http://evolve.elsevier.com/McCuistion/pharmacology/
Over the years, immunizations have prevented global epidemics, such as smallpox in 1977 and the eradication of polio in the United States in 1979. The U.S. National Immunization Survey (NIS; www.cdc.gov/vaccines) tracks vaccination coverage of children 19 to 35 months and teens 13 to 17 years. In 2014, vaccine coverage was high (at 90%) for 19- to 35-month-old children who received the recommended doses of diphtheria, tetanus, acellular pertussis (DTaP; for children <7 years old); Haemophilus influenzae type B (Hib); measles, mumps, and rubella (MMR); polio; hepatitis B (HepB); pneumococcal conjugate vaccine (PCV); varicella; rotavirus (RV); and hepatitis A (HepA). The survey also estimated that 0.8% of children below the federal poverty level had lower coverage for all vaccinations compared with children at or above the poverty level.
The 2014 national coverage also indicated that the Healthy People 2020 target goal of 90% was met for children aged 19 to 35 months who received the recommended doses of DTaP, polio, MMR, Hib, HepB, PCV, and varicella vaccines.
The NIS estimated that from 2013 to 2014, vaccination coverage among teens aged 13 to 17 years increased from 84.7% to 87.6% for tetanus, diphtheria, and acellular pertussis (Tdap; for individuals >11 years old), and from 76.6% to 79.3% for meningococcus 4-valent conjugate (MenACWY). Although human papillomavirus (HPV) vaccination increased for females (from 56.7% to 60%) and males (from 33.6% to 41.7%), vaccination coverage remained low. These results indicate an increase in all vaccinations for teens, with a need to improve HPV coverage.
Immunity
Active Immunity
The body can obtain immunity in different ways. Active immunity occurs when the body’s immune response is stimulated by an antigen or when a pathogen enters the body. The body recognizes this pathogen as a foreign substance and produces antibodies, also called immunoglobulins, which defend the body against pathogens. The immune response is slow, taking several days or weeks to develop immunity. Yet the immunity is often long lasting. During this process, the immune system retains memory of the pathogen then produces antibodies to defend against the disease. Natural acquired active immunity occurs from exposure to a pathogen or disease. Active acquired artificial immunity occurs when a weakened antigen or immunoglobulin (Ig) is injected into an individual as a vaccination, which then stimulates an immune response.
Passive Immunity
When an individual is unable to make antibodies and memory cells, antibodies are given from another source to provide passive immunity. These antibodies may be produced using recombinant deoxyribonucleic acid (DNA) technology or pooled antigens from several human or animal sources that have been exposed to disease-causing pathogens.
Passive immunity can be natural, in which case the body produces its own antibodies, or it can be acquired—that is, the body receives antibodies from an outside source. Either way the immunity is immediate and short lived, lasting no more than several weeks to a few months; the recipient does not induce his or her own immune response. One example of natural immunity passively acquired is in infants, who are unable to protect against disease because of immature immune systems but instead require antibodies from an outside source, such as the mother’s placenta and breast milk. Another example is receiving an Ig to provide antibodies against a specific disease. Passive acquired immunity is essential when (1) time does not permit active vaccination alone, (2) the exposed individual is at high risk for complications of the disease, or (3) the individual suffers from an immune system deficiency that renders that person unable to produce an effective immune response.
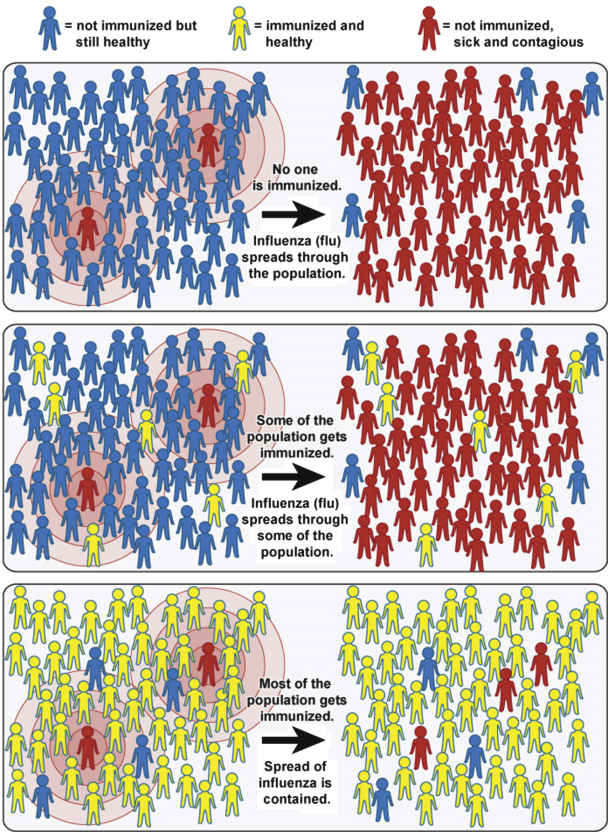
Community Immunity
Community immunity, also known as herd immunity, occurs when most of the community is immunized against contagious diseases, allowing protection of those not immunized. In contrast, when most of the community is not immunized, there is an increased risk for the spread of contagious disease within the community (Fig. 31.1). For more information, see www.vaccines.gov/basics/protection/.
Vaccines
Vaccination involves the administration of a small amount of antigen, which although capable of stimulating an immune response does not typically produce the disease. Different types of vaccines are available, but the type used in vaccinations depends on the person’s immune response. The antigen in vaccines may be produced in several ways. Traditional vaccines contain the whole or components of an inactivated (killed) microorganism. Other vaccines are attenuated viruses composed of live, attenuated (weakened) microorganisms. Persons who are immunocompromised because of illness or who take medication that causes immunosuppression should avoid live vaccines. Toxoids are inactivated toxins that can no longer produce harmful diseases but do stimulate formation of antitoxins, which produces active immunity (e.g., tetanus toxoid).
Newer vaccines, called conjugate vaccines, require a protein or toxoid from an unrelated organism to link to the outer coating of the disease-causing microorganism. This linkage creates a substance that can be recognized by the immature immune system of young infants. One example is H. influenzae type B.
Recombinant subunit vaccines involve the insertion of some of the genetic material (e.g., DNA) of a pathogen into another cell or organism, where the antigen is then produced in massive quantities. These antigens are then used as a vaccine in place of the whole pathogen. The HepB vaccine is an example of this type of vaccine.
An adjuvant—often an aluminum salt such as aluminum hydroxide, aluminum phosphate, or aluminum potassium sulfate—is a substance added to a vaccine to increase the body’s immune response to the vaccine. One value of adding adjuvant to a vaccine is to reduce the amount of antigen needed to produce a dose of vaccine. Vaccines with adjuvants are rigorously tested for safety before being licensed. In the United States, vaccines against measles, mumps, rubella, chickenpox, rotavirus, polio, and seasonal influenza do not contain adjuvants.
Regardless of the composition of the vaccine, each vaccine is designed to stimulate an immune response against a specific pathogen. Some vaccines require booster doses to maintain sufficient immunity. The immune system’s memory responds rapidly to prevent disease when exposed to a booster, which provides an active albeit artificially acquired immunity.
Vaccine-Preventable Diseases
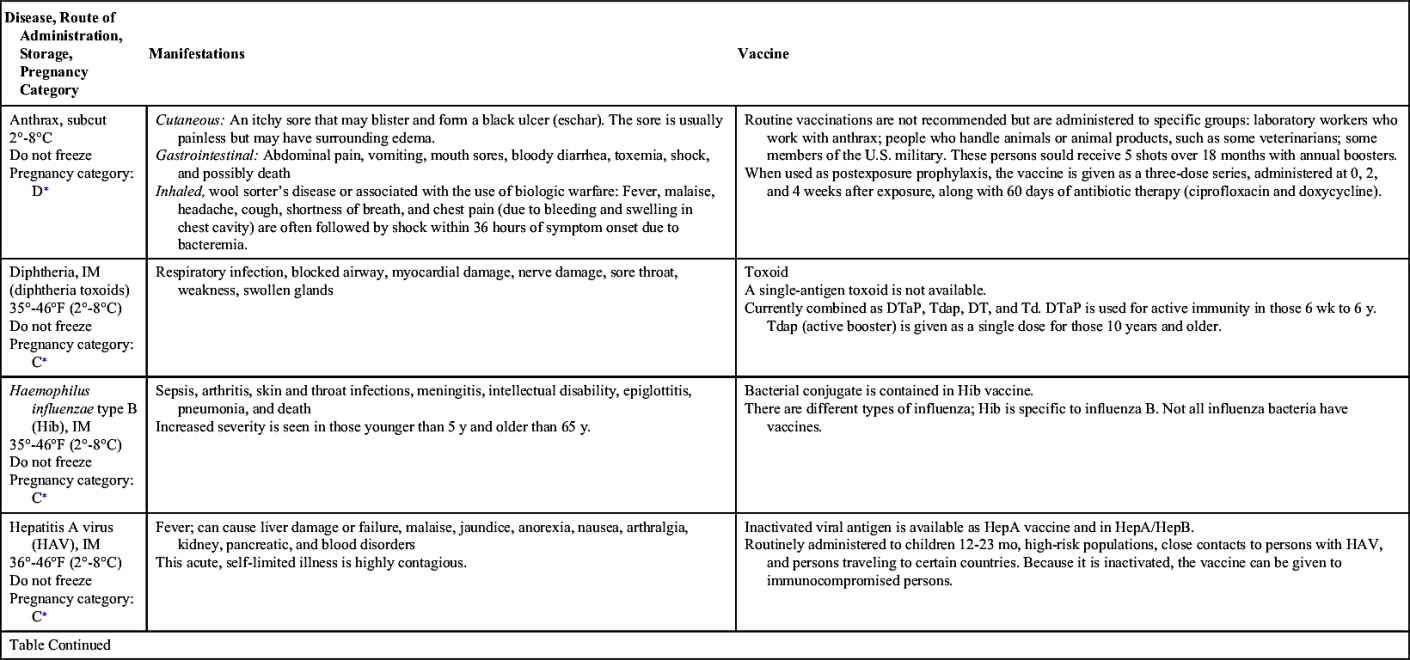
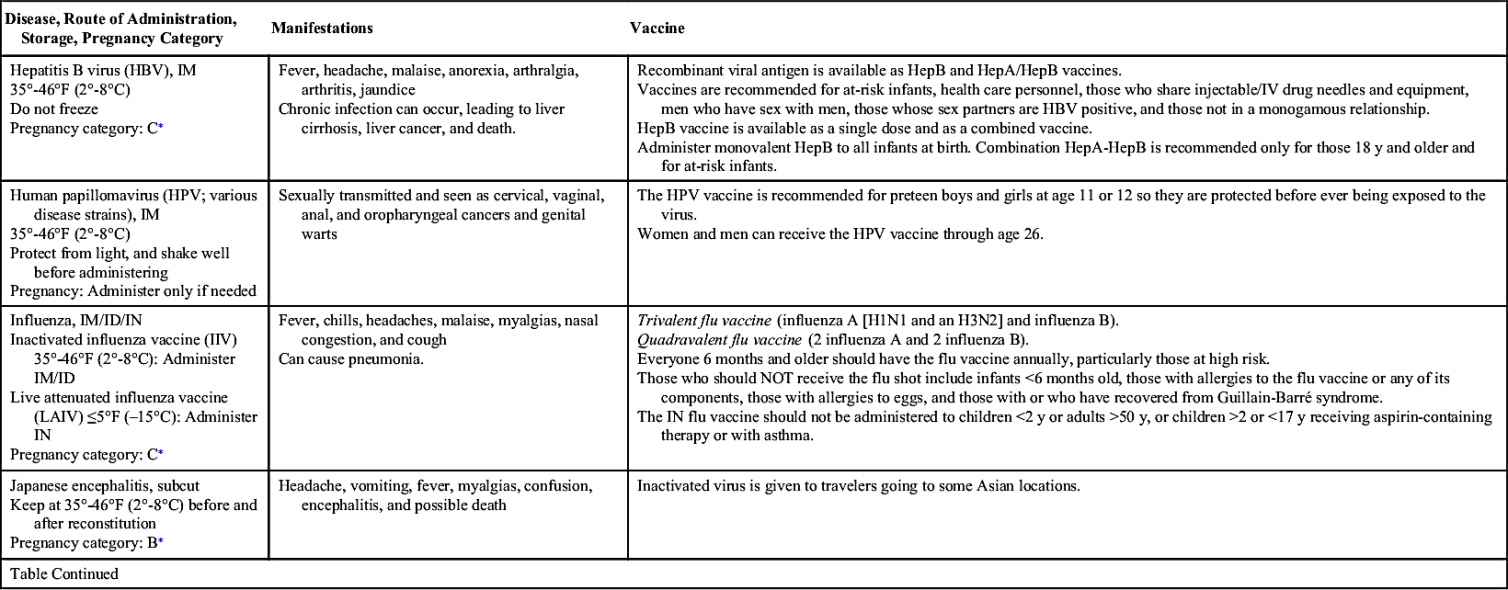
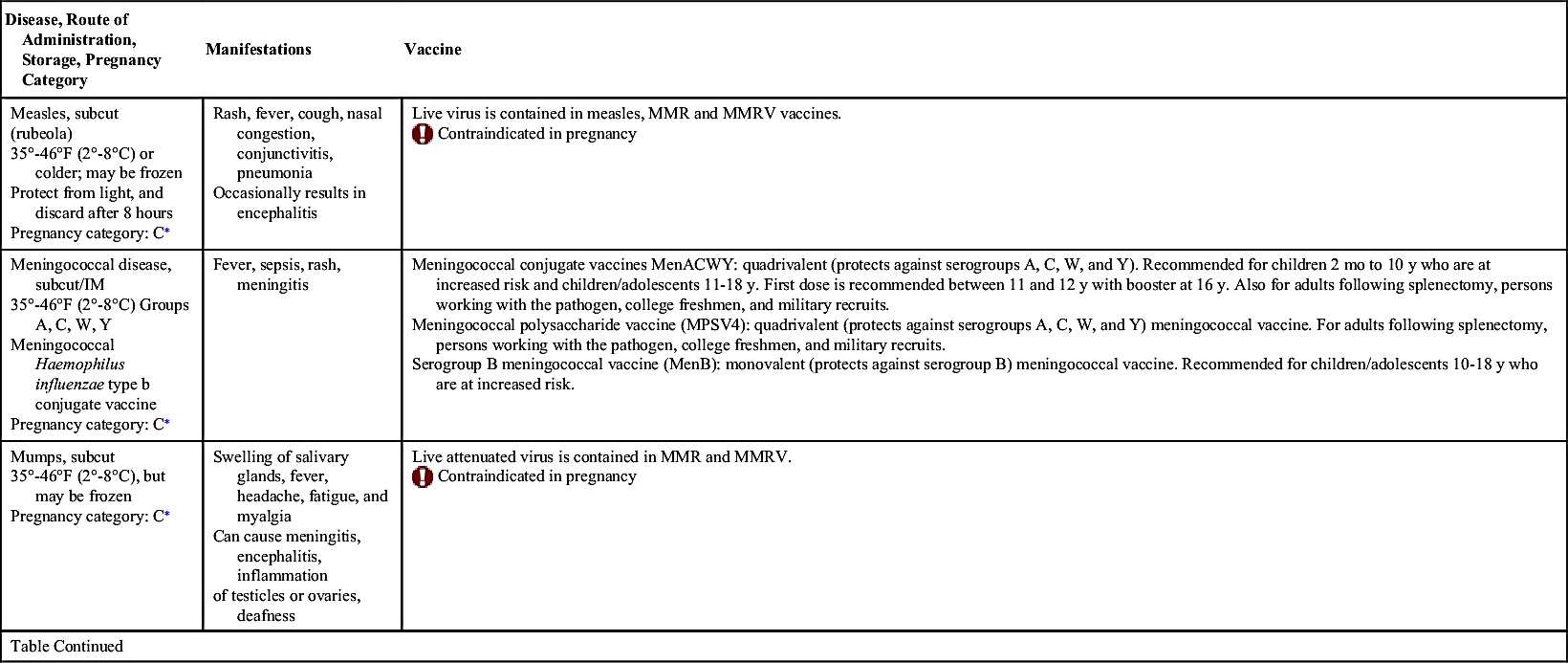
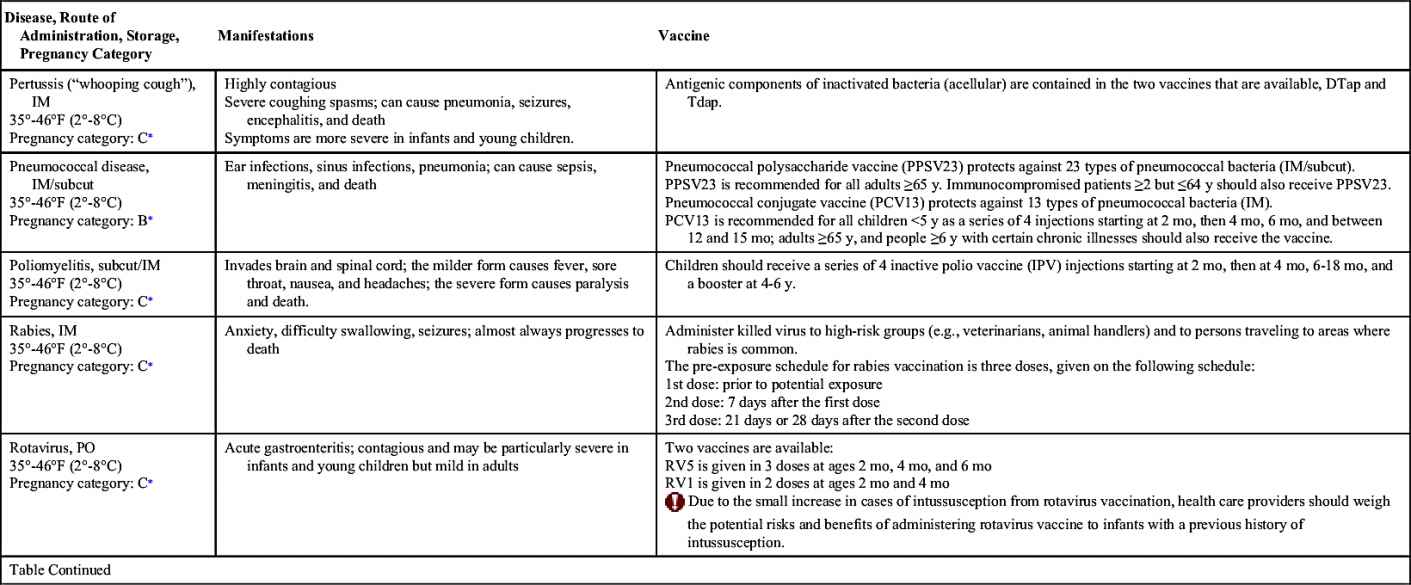
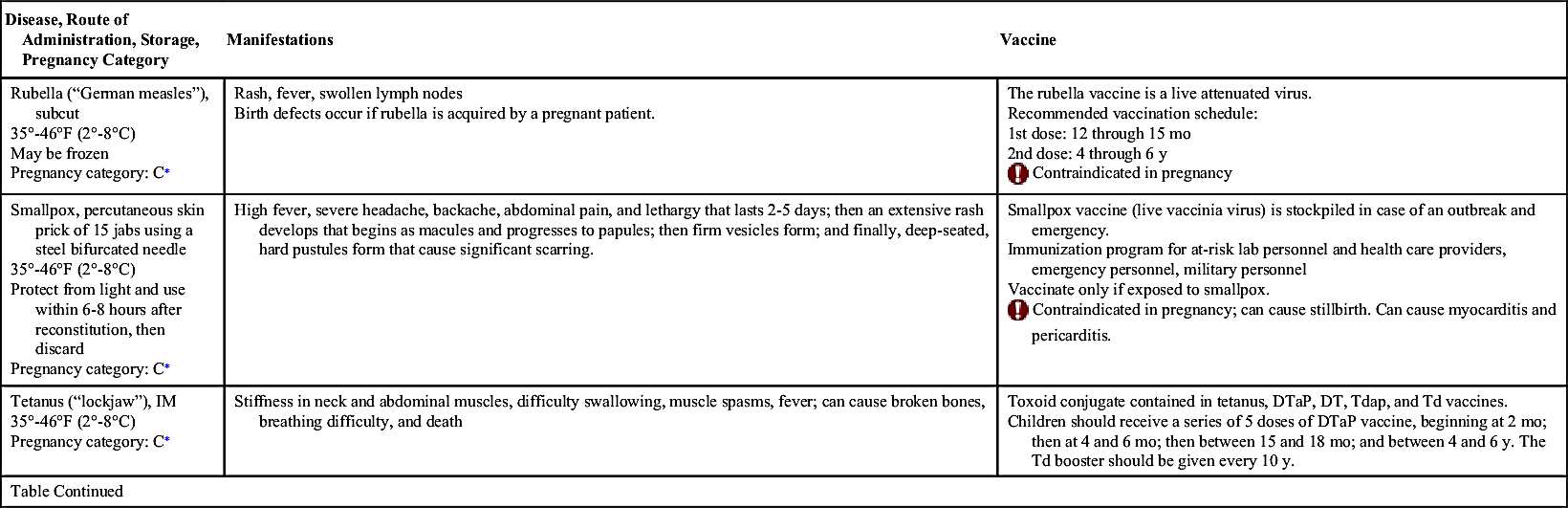
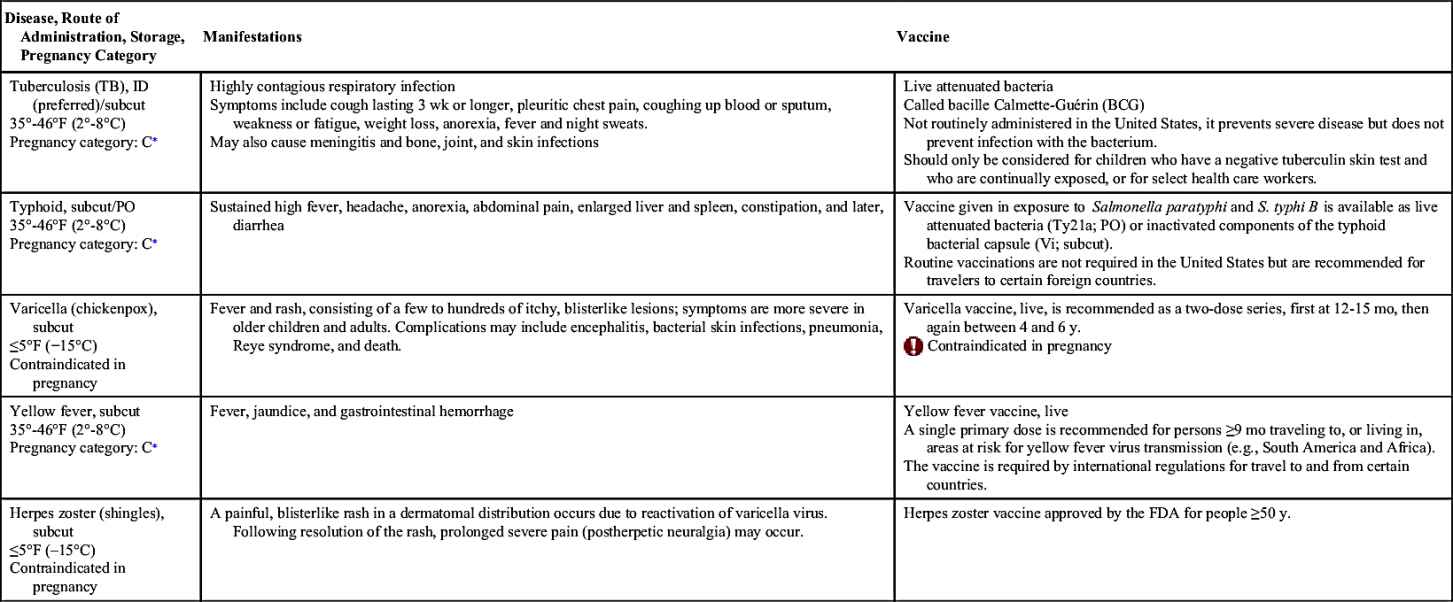
Vaccinations maintain health by preventing disease. In the United States, more than 20 infectious diseases may be prevented by active vaccination. Many of these vaccines are routinely administered to healthy children and adults. Others are reserved for special populations such as health care providers, military personnel, immigrants, adults with special health needs, the chronically ill, and travelers to certain foreign countries. Table 31.1 provides an overview of the disease manifestations and vaccine information, including route of administration and storage temperature. Vaccine-preventable diseases for children, adolescents, and adults include—but are not limited to—anthrax, diphtheria, H. influenzae type B, hepatitis A and B, human papillomavirus, influenza (flu), Japanese encephalitis, measles, meningococcal disease, mumps, pertussis, pneumococcal disease, poliomyelitis, rabies, rotavirus, rubella, smallpox, tetanus, tuberculosis (TB), typhoid, varicella, yellow fever, and herpes zoster.
Vaccination Recommendations
Immunization schedules are approved by the Advisory Committee on Immunizations (ACIP). Each schedule identifies recommended vaccinations, ages to vaccinate, dosage, and route for children, adolescents, and adults. For the most current information on childhood immunizations, consult the Centers for Disease Control and Prevention (CDC) website at www.cdc.gov/vaccines. A vaccine information statement (VIS) is also produced by the CDC for each vaccine, and provides information on the (1) route of administration, (2) schedule for routine vaccine administration, (3) minimum dosing intervals, (4) contraindications, and (5) standing orders for administering vaccines.
The childhood immunization schedule from birth to 6 years recommends HepB, RV, DTaP, Hib, PCV, inactivated polio virus (IPV), MMR, varicella, and HepA. Before immunizations are administered, screen for medical conditions that put the child at risk, use of prescription and over-the-counter (OTC) drugs to include herbal preparations, and any food or drug allergies (Complementary and Alternative Therapies 31.1).
The adolescent immunization schedule from ages 7 to 18 years recommends Tdap, influenza, HPV, and meningococcal vaccinations. Adolescents may also need to catch up on any vaccines missed, such as MMR, HepA, HepB, IPV, and varicella (chickenpox).
A catch-up immunization schedule is available for those up to 18 years of age who fall behind or start late with immunizations. Childhood and adolescent immunization schedules are also available as a combined schedule (birth to 18 years).
Adult (19 years and older) vaccination rates remain low, which indicates a need to improve. One way is to increase awareness that routine vaccines for adults are important for well-being, to provide information on how vaccines protect from diseases, and to assess immunization status during clinical visits. Adult vaccines are based on factors such as age and health status and include Tdap, tetanus-diphtheria (Td) booster, influenza, pneumococcal polysaccharide vaccine (PPSV23), HPV, MMR, varicella vaccine (chickenpox), and zoster vaccine (shingles). In certain situations, adults may also be immunized with certain additional vaccines, including HepA, HepB, smallpox (2016 update: routine for lab personnel handling vaccine cultures), and meningococcal vaccine. Current recommendations for adult immunization can be found at www.cdc.gov/vaccines/schedules/.
Immunization Before International Travel
International travel warrants the updating of routine vaccines based on age and immunization history, and travelers to some areas should consider additional vaccines. For example, the CDC currently recommends U.S. travelers 6 months and older to have an updated MMR vaccine based on the travel destination.
Travelers may need to consider vaccination against typhoid and yellow fever. Typhoid fever is caused by a bacterium, Salmonella typhi, which is generally spread via contaminated food and water (see Table 31.1). Risk of contracting this infection is greatest for travelers to India, Pakistan, Mexico, Bangladesh, the Philippines, and Haiti. Even stays of less than 2 weeks pose significant risk. Two forms of typhoid vaccine are available for use in the United States. Typhoid vaccine live oral contains the Ty21a strain of S. typhi, and is a live attenuated vaccine, which can be administered to persons 6 years of age and older and consists of four capsules, one taken every 48 hours, with the series completed 1 week before potential exposure. A booster dose consisting of the same four-capsule regimen is recommended every 5 years. The other vaccine, parenteral typhoid Vi polysaccharide vaccine, is an inactivated vaccine that contains purified cell surface polysaccharide antigens extracted from S. typhi of the Ty2 strain, and may be administered to travelers 2 years of age and older. It is administered at least 2 weeks before expected exposure as a single, intramuscular (IM) injection. A booster dose is recommended every 2 years for those who remain at risk.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree