the vaginal route for hormonal contraception [38]. Vaginal rings for steroids release have also been proposed when both local and systemic effects are intended, as is the case for estrogens to treat atrophic conditions, including related vaginitis [39,40]. Moreover, distinct applications may emerge in the future for vaginal rings. For instance, it may contribute to the development of a new delivery approach for spermicides, which are classically administered through semi-solid dosage forms and sponges. New spermicide molecules have also been developed and classic dosage forms have been proposed [41,42]. Regarding topical antimicrobial treatment, therapeutic strategies for the most common vaginal infections, namely bacterial vaginosis (BV) and vulvovaginal candidosis (VVC), include drug products for vaginal application [43]. Clindamycin cream and metronidazole gel are two available therapeutic options to treat BV [44]. However, due to increasing bacterial resistance and infection-related complications, acid-buffering gels and vitamin C tablets for vaginal application have been proposed, alone or combined with oral therapy [45–47]. Additionally, recognized antimicrobial molecules, such as fenticonazole, garenoxacin and rifaximin, have been revisited and their possible topical application for BV treatment considered [48–50]. Vaginal administration of natural products to control and eradicate genital infections is very popular amongst women and arises as a possible alternative to overcome antibiotic resistance. Distinct plant extracts and essential oils have been proposed as valuable therapeutic alternatives for both BV and VVC, and have been studied in vitro and in animal models [51–54]. These natural products seem to be valuable for topical therapy especially in recurrent and resistant cases. In fact, VVC is treated very effectively with both oral and topical azoles unless a suspected azole-resistant strain is identified [55]. Topical azoles are recognized as safe and show comparable efficacy to oral therapy in uncomplicated VVC cases [56]. However, in addition to the limited number of available antifungals, the restrictions to its use (insufficient bioavailability, drug-related toxicity) and the increasing number of resistant cases stress the need for the development and validation of new therapeutic strategies exhibiting distinct mechanisms of action and/or evasion of resistance [57]. For instance, a vaginal cream associating amphotericin B (100 mg) and flucytosin (1 g) was proposed as an alternative topical treatment, especially for non-albicans infections [58]. Also, lidocaine and nitroglycerine, drugs with other main clinical applications, have been tested in combination for their antifungal activity as a step in the development of a new preparation for genital fissures treatment [59]. Antidepressive drugs that are often prescribed for pre-menstrual syndrome seem to contribute to control yeast infections [60]. For example, the anti-Candida in vitro activity of serotonin and fluoxetine was tested and both exhibited a rapid fungicidal effect [61,62]. Additionally, classical local therapies, namely gentian violet solution and boric acid vaginal capsules, have been revised [63,64]. Vaginal immunomodulation therapeutics is another important field for investigation that encourages more research. Intravaginal administration of vaccines was shown to promote local immunoglobulin production, standing up as a valuable route for the prevention of sexually transmitted diseases [65,66]. Enhancing vaginal innate and acquired defence mechanisms to treat VVC has been tested by the topical use of mannose-binding lectin and administration of Candida antigens and antibodies against yeasts’ virulence traits [67–69]. An immunomodulatory effect has been reported for Echinacea purpurea plant extract that is widely used for respiratory and urinary infections [70]. Some home-made preparations propose the topical administration of Echinacea spp. to control yeast infections. However, further scientific work testing its effect and proper delivery is required. Also, an anti-allergic therapeutic approach associating oral cetirizine and fluconazole was tested and showed to be helpful in women suffering from recurrent VVC with persistent pruritus [71]. Vaginal application of these two drugs may arise as a possible therapy. Local therapy is also very common in human papillomavirus (HPV) infections as systemic therapy is highly ineffective [72]. Podophyllin and podophyllotoxin topical delivery systems are available and widely used for localized treatment of genital lesions, despite adverse reactions and high recurrence rate being reported frequently. Other options, such as trichloroacetic acid solution and 5-fluorouracil, can also be used [73]. These drugs, frequently used in the past for vulvar infections, must be very carefully used when applied in the vagina: applications must be restricted to lesions, not healthy tissue. Topical immunomodulation therapeutic approaches are also available for HPV genital lesions, namely with topical imiquimod. Recently, new possibilities for the management of HPV infection have been proposed. For example, the therapeutic efficacy of topical cidofovir, an antiproliferative agent, was reported [74]. Additionally, lopinavir stands up as a future molecule for topical application [75]. Furthermore, polyphenon E, an extract from green tea (Camellia sinensis) leaves that induces cell apoptosis, was proposed as a valuable therapeutic agent [76]. Since the 1980s topical therapy has been used to control herpes simplex virus (HSV) genital infections, when first reports showed the efficacy of topical acyclovir [77]. However, due to pharmacokinetics limitations, new technologic formulations are required in order to improve acyclovir bioavailability. In the search for alternatives, different plant products with anti-HSV activity have been tested in vitro and in animal models. For example, eugenol exhibited an interesting microbicide effect upon this virus [78,79]. Immune response modifier molecules, such as immunostimulatory oligonucleotides and resiquimod, are also anticipated to be a valuable vaginal topical therapeutic strategy to treat HSV genital infections and reduce the frequency of recurrences [80,81]. A recent study reported the protective anti-HSV effect in animal models of several microbicides that are under development and clinical trials. Promising results were obtained, especially for carrageenan formulations, highlighting the importance of additional studies in this field [82]. Further, the development of microbicides, products intended to prevent sexual transmission of pathogens, in particular HIV, stands as a promising and exciting investigation topic in the field of vaginal drug delivery (more information is given in Section 5.6). The administration of antimicrobials by the vaginal route must take into account the preservation of probiotic lactic acid bacteria (LAB). Topical administration of protective microorganisms, especially Lactobacillus spp., has been proposed to restore the vaginal microbiota after insult and as an alternative or coadjuvant treatment for urogenital infections [83,84]. Clinical trials have shown vaginal probiotics formulations to be safe with high rates of acceptability [85] but data on efficacy of these formulations are still controversial, mostly related to limitations such as small samples and lack of product stability [86]. Ideally, drug dosage forms should be easy to use, allow self-administration, painless upon administration and use, comfortable, discreet, and removable if needed [2]. Also, they should not interfere with vaginal physiology and daily life, while allowing high drug bioavailability (either local or systemic) to be obtained with little variability. Two critical issues of dosage forms are their pH and osmolarity or, in particular, their influence on these properties after vaginal application. Hyperosmolar vaginal formulations have been associated with mucosal damage and local side effects [87,88]; in addition, formulations capable of lowering the normal vaginal pH may cause vaginal mucosal damage [89] while alkaline products may potentially lead to decreased levels of protective Lactobacilli [90]. Of course, achieving all such properties remains challenging but substantial efforts have been performed in order to optimize the wide variety of existing dosage forms while new ones have been proposed. The most traditionally used vaginal dosage forms comprise suppositories, tablets, capsules, gels, creams and liquids (solutions or lotions), and have been mainly used as vehicles for drugs such as anti-infective agents or contraceptives [27]. Conversely, over the last decades, other dosage forms such as rings and films have also gained popularity amongst pharmaceutical developers, clinicians and users, and are now the focus of intense study. These and other vaginal dosage forms are discussed in the following subsections. Solid systems commonly administered by the vaginal route include tablets, capsules and vaginal suppositories, presenting up to 2–3 g. Vaginal tablets offer those typical advantages of other tablets, such as portability, precise dosing, ease of storage, handling and administration, possibility of large scale production, and low cost [91]. These tablets are usually designed to allow the rapid release and the solubility of the active substances to be promoted (e.g. by using effervescent formulations), while offering the potential for improved stability. Although very similar to oral tablets, these systems present some particularities, such as being round or oval-shaped and devoid of sharp edges, in order to avoid mucosal damage [27]. Vaginal tablets have been typically used for delivering antimicrobial drugs [92,93] and hormones [94]. Also, a number of anti-HIV compounds (e.g. cellulose sulfate, dapivirine, tenofovir and UC-781) may find in these systems suitable vehicles for developing microbicide products [91]. Another interesting use for tablets is the delivery of different species of probiotics in order to allow the normal vaginal microbiota to be restored [95,96]. This type of tablets usually requires specific proceedings to ensure the viability of bacteria and stability of the final product [97]. Vaginal suppositories, also referred to as ovules or pessaries, are ovoid-shaped solid dosage forms specifically designed for vaginal administration. Unlike tablets, these systems are typically prepared by melting and moulding although, in some particular cases, special compression processes can be used [27]. The major advantages of vaginal suppositories are their reduced cost and ease of production. However, vaginal suppositories present some usual inconveniences, such as messiness upon application, poor retention in the vagina, and reduced shelf-life stability. Several excipients or mixtures, also referred to as bases, have been used in the formulation of vaginal suppositories, namely gelatine and glycerine, cocoa butter, semi-synthetic glycerides, and poly(ethylene glycol)s (PEGs) [27]. Upon vaginal administration, vaginal suppositories dissolve in vaginal fluids or melt at body temperature, typically resulting in rapid release of drugs. Sustained-release formulations have been proposed in order to circumvent this last problem [98,99]. Semi-solid dosage forms are very popular and frequently used for vaginal delivery of drugs. These systems are easy to use, have good acceptability and provide relatively inexpensive options for drug therapy. However, leakage, messiness and discomfort during application are recognized as important limitations. In the particular case of leakage, night administration is usually recommended. Vaginal creams present the possibility of easily dissolving both hydrophobic and hydrophilic drugs even in the same formulation. Their main application has been in the delivery of hormones and antimicrobials [100,101]. In the case of vaginal gels, the main advocated advantages are their ability to provide high bioavailability, biocompatibility and spreadability [102]. Also, the use of polymeric gelling agents usually provides mucoadhesive properties to these dosage forms; this can increase vaginal retention and reduce leakage. Mostly hydrophilic in nature, vaginal gels are generally easy to use, inexpensive and highly accepted by women, being usually associated with a refreshing effect due to its high water content. Aqueous and nonaqueous vaginal gels have been the main dosage forms used for developing microbicides [103,104]. Liquid preparations, mostly solutions, may also be useful for vaginal drug delivery. Commonly, commercially available or simple home-made solutions are used for cleansing purposes only, being administered as douches. However, current knowledge generally discourages douching as this practice may have deleterious effects on the normal vaginal milieu [105]. Vaginal foams (aerosols) present some distinctive advantages for vaginal drug administration including high spreadability and ease of application, which typically results in enhanced drug delivery efficiency and user comfort [106,107]. Also, most foam bases are nonirritating to the vaginal mucosa. However, there are some limitations, such as the need for special containers, low mucoadhesiveness, and quick swelling and breaking of foam upon application. Thus, vaginal foams have had only limited success so far. Vaginal tampons have been adapted to deliver therapeutic agents. Similar to those used during menses, medicated tampons are impregnated with the drug(s) of interest, being either produced as such or immersed in a solution of drug(s) immediately before use [108]. Also, multifunctional tampons presenting different compartments for drug(s) delivery and absorption of menstrual fluids have been proposed [109]. One such tampon (RepHresh® Brilliant™ pH tampons, Lil’ Drug Store Products, Inc.) is currently marketed in the USA. Vaginal rings are doughnut-shaped polymeric dosage forms that were initially developed in the 1970s for the delivery of hormones with contraceptive purposes [110]. At present, one combination vaginal ring (etonogestrel/ethinyloestradiol) is commercially available worldwide for contraception (Nuvaring®, Organon) while two others containing oestradiol (base or acetate) are used for hormonal replacement therapy in post-menopausal women (Estring®, Pfizer and Femring®, Warner Chilcott). Currently, vaginal rings are being developed for the delivery of antiretroviral drugs to be used as microbicides and nonhormonal contraceptives [111,112]. Vaginal rings have adequate flexibility and dimensions in order to allow comfortable insertion and retention in the vagina (Figure 5.2a). Different cross-sectional configurations have been proposed presenting diverse properties, particularly related with their ability to provide controlled drug release, and requiring different manufacture processing (Figure 5.2b) [27]. Also, nonmedicated rings have been proposed as simple holders for medicated rods or tablets, which allows for multiple use of the same ring by inserting only rods/tablets in specific spots (Figure 5.2b). Advantages such as cost savings, multiple drug delivery or facilitated drug processing (e.g. avoidance of high temperature exposure) have been claimed for these last [111]. Additionally, multisegmented rings, that is presenting different sections along the circumference of the device, have been proposed has a versatile strategy to formulate different drugs in one ring [113]. Figure 5.2 The vaginal ring. (a) NuvaRing® vaginal ring made from poly(ethylene-vinyl acetate) (reservoir design ring with 5.4 cm diameter and 0.4 cm cross-section); (b) Different cross-section designs of vaginal rings: (1) matrix design, (2) reservoir design, (3) sandwich design, and (4) rod/tablet-insert design. As one of the main advantages of vaginal rings is their ability to release one or more drugs in a sustained fashion for long periods (up to one year), they obviate problems such as compliance or daily fluctuation of drug levels, as in the case of oral contraceptives [114]. Also, rings retain their shape throughout the time of application and can be removed if needed (e.g. in case of adverse effects, during menses, for gynaecological examination). Common polymers used in the production of vaginal rings include silicones [poly(dimethylsiloxane), poly(dimethylsiloxane-vinylmethylsiloxane)] and poly(ethylene-vinyl acetate) (EVA) but other materials such as poly(styrene-butadiene-styrene) [115], polyurethanes [116], Acacia gum and methacrylates [117] have also been proposed. Industrial manufacturing of rings comprising silicones or EVA is performed by hot-melt extrusion (or co-extrusion when the ring comprises different layers), which requires that all materials, including active drugs, be stable at high temperatures, at least during the time required for processing. Vaginal films comprise solid and flexible thin sheets in which one or more drugs are dispersed or dissolved in the matrix, usually polymeric in nature (Figure 5.3). Matrix-forming materials used in film manufacturing include poly(vinyl alcohol) (PVA), cellulose derivatives, polyacrylates and chitosan [118–120]. The inclusion of plasticizers (e.g. PEG, glycerine) is usually required in order to confer flexibility to films. Films are usually square with an approximate side of 5–10 cm but other shapes and sizes may also be desirable. The most frequent method of production is by casting. Typically, films are to be folded in four parts and inserted into the vagina with the aid of two fingers. Once in place, films are hydrated by local moisture, and disperse/dissolve and adhere to the mucosa. Their large surface allows for immediate extensive coverage of the vaginal canal. Vaginal films have been traditionally used for delivering spermicide compounds (e.g. the 28% nonoxynol-9-containing VCF® Vaginal Contraceptive Film, Apothecus) [121] or simply as deodorants or lubricants (VCF® Dissolving Feminine Deodorant Film and VCF® Dissolving Vaginal Lubricant Film, Apothecus) but have recently attracted the interest of scientists involved in the development of anti-HIV microbicides [122,123]. Figure 5.3 A vaginal film (VCF® Vaginal Contraceptive Film) folded in half. Vaginal sponges are also interesting dosage forms that can be be impregnated with one or more drugs in order to be inserted in the vagina for a limited amount of time, usually up to 24 h. Their application is essentially as nonhormonal contraceptives [124] but other uses have also been proposed (e.g. in the treatment of bacterial vaginosis [125]). One commercially available example is the Today® sponge (Almatica Pharma, Inc.), which comprises a soft polyurethane sponge (7.6 × 3.8 cm) impregnated with one gram of the spermicide nonoxynol-9. Other types of vaginal barrier devices, namely diaphragms or cervical caps, have also been modified in order to deliver spermicides or microbicides [126–128]. Drugs may be impregnated in the matrix of the device, or incorporated into a specific reservoir or simply placed on top of the device prior to insertion. Other proposed dosage forms include vaginal patches similar to those used on the skin but with adequate size for treating small mucosal areas, namely localized cervical neoplastic lesions [129]. Finally, vaginal inserts containing dinoprostone have been long used in the clinics for inducing labor (Propess®, Ferring, and Cervidil®, Forest Laboratories) [130]. The full insert comprises a thin rectangular PEG matrix containing the drug (10 mg), which is involved in a knitted polyester net ending in a long tail in order to allow retrieval of the system. Once applied in the vagina, the polymeric matrix allows for controlled release of dinoprostone at an approximate rate of 0.3 mg/h over 12 h [131]. The assessment of which excipients are suitable to be included in the formulation of vaginal drug dosage forms or delivery systems is of paramount importance. It is well known that excipients have the ability to impact on the performance of pharmaceutical drug products and interact differently with mucosal tissues. In the particular case of vaginal drug delivery, concerns about the safety of used excipients have been raised over the last decade and addressed in recent years. A list of materials proposed for or already used in commercially available vaginal products, alongside their typical concentration ranges, has been compiled by Garg et al. [132]. However, further studies have shown that several commonly used excipients may present deleterious effects to the vaginal mucosa [133,134] and thus their use (or typical concentration limits) in vaginal formulations should be reconsidered. Safety issues seem to be of particular importance when chronic application is intended, such as in the case of contraceptives and microbicides. In the latter, deleterious effects to the vaginal epithelial have been implicated with higher transmission of HIV or other pathogens in both nonclinical and clinical trials [135–137], reinforcing the need for safety assessment of both vaginal formulations and their individual components [138–140]. Some efforts have been made in order to develop standard formulations which are considered safe as evaluated in clinical trials. One such example is the ‘Universal Placebo’ gel; this gel, comprising hydroxyethylcellulose (2.7 g), sodium chloride (0.85 g), sorbic acid (0.1 g), caramel colour (0–0.02 g), sodium hydroxide (e.q. pH 4.4), and water (e.q. 100 g) has been shown safe in clinical trials [141,142] and is considered a golden standard in microbicide gels testing. The relatively low accessibility to the vaginal canal may justify the use of applicators in order to correctly administer a dosage form. In the case of foams, semi-solids and liquids this is mandatory, namely when deep insertion is required; as for solid systems, their use may be optional or even unnecessary. Special applicator tips can also be attached to a tube containing a liquid, semi-solid or foam in order to be inserted in the vagina. In all cases, applicators (or applicator tips) may present different designs according to specific needs or women’s preferences. In general these medical devices should be made of nontoxic materials (e.g. polypropylene, polyethylene), allow comfortable administration to the proposed site within the vagina (e.g. placement near the cervix, allow optimal distribution throughout the mucosa), and avoid any damage to the mucosa [27]. Also, affordability may be an important question particularly when considering products intended to be used in low-resource settings. For instance, re-usable paper applicators have been recently proposed as low-price alternatives to pre-filled plastic ones for the administration of tenofovir microbicide gels [143]. Different interesting new approaches have been developed and investigated over the last few years in order to advance vaginal drug delivery. New drug delivery systems have been proposed in order to respond to the challenges posed by the vaginal anatomy and physiology. One interesting strategy has been the development of stimuli-sensitive systems, which can modify their behaviour (e.g. drug release rate, physical state) once environmental conditions change. A straightforward approach is to take advantage of the increase in temperature upon vaginal administration. Different thermosensitive formulations based on poloxamer have been proposed for vaginal drug delivery [144–146]. These systems are liquid at room temperature but become gels at near body temperature. This allows for easy administration and intravaginal distribution typical of a liquid but with enhanced retention due to gelation. Furthermore, thermosensitive systems usually comprise mucoadhesive polymers that strengthen their ability to reside intravaginally. Modified drug release is also typically observed for this type of systems. Alongside temperature, systems that can respond to changes in vaginal pH can be an interesting means to modulate drug release. For instance, Gupta et al. developed a thermo- and pH-sensitive system based on a terpolymer of N-isopropyl acrylamide, acrylic acid, and butyl methacrylate that allows adequate administration, distribution and retention due to its thermosensitive nature (as described above) [147]. Moreover, in vitro experiments showed that an increase in pH, such as observed upon vaginal ejaculation, was able to dissolve the gel, thus triggering burst release of different model compounds (acid orange dye and FITC-dextran). This study provides evidence that this type of environment-sensitive system might be an interesting approach for developing smart microbicide products for vaginal protection from viral transmission. Other researchers have recently applied the same principle of pH rise upon ejaculation in order to develop pH-sensitive polymeric nanocarriers based on methacrylic acid/methyl methacrylate copolymers and containing microbicide drugs [148,149]. The use of microparticles has been proposed for drugs intended to be administered by the vaginal route. The main advocated advantages for these systems are the possibility of obtaining control over drug release, provide enhanced retention by using mucoadhesive polymers and protect the drug payload [150–152]. Typical processing of developed microparticles into tablets or gels has been proposed in order to allow administration. Further, larger starch-based particles (pellets) have been proposed by Vervaet and collaborators [153,154], which showed they provided an interesting approach for complete distribution and prolonged retention of drugs intravaginally as assessed in both sheep and humans. Nanotechnology-based solutions for improving vaginal drug delivery have been increasingly proposed over the last years, particularly to be used in microbicide development [155,156]. Developed systems include liposomes and proliposomes [157–159], niosomes [160], polymeric nanoparticles [161–163], and solid lipid nanoparticles [164]. Claimed advantages of nanosystems include the ability to allow protection of sensitive drug payloads (e.g. peptides, proteins and genetic material), improving solubility, obtaining controlled/sustained drug release, and achieving mucosal penetration and targeting special cell types (e.g. HIV-target cells) [155]. So far, a few animal in vivo studies provide evidence that adequately engineered polymeric nanoparticles, particularly related to their interaction with mucus (further information is given in Section 5.5), may provide interesting platforms for enhanced drug delivery. For example, Woodrow et al. [162] demonstrated the ability of poly(lactide-co-glycolide)-based nanoparticles to deliver siRNA against the MAPK1 gene, provide deep tissue penetration and cause efficient and sustained gene silencing. Further, two recent studies demonstrated the higher efficacy of acyclovir [165] and siRNA against nectin-1 [166] in protecting mice from HSV-2 vaginal challenge when associated to different polymeric nanoparticles over nonformulated compounds. Alongside the use of probiotics for prevention and therapeutic purposes, the vaginal microbiota provide interesting opportunities to vaginal drug delivery. Indeed, engineered commensal bacteria may provide an interesting live ‘platform’ for the vaginal delivery of active substances. In particular, bacteria producing antiviral compounds have been tested in order to prevent the sexual transmission of HIV [167–169] and for vaccine development [170]. This strategy seems to be particularly interesting for delivery of peptides and proteins, which usually require special formulation in order to assure activity and stability. Also, colonization and continuous production of the compound(s) of interest by bacteria allows for ‘sustained release’ and, ideally, a single administration would provide weeks to months of protection [171]. For example, a recent study in macaques provided evidence that Lactobacilli jensenii modified in order to produce cyanovirin-N, an antiviral protein, was able to reduce substantially the transmission of chimaeric simian/HIV upon repeated vaginal challenges [172]. However, and even if this strategy seems quite attractive, issues such as the use of genetically-modified bacteria, and the complex and variable interaction between host and vaginal microbiota [173], raise some reservations towards the future applicability of this biotechnological approach. One common challenging problem in drug formulation is the low solubility of most active substances. In the particular case of the vaginal route, the limited amount of fluid present in the vagina and relatively low amount/size of a product that can be administered intravaginally may enhance solubility issues. In order to overcome such problems, formulations comprising the use of cyclodextrins [145], microparticles [174] or multiple emulsions [175] have been successfully used. However, a great deal of work is still required in the field. The topic of mucoadhesion is highly relevant when considering vaginal drug delivery. Although the underlying mechanisms of mucoadhesion at the vagina are common to those applicable to other mucosal routes [176], several features are particular to this mucosal environment and deserve special attention. The natural mild slope of the vaginal canal, in association with its self-cleansing mechanisms (e.g. fluid secretion) and possible mechanical stress (e.g. during penile penetration), contributes to the expulsion of products placed in the vagina. Another important issue impacting the mucoadhesion phenomenon is related to the variability of the vaginal fluid with the menstrual cycle, vaginal practices (e.g. douching) or sexual intercourse. Vaginal fluid can undergo either quantitative or qualitative changes, namely in pH, mucin content and rheology. These factors influence the interaction of mucoadhesives with mucin, namely by changing the conformation and properties of the network formed by mucin within the vaginal fluid [177]. Apart from those forms relying on specific tensions exerted against the mucosal wall in order to be retained intravaginally (e.g. rings, diaphragms, sponges), vaginal dosage forms require additional mechanisms in order to increase retention after application. Mucoadhesive dosage forms or delivery systems can contribute to prolonged in situ
Vaginal Mucosa and Drug Delivery
5.1 Introduction
5.2 Drug Delivery and the Human Vagina
5.2.1 Anatomical and Physiological Considerations
5.2.2 Present and New Therapeutic Uses
5.3 Vaginal Drug Dosage Forms
5.3.1 General Properties
5.3.2 Specific Vaginal Drug Dosage Forms
5.3.3 Considerations About Excipients
5.3.4 Applicators
5.4 Novel Strategies for Enhanced Vaginal Drug Delivery
5.5 Mucoadhesion and the Vaginal Environment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


