http://evolve.elsevier.com/McCuistion/pharmacology/
The drugs described in this chapter are first-line agents used to treat various medical emergencies. Nurses must have knowledge of the indications and actions of these drugs because medical and surgical emergencies can occur in virtually any area of nursing practice. Learning key nursing implications before a crisis situation enables the nurse to function at the highest possible level when the patient requires life-saving intervention.
At the end of the discussion of each group of emergency drugs is a summary prototype drug chart that contains dosages and indications. Common adult dosages are listed in the drug charts; pediatric dosages may vary widely depending on the child’s age and weight. For the purpose of drug dosing, advanced life support (ALS) guidelines consider adults to be older than 8 years and children to be 8 years or younger; infants are younger than 1 year. The drug charts list only the most common indications and dosages for the emergency drugs discussed; they do not describe all possible uses and dosing regimens for each drug.
Oxygen as an Emergency Drug
Oxygen can be classified as a drug because it can have both beneficial and adverse effects on the body based on the amount and manner in which it is administered. Oxygen is essential to life—without it, brain death begins within 6 minutes. Inadequate oxygenation produces hypoxemia, inadequate oxygen in the blood, and leads to significant physiologic sequelae to all body systems; therefore oxygen is a first-line drug for all emergency situations in which hypoxemia poses a physiologic threat. Depending on the circumstances, adequate oxygenation may be all that is necessary to effectively treat physiologic disturbances that arise from hypoxemia, such as chest pain, bradycardia, and cardiac dysrhythmias. Oxygen also acts as a potent pulmonary vasodilator and may be beneficial for patients in heart failure.
Before the other pharmacologic drugs discussed in this chapter are administered, ensure that the patient’s airway and breathing are addressed to promote normoxemia, oxygen saturation between 94% and 99%. Giving a drug to treat a disorder brought on by hypoxemia without effectively correcting the cause of the hypoxemia is ineffective and ultimately does not produce the desired outcome. Pulse oximetry, which provides a digital display of oxygen saturation, is an essential monitoring tool that should be used in emergency situations to assess the adequacy of oxygenation and guide further interventions. Ideally oxygen saturation should be kept in a range between 94% and 99%. It is important to recognize, however, that certain pathophysiologic states can make pulse oximetry readings inaccurate. These conditions include vasoconstriction, severe anemia, hypothermia, carbon monoxide poisoning, and shock.
The ambient room air contains approximately 21% oxygen. When patients breathe room air, the oxygen they inspire constitutes 21% of the total volume of gas they take in with each breath. This measure is termed the fraction of inspired oxygen (FiO2). Patients with hypoxemia due to severe physiologic stress from shock states, major traumatic injury, acute myocardial infarction (AMI) with hemodynamic instability, and cardiac arrest initially require supplemental oxygen in high concentrations (i.e., an FiO2 close to 100%). The initial emergency oxygen delivery devices of choice for these conditions include a nonrebreather mask with an oxygen reservoir (oxygen flow rate set at 10 to 15 L/min) for spontaneously breathing patients and a bag-valve-mask device attached to an oxygen source at a flow rate of 15 L/min for patients who require assisted ventilation until definitive airway management and a mechanical ventilator are available.
Although caution must be exercised for patients with chronic obstructive pulmonary disease (COPD), who may lose their hypoxic respiratory drive when given oxygen in high concentration, oxygen should never be denied to a patient who needs it. In the case of COPD, the nurse should be prepared to ventilate the patient manually with a bag-valve-mask if respiratory depression or arrest occurs. Some patients in respiratory distress as a result of obstructive pulmonary disease or acute cardiogenic pulmonary edema may also benefit from oxygen therapy delivered through noninvasive mask ventilation via a continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) circuit; these devices deliver oxygen via a positive-pressure mechanism.
Whatever the underlying injury or disease process, as the patient’s condition stabilizes, the oxygen concentration should be decreased to achieve an arterial oxygen saturation between 94% and 99%. An FiO2 above 50% for a prolonged period can lead to oxygen toxicity and other detrimental effects in both adults and children.
For situations that do not involve severe physiologic stress (e.g., angina, dysrhythmias, pulmonary disease), supplemental oxygen delivered by nasal cannula at 1 to 6 L/min or by simple face mask at 6 to 10 L/min may be considered. Young children may better tolerate a face tent with a high oxygen flow of 10 to 15 L than a face mask. Current evidence suggests that oxygen administration to hemodynamically stable patients without heart failure who are normoxemic may offer no therapeutic benefit and should be avoided.
Emergency Drugs for Cardiac Disorders
Drugs described in this section are indicated for cardiac emergencies such as angina, myocardial infarction ([MI] heart attack), disturbances of cardiac rate or rhythm, and cardiac arrest. In a resuscitation situation, the foundation of patient therapy is based upon proper oxygenation and ventilation, performance of optimal cardiopulmonary resuscitation (CPR), and application of electrical therapy (cardioversion and defibrillation) according to established treatment algorithms and standards. Pharmacologic drugs are used as adjuncts in synchrony with these efforts when indicated to enhance the likelihood of a successful outcome. These drugs often must be prepared and administered rapidly. A sound knowledge base and easy access to the drugs and necessary equipments are essential for the best patient response in a cardiac emergency. Usually in an emergency, detailed personal, medical, drug, and herbal histories are unavailable (Complementary and Alternative Therapies 55.1). Treatment is based on patient presentation.
Aspirin
The nonenteric form of aspirin is a first-line emergency drug used to decrease platelet aggregation in the management of acute coronary syndromes and MI. It is best administered immediately upon the onset of chest pain in doses of 162 to 325 mg orally. The patient should be asked to chew the tablet to speed absorption of the drug instead of swallowing the tablet whole. For patients experiencing nausea and vomiting, an aspirin rectal suppository in a dose of 300 mg can be given as an alternative. Aspirin is also indicated in the initial treatment algorithm for patients suffering from acute ischemic stroke who are not candidates for fibrinolytic therapy. Contraindications to aspirin administration include a true drug allergy, presence of cerebral hemorrhage on computed tomography (CT) scan, and recent gastrointestinal (GI) bleeding.
Nitroglycerin
Nitroglycerin dilates coronary arteries and improves blood flow to an ischemic myocardium. It is therefore the treatment of choice for angina pectoris (chest pain) and MI. Nitroglycerin is also considered a first-line drug in the management of patients with acute cardiogenic pulmonary edema due to its ability to decrease both preload and afterload. A focused prescription drug history is essential prior to administration, even in emergency situations, because nitroglycerin in combination with drugs for erectile dysfunction (i.e., sildenafil, vardenafil, tadalafil) causes profound hypotension that may be refractory to treatment when taken within a 24- to 48-hour period. This combination is contraindicated. Nitroglycerin is available in sublingual, translingual aerosol spray, oral, topical, and intravenous (IV) forms. Only the sublingual, translingual aerosol spray, and IV preparations are discussed.
Sublingual nitroglycerin (0.3 to 0.8 mg) and the translingual aerosol spray (0.4-mg metered dose) preparations are indicated for patients experiencing an acute anginal attack who have a systolic blood pressure above 90 mm Hg. The patient is taught to sit or lie down and moisten 1 sublingual nitroglycerin tablet with saliva and then place it under the tongue to allow it to dissolve slowly. If the chest pain is not relieved, sublingual nitroglycerin may be repeated at 3- to 5-minute intervals—as long as the patient’s systolic blood pressure remains above 90 mm Hg—until a total of 3 tablets have been taken. Patients prescribed the translingual aerosol preparation should be reminded that the spray should not be inhaled. Instead, it should be sprayed onto or under the tongue. The patient should be instructed not to swallow for approximately 10 seconds to allow absorption of the drug. As with sublingual nitroglycerin, up to 3 doses may be taken within 15 minutes. If pain persists despite 3 doses of the sublingual or aerosol forms, further interventions are necessary in an emergency or critical care setting. An ambulance should be called if the patient is outside the hospital, and blood pressure and heart rate must be monitored closely. Hypotension is a common adverse effect, especially the first time a patient takes nitroglycerin. Tachycardia, an abnormally high heart rate in adults (>100 beats/min), or uncommonly bradycardia, a slow heart rate, also may occur. Patients who take sublingual or translingual aerosol spray nitroglycerin while wearing a nitroglycerin patch may be at higher risk for hypotension. This situation warrants caution. However, individuals who take daily nitroglycerin can develop tolerance to the drug, which can provide some protection against hypotension. If hypotension persists or worsens, the nitroglycerin patch may need to be removed. To prevent arcing and the potential for skin burns, the nitroglycerin patch must be removed prior to cardioversion or defibrillation.
IV nitroglycerin is reserved for patients with unstable angina or AMI. A continuous infusion is usually initiated at a rate of 5 mcg/min and titrated by 5 mcg/min every 3 to 5 minutes based on chest pain and blood pressure response. Maximum rate of titration is 20 mcg/min every 3 to 5 minutes. Continuous blood pressure and heart monitoring are required because hypotension is a common adverse effect. Hypotension usually is treated by reducing or discontinuing the nitroglycerin infusion (see Chapter 37) and by placing the patient in a supine position with legs elevated if tolerated.
Morphine Sulfate
Morphine sulfate, a narcotic analgesic, is used to treat the chest pain associated with ST-segment elevation myocardial infarction (STEMI). It also is indicated for acute cardiogenic pulmonary edema. Morphine relieves pain, dilates venous vessels, and reduces the workload on the heart. The standard dosage of morphine sulfate is 1 to 4 mg IV over 1 to 5 minutes, repeated every 5 to 30 minutes until chest pain is relieved. Because respiratory depression and hypotension are common adverse effects, the drug must be administered slowly and must be carefully titrated to achieve the desired therapeutic effects. Close patient monitoring is essential. It is important to realize that although morphine can produce respiratory depression, this drug can relieve the dyspnea caused by pulmonary edema. In this situation, respiratory distress is not a contraindication to morphine administration. The narcotic antagonist naloxone may be ordered to reverse the action of morphine if adverse effects pose a significant risk to the patient. The dose is 0.4 to 2 mg every 2 minutes as indicated (see Chapter 25).
Atropine Sulfate
Atropine sulfate is the primary drug indicated for the treatment of hemodynamically significant bradycardia and some types of heart block (e.g., atrioventricular [AV] block at the nodal level). Atropine acts to increase heart rate by inhibiting the action of the vagus nerve (parasympatholytic effect). Atropine sulfate is also used as an emergency drug to reverse the toxic effects of organophosphate pesticide and nerve agent exposure, which includes bradycardia and excessive secretions.
In symptomatic bradycardia, atropine is administered IV in 0.5-mg doses at 3- to 5-minute intervals until the desired heart rate is achieved or until 0.04 mg/kg (not more than 3 mg) is given. Dosing in this manner may be repeated every 3 to 5 minutes up to a limit of 0.04 mg/kg (usually not more than 3 mg IV).
The adult IV atropine dose should never be less than 0.5 mg. Doses below 0.5 mg may produce a paradoxical bradycardia; at doses of 0.04 mg/kg or greater, vagal activity is considered completely blocked, and further atropine administration may have no benefit. However, in the case of organophosphate insecticide or nerve agent poisoning, very high doses of atropine may be necessary to counteract the pathophysiologic effects of these toxins. Therefore the typical dosing range and limits do not apply under these circumstances.
If venous access is not available in an emergency situation, atropine sulfate should be administered through the intraosseous (IO) route. As a last resort, atropine may be given via the endotracheal tube (ETT) route if venous or IO access cannot be achieved. The dose for endotracheal administration is 2 to 2.5 times the venous dose, diluted with normal saline or sterile water. Accordingly, 2 to 3 mg of atropine would be diluted in 10 mL of normal saline or sterile water and instilled deep into the ETT via a feeding tube attached to a syringe. After endotracheal administration, the patient should be ventilated vigorously with a bag-valve device to enhance absorption of the drug.
Continuous cardiac and blood pressure monitoring is essential for the patient who receives atropine sulfate. Significant adverse effects include cardiac dysrhythmias, tachycardia, myocardial ischemia, restlessness, anxiety, mydriasis, thirst, and urinary retention. See Chapter 16 for more information on atropine and other anticholinergics.
Pediatric Implications
The definition of bradycardia is variable and age specific for the pediatric population. Knowledge of normal ranges is essential. Because cardiac output is dependent on heart rate in infants younger than 6 months, bradycardia (heart rate <100 beats/min for infants) must be treated. In fact, a heart rate less than 60 beats/min in an infant requires performance of CPR. Before administration of drugs, efforts always should be targeted first toward restoring adequate ventilation and oxygenation. For the neonate with a spontaneous heart rate of less than 80 beats/min, epinephrine 0.01 mg/kg IV or IO every 3 to 5 minutes as indicated should be given prior to atropine to elevate the heart rate because stressed neonates quickly deplete their own stores of catecholamines. If these interventions do not produce the desired clinical response, atropine is indicated in the presence of increased vagal tone or AV block. Other pediatric indications for atropine include management of organophosphate toxicity and as pretreatment to prevent bradycardia after succinylcholine administration during rapid-sequence intubation, although this specific indication is currently controversial.
The pediatric dose of atropine is 0.02 mg/kg IV or IO. It is important to be cognizant that in the pediatric population, the minimum single dose is 0.1 mg, and the maximum single dose is 0.5 mg IV. The maximum total pediatric dose is 1 mg in a child and 3 mg in an adolescent (defined as an individual who has reached puberty). Note that when referring to general age groupings of patients, infants are considered to be younger than 1 year; a child is considered to be age 1 year to adolescence (puberty), and an adult is considered to be an adolescent or older. See Chapters 5 and 11 for more information on pediatric medication dosing and monitoring.
Adenosine
Adenosine is the first-line drug of choice to treat paroxysmal supraventricular tachycardia (PSVT), a sudden, uncontrolled, rapid rhythm that exceeds 150 ventricular beats/min in adults and originates above the ventricles. The goal is to convert PSVT to sinus rhythm. Adenosine, a natural substance found in all body cells, slows impulse conduction through the heart’s AV node, interrupts dysrhythmia-producing reentry pathways, and restores a normal rhythm in patients with PSVT. Because the half-life is less than 5 seconds, adenosine is best administered rapidly via a peripheral IV site in the port most proximal to the patient as a 6-mg IV bolus over 1 to 3 seconds followed by a 20-mL saline flush. A 12-mg bolus may be given 1 to 2 minutes after the initial dose if PSVT persists. Higher doses are not recommended.
Nursing considerations include continuous cardiac monitoring and frequent assessment of vital signs. Adenosine is inhibited by methylxanthines such as caffeine and theophylline, so higher doses may be needed. Although usually transient, ventricular ectopy, bradycardia, flushing, chest pain, and dyspnea may occur. In addition, a short period of asystole may follow injection of adenosine (up to 15 seconds). Spontaneous cardiac activity typically resumes. Adenosine is contraindicated in patients with poison- or drug-related tachycardia, second- and third-degree heart block, and in patients with sick sinus syndrome except those with functioning pacemakers. If the tachycardia originated in the ventricles, the patient could deteriorate and become hypotensive after adenosine administration. See Chapter 37 for more information on antidysrhythmic drugs.
Diltiazem
Diltiazem is a calcium channel blocker, and it is administered as an IV bolus to treat PSVT and to slow the ventricular response rate in atrial fibrillation or flutter. It is considered a second-line agent after adenosine. Diltiazem has less of a negative inotropic effect than other, similar calcium channel blockers, but it has strong negative chronotropic actions. Therefore IV diltiazem is less likely to cause cardiac depression but is very effective in controlling heart rate.
The usual initial bolus dose of IV diltiazem is 0.25 mg/kg given over 2 minutes. If the supraventricular tachycardia does not convert to a normal sinus rhythm in 15 minutes, a second IV bolus of 0.35 mg/kg over 2 minutes may be necessary. For ongoing control of the ventricular rate in patients with atrial fibrillation or flutter, a continuous infusion of diltiazem is indicated at a dose range of 5 to 15 mg/hour, titrated according to the desired heart rate.
The nurse must carefully monitor blood pressure and heart rate and rhythm after administering IV diltiazem. Arrhythmias, bradycardia, heart block, and hypotension may develop. Diltiazem can elevate serum digoxin levels, predisposing the patient to digitalis toxicity. Simultaneous use of calcium channel blockers and beta blockers is contraindicated because their negative inotropic and negative chronotropic effects are synergistic, causing myocardial depression and bradycardia. Other contraindications include preexisting heart failure, Wolff-Parkinson-White syndrome, and heart block or sick sinus syndrome in the patient without a pacemaker.
Amiodarone
The IV form of amiodarone is considered a first-line agent in the ALS algorithms for the treatment of life-threatening ventricular dysrhythmias and cardiac arrest. It has alpha- and beta-adrenergic blocking effects and acts on sodium, potassium, and calcium channels. Indications for use include pulseless ventricular tachycardia and ventricular fibrillation (after defibrillation and epinephrine), hemodynamically stable ventricular tachycardia, PSVT refractory to adenosine, ventricular rate control in atrial fibrillation, and pharmacologic treatment of atrial fibrillation.
Amiodarone is especially good for patients with impaired heart function who have atrial and ventricular dysrhythmias. It has been found to be more effective and to have fewer proarrhythmic properties than other agents with similar actions.
For patients who have a pulse (i.e., those not in cardiac arrest), amiodarone 150 mg IV is given over 10 minutes, followed by a continuous infusion of 1 mg/min for 6 hours, then 0.5 mg/min over 18 hours as a maintenance infusion. For patients in cardiac arrest because of pulseless ventricular tachycardia or ventricular fibrillation, a dose of 300 mg diluted in 20 to 30 mL D5W is given as a rapid infusion followed by a continuous infusion as described earlier. Additional doses of 150 mg may be given by rapid infusion if ventricular fibrillation or ventricular tachycardia recurs. The maximum daily dose is 2.2 g per 24-hour period.
Significant adverse effects include hypotension and bradycardia, therefore the nurse should slow the infusion rate to prevent or treat these effects and should be prepared to administer IV fluids, vasopressors, and agents to increase heart rate. A temporary pacemaker may be needed. Amiodarone has a very long half-life. It should not be given concurrently with pharmacologic drugs that prolong QT interval on electrocardiography (ECG), such as procainamide.
Pediatric Implications
Amiodarone has an off-label use among pediatrics. It is given for pulseless ventricular tachycardia and ventricular fibrillation as a 5-mg/kg rapid IV/IO bolus, which can be repeated up to a maximum dose of 15 mg/kg per 24 hours. For responsive children who have supraventricular (junctional and atrial) tachycardia and ventricular dysrhythmias with pulses present, amiodarone is given as a 5 mg/kg IV/IO loading dose (300 mg maximum) over 20 to 60 minutes and repeated to a maximum daily IV dose of 15 mg/kg per 24 hours.
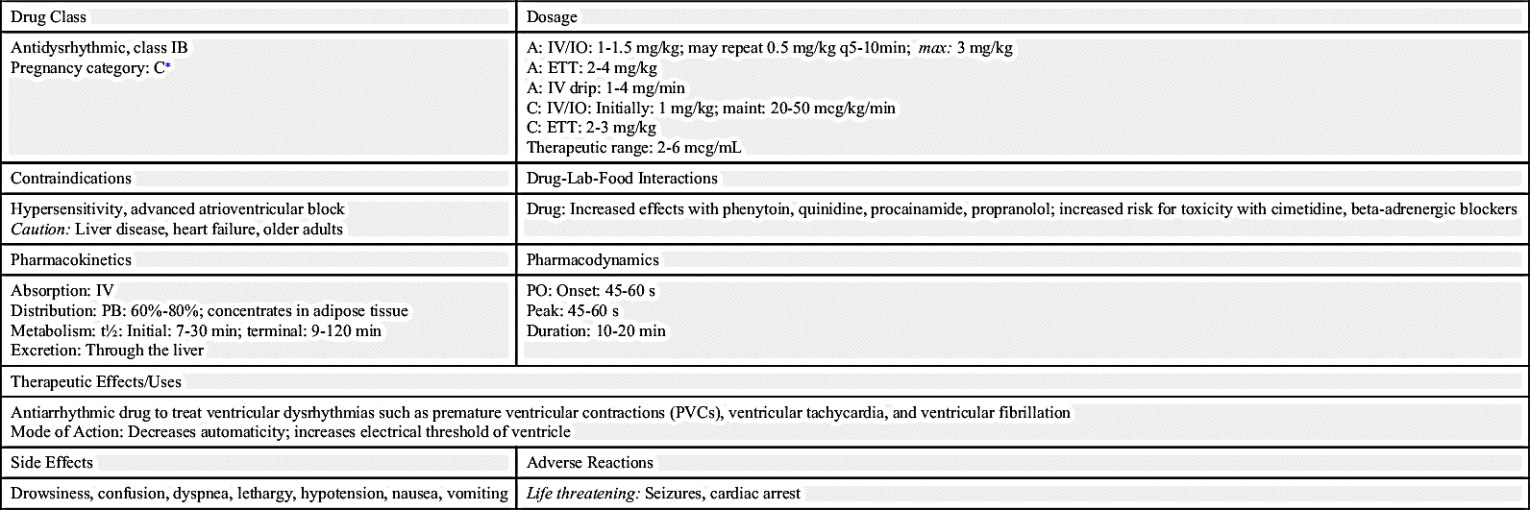
Lidocaine
As an alternative to amiodarone, lidocaine may be used to treat significant ventricular dysrhythmias, or irregular heartbeats, such as frequent premature ventricular contractions (PVCs), ventricular tachycardia, and ventricular fibrillation. Lidocaine exerts a local anesthetic effect on the heart, thus decreasing myocardial irritability. Typically, a patient with ventricular dysrhythmias is given a 1- to 1.5-mg/kg bolus of lidocaine, followed by 0.5 mg/kg to 0.75 mg/kg every 5 to 10 minutes until the dysrhythmia is controlled or a total dose of 3 mg/kg has been administered via the IV or IO route. A continuous lidocaine infusion is initiated at a rate of 1 to 4 mg/min to maintain a therapeutic serum level. Lidocaine may also be administered via the endotracheal route in doses of 2 to 4 mg/kg.
Important nursing considerations for the patient receiving lidocaine include continuous cardiac monitoring and assessment for signs and symptoms of lidocaine toxicity (e.g., confusion, drowsiness, hearing impairment, cardiac conduction defects, myocardial depression, muscle twitching, and seizures). Because lidocaine is metabolized by the liver, patients with hepatic impairment, heart failure, shock, and advanced age (>70 years) are at higher risk for toxicity. In these patients, the lidocaine dose may need to be reduced by as much as 50% (see Chapter 37). Lidocaine is contraindicated as a prophylactic agent to prevent ventricular dysrhythmias following AMI.
Pediatric Implications
Ventricular ectopy is uncommon in children, therefore metabolic causes should be suspected if ventricular dysrhythmias occur. The pediatric dose of lidocaine is 1 mg/kg IV or via the IO route, and the ETT dose is 2 to 3 mg/kg. A maintenance infusion of 20 to 50 mcg/kg/min is recommended following the bolus dose. Prototype Drug Chart 55.1 lists the pharmacologic data for lidocaine.
Procainamide
Procainamide is an antidysrhythmic drug prescribed for ventricular tachycardia, PVCs, and rapid supraventricular dysrhythmias unresponsive to adenosine. The typical IV loading dose of procainamide is 20 to 50 mg/min until the dysrhythmia is successfully treated. Other end points to procainamide administration include a total administration of 17 mg/kg of the drug, development of hypotension, and specific changes on the ECG (e.g., widening of the QRS complex by 50% or more). A continuous maintenance infusion of 1 to 4 mg/min may be ordered following the loading dose.
The nurse must monitor vital signs and the ECG with particular attention to heart rate and rhythm, blood pressure, and the width of the QRS complex. Procainamide administration can cause severe hypotension. Heart block, rhythm disturbances, and cardiac arrest can also occur. Procainamide is contraindicated in patients with torsades de pointes, an unusual polymorphic ventricular tachycardia often associated with a prolonged QT interval. Procainamide is eliminated via the kidneys, therefore patients with renal failure are at higher risk of adverse effects and often require a lower dosage.
Pediatric Implications
Procainamide has an off-label use among pediatrics and is given to children for ventricular tachycardia that is recurrent or refractory to other measures and for supraventricular tachycardia. The loading dose is 15 mg/kg IV or IO given over 30 to 60 minutes. The same monitoring guidelines, adverse effects, and contraindications described for adults are relevant in the pediatric population.
Magnesium Sulfate
Magnesium is an essential element in multiple enzymatic reactions in the body, including function of the sodium-potassium adenosine triphosphatase (ATPase) pump. Its physiologic effects can be likened to a calcium channel blocker with neuromuscular blocking properties. Hypomagnesemia is associated with the development of atrial and ventricular dysrhythmias.
The primary indications for emergency administration of magnesium sulfate are refractory ventricular tachycardia, refractory ventricular fibrillation, cardiac arrest associated with hypomagnesemia (low serum magnesium level), and life-threatening ventricular dysrhythmias from digitalis toxicity. It is also the drug of choice for the treatment of torsades de pointes.
Magnesium is administered by diluting 1 to 2 g (2 to 4 mL of a 50% solution) in 10 mL of D5W. For cardiac arrest caused by hypomagnesemia or torsades de pointes, magnesium is given by direct IV push or via the IO route over 5 to 20 minutes. For patients experiencing torsades de pointes who are not in cardiac arrest, a magnesium infusion of 1 to 2 g diluted in 50 to 100 mL of D5W can be given IV/IO over 5 to 60 minutes followed by a continuous infusion of 0.5 to 1 g/h.
Although magnesium toxicity is rare, the nurse should monitor the patient’s response to magnesium administration. Hypotension is the most common adverse effect when magnesium is given by rapid IV push. Other effects include mild bradycardia, flush, and sweating. True hypermagnesemia can cause diarrhea, respiratory depression, deep tendon reflex impairment, flaccid paralysis, and circulatory collapse. Because magnesium is eliminated via the kidneys, it should be administered with caution in patients with renal impairment.
Pediatric Implications
Indications for magnesium sulfate in pediatric patients include torsades de pointes, hypomagnesemia, and status asthmaticus that is unresponsive to beta-adrenergic drugs. The magnesium sulfate dose is 25 to 50 mg/kg IV/IO (maximum dose of 2 g) given as a bolus for pulseless ventricular tachycardia, slowly over 10 to 20 minutes for ventricular tachycardia with pulses and over 15 to 30 minutes for status asthmaticus.
Epinephrine
Epinephrine is a catecholamine with alpha- and beta-adrenergic effects. It has multiple uses. Emergency cardiac indications for administration of IV/IO epinephrine include profound bradycardia and hypotension, asystole, pulseless ventricular tachycardia, and ventricular fibrillation. Epinephrine is thought to improve perfusion of the heart and brain in cardiac arrest states by constricting peripheral blood vessels. In addition, epinephrine increases the chances for successful electrical countershock (defibrillation) in ventricular fibrillation. It is important to be aware that epinephrine  is available in two primary concentrations: 1:1000 and 1:10,000. The 1:10,000 concentration is used when administering a single IV/IO dose of epinephrine. The 1:1000 form is used when preparing a continuous epinephrine infusion or when giving epinephrine via the intramuscular (IM) route. The subcutaneous (subcut) route should not be used for emergency epinephrine administration because absorption is unpredictable.
is available in two primary concentrations: 1:1000 and 1:10,000. The 1:10,000 concentration is used when administering a single IV/IO dose of epinephrine. The 1:1000 form is used when preparing a continuous epinephrine infusion or when giving epinephrine via the intramuscular (IM) route. The subcutaneous (subcut) route should not be used for emergency epinephrine administration because absorption is unpredictable.
 is available in two primary concentrations: 1:1000 and 1:10,000. The 1:10,000 concentration is used when administering a single IV/IO dose of epinephrine. The 1:1000 form is used when preparing a continuous epinephrine infusion or when giving epinephrine via the intramuscular (IM) route. The subcutaneous (subcut) route should not be used for emergency epinephrine administration because absorption is unpredictable.
is available in two primary concentrations: 1:1000 and 1:10,000. The 1:10,000 concentration is used when administering a single IV/IO dose of epinephrine. The 1:1000 form is used when preparing a continuous epinephrine infusion or when giving epinephrine via the intramuscular (IM) route. The subcutaneous (subcut) route should not be used for emergency epinephrine administration because absorption is unpredictable.For profound bradycardia or hypotension, an epinephrine infusion may be ordered at 0.1 to 0.5 mcg/kg/min. For asystole, pulseless ventricular tachycardia, and ventricular fibrillation, epinephrine is administered in 1-mg doses (1:10,000 solution) IV/IO every 3 to 5 minutes until the desired clinical response—usually return of effective cardiac activity—is achieved. Epinephrine also may be given via the ETT route in doses of 2 to 2.5 mg diluted in 10 mL of normal saline.
Nursing implications for patients receiving epinephrine include constant cardiac and hemodynamic monitoring. Epinephrine can cause myocardial ischemia and cardiac dysrhythmias. It should never be administered in the same site as an alkaline solution, such as with sodium bicarbonate, because alkaline solutions inactivate epinephrine. In addition, the presence of metabolic or respiratory acidosis decreases the effectiveness of epinephrine. All efforts should be made to correct acid-base imbalances in the patient. More drug information about epinephrine and other adrenergic drugs can be found in Chapter 12.
Pediatric Implications
The pediatric dose of epinephrine is 0.01 mg/kg (1:10,000 solution) given every 3 to 5 minutes IV/IO for cardiac arrest. The ETT dose of 0.1 mg/kg should be given using the 1:1000 solution every 3 to 5 minutes.
Sodium Bicarbonate
Sodium bicarbonate is prescribed to treat severe metabolic acidosis that may accompany cardiac arrest and the hyperkalemia and acidotic states related to specific drug overdose situations. The current standard in resuscitation is to give sodium bicarbonate only after adequate ventilation, chest compressions, IV fluids, and drug therapy fail to correct the acidotic state. Sodium bicarbonate is not considered a first-line drug for the treatment of cardiac arrest; it is preferentially given based on results of arterial blood gas analysis when acidosis is severe. The standard initial IV dose of sodium bicarbonate is 1 mEq/kg. Subsequent dosing depends on arterial blood gas analysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree