1 Introduction
People rarely suffer from the same infectious disease twice. Reinfections normally occur, primarily (1) when the infectious agent exhibits antigenic plasticity such as with the common cold and influenza; (2) if the patient is immunocompromised, due for example to immunosuppressive therapy or immunological disorders; or (3) when a significant amount of time has passed after the first infection. Alternatively, the patient may have failed to eliminate the primary infection which remained latent and emerged later in a modified of similar form as for example with herpes simplex (oral and genital herpes), herpes zoster, (chickenpox) and HIV/AIDS.
Immunity against reinfection was recognized long before the discovery of the causal agents of infectious disease. Consequently, efforts were made towards developing treatment strategies that could generate immunity to infection without the individual suffering the infection. An early development was the attempted prevention of smallpox (variola major) through the dermal inoculation of healthy individuals with material taken from active smallpox lesions. Such treatments often produced single localized lesions and commonly, but not always, protected the recipient from contracting full-blown smallpox. The process became known as variolation and, unknown to its practitioners, protected against the disease by changing the route of infection of the causal organism from respiratory transmission to cutaneous. Unfortunately, occasional cases of smallpox resulted from such practices and variolated individuals could also (rarely) infect others, resulting in infection. Further developments recognized that immunity developed towards one pathogen may be associated with crossimmunity towards related infectious agents. Cowpox is a disease of cattle that can be transmitted to humans. The symptoms are similar to those of smallpox, but considerably less severe. Following the observation that individuals exposed to cowpox were conferred protection against smallpox, Edward Jenner substituted material taken from active cowpox (vaccinia) into the variolation procedures. This conferred much of the protection against smallpox that had become associated with variolation but without the associated risks. This discovery, made over two centuries ago, became known as vaccination and heralded a new era in disease control. The term vaccination was originally used to refer to prophylactic measures that use living microorganisms or their products to induce immunity, but the term is now used to refer to all immunization procedures.
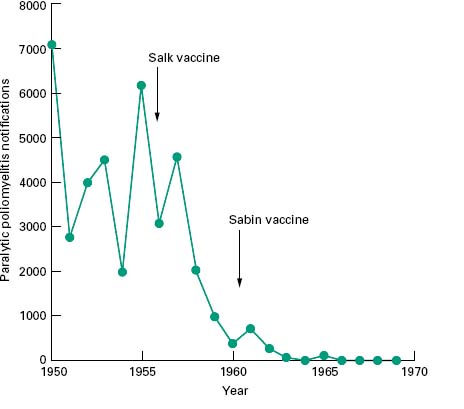
Vaccination is used to protect individuals against infection and also to protect communities against epidemic disease. Such public health measures have met with spectacular success and in instances where there is no reservoir of the pathogen other than in infected individuals and survival of the pathogen outside the host is therefore limited, vaccination has the potential to eradicate the disease permanently. This has already been achieved for smallpox where the coordinated deployment of an effective vaccine over many decades led to the eradication of this disease. The global eradication of smallpox was endorsed by the World Health Assembly on 8 May 1980. Another candidate disease for global eradiation by vaccination is poliomyelitis, where effective vaccination programmes have reduced the annual incidence to fewer than 2000 cases. The virus persists, however, in India, Pakistan, Afghanistan and Nigeria. The Global Polio Eradication Initiative, spearheaded by the World Health Organization (WHO), Rotary International, the U.S. Centers for Disease Control (CDC) and the United Nations Children’ s Fund (UNICEF) is now actively working towards the eradication of this virus. It has been calculated that eradication is a more costeffective option than containing the disease, as well as reducing morbidity associated with the residual cases. The effectiveness of poliomyelitis vaccines is clearly indicated by data shown in Figure 10.1.
Figure 10.1 Reported incidence of paralytic poliomyelitis in England and Wales during the 1950s and 1960s. After the introduction of vaccination programmes the incidence of this disease dropped from an endemic incidence of around 5000 cases per year to fewer than 10.
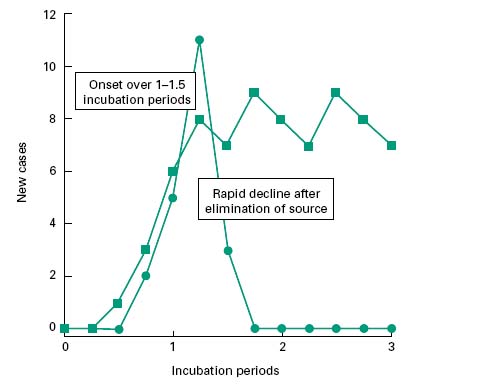
Infectious diseases may either be spread from a common reservoir (common source) of the infectious agent that is distinct from the diseased individuals, or through a population by serial transfer from diseased to healthy, susceptible individuals (propagated source; Figure 10.2).
Figure 10.2 Incidence pattern for common-source outbreaks of infection where the source persists ( ) and where it is short-lived (
) and where it is short-lived ( ).
).
2.1 Common-source infections
In common source infections, potential reservoirs of infection include infected drinking-water, contaminated water vapour from a cooling tower, or contaminated food. In the simplest of cases the source of the infection is transient (i.e. food sourced to a single retail outlet, or to an isolated event such as a dinner party). In such instances, the onset of new cases will be phased over a timescale approximately equivalent to one incubation period, and the decline in new cases closely follows the elimination of the source (Figure 10.2). This leads to an acute outbreak of infection, limited to those linked with the source. Such incidents are epitomized by the 1996 outbreak of Escherichia coli O157 infections in Lanarkshire, Scotland, that resulted in 5 deaths and left 280 people ill. Similarly, in Clemenstone, South Wales, an outbreak resulted in 157 cases of E. coli including the death of a 5-year-old boy in 2005. Both of these outbreaks were linked to a single retail outlet.
If the source of the infection persists beyond the onset, then the incidence of new cases may be maintained at a level that is commensurate with the infectivity of the pathogen and the frequency of exposure of individuals. For those infectious diseases that are transmitted to humans via insect vectors that may act as reservoirs of infection, onset and decline phases of epidemics are rarely observed, other than as reflections of the seasonal variation in the prevalence of the insect. Diseases such as these are generally controlled by public health measures and environmental control of the insect vector with vaccination and immunization being deployed to protect individuals (e.g. yellow fever vaccination).
2.2 Propagated-source infections
Propagated outbreaks of infection relate to the direct transmission of an infective agent from a diseased individual to a healthy, susceptible one. Mechanisms of such transmission have been described in Chapter 7 and include inhalation of infective aerosols such as with measles, mumps and diphtheria; direct physical contact such as may occur with syphilis, herpesvirus and human papillomavirus; and, where sanitation standards are poor, through the introduction of infected faecal material into drinking-water (i.e. cholera and typhoid fever) or onto food (e.g. Salmonella and Campylobacter). The ease of transmission, and hence the rate of onset of an epidemic, relates to the susceptibility status and general state of health of the individuals concerned, the virulence properties of the organism, the route of transmission, the duration of the infective period associ-ated with the disease, behavioural patterns, age of the population group and the population density (i.e. urban versus rural).
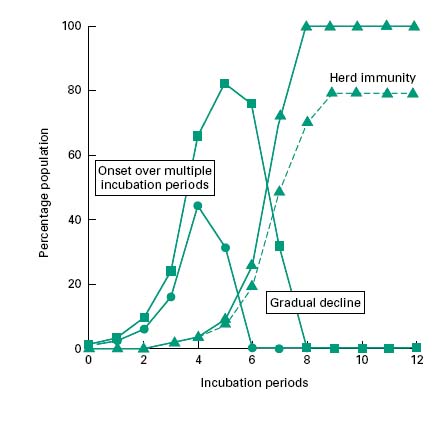
Each infectious individual will be capable of transmitting the disease to the susceptible individuals that they encounter during their infectious period. The number of persons to which a single infectious individual might transmit the disease and hence the rate of occurrence of the infection within the population will depend upon the population densities of susceptible and infective individuals, the degree and nature of their social interaction and the duration and timing of the infective period. If infectivity precedes the manifestation of disease, then spread of the infection may be greater than if these were concurrent. As each infected individual will in turn, become a source of infection, this leads to a near exponential increase in the incidence of disease. Figure 10.3 shows the incidence of disease within a population group. This group is perfectly mixed and all individuals are susceptible to the infection. The model infection has an incubation period of 1 day and an infective period of 2 days commencing at the onset of symptoms with recovery occurring 1 day later. For the sake of this illustration it has been assumed that each infective individual will infect two others per day until the entire population group has contracted the disease (solid lines). In reality, the rate of transmission will decrease as the epidemic progresses, because recovered individuals may become immune to further infection, reducing the population density of susceptible individuals, and thereby the likelihood of onward transmission. Epidemics therefore often cease before all members of the community have been infected (Figure 10.3, dotted line). If the proportion of immune individuals within a population group can be maintained above this threshold level then the likelihood of an epidemic arising from a single isolated infection incident is small (this is referred to as herd immunity). The threshold level itself is a function of the infectivity of the agent and the population density. Outbreaks of measles and chickenpox therefore tend to occur annually in the late summer among children attending school for the first time. This has the effect of concentrating all susceptible individuals in one, space and thereby reducing the proportion of immune subjects to a value below the threshold for propagated transmission. An effective vaccination programme that maintains the proportion of individuals who are immune to a given infectious disease above the critical threshold level for herd immunity. Such a programme will not prevent isolated cases of infection but will prevent these from becoming epidemic.
Figure 10.3 Propagated outbreaks of infection showing the incidence of new cases ( ), diseased individuals (
), diseased individuals ( ) and recovered immune individuals (
) and recovered immune individuals ( ). The dotted line indicates the incidence pattern for an incompletely mixed population group.
). The dotted line indicates the incidence pattern for an incompletely mixed population group.
3 Objectives of a vaccine/immunization programme
There is the potential to develop a protective vaccine/ immunization programme for all infectious diseases, although some pathogens are considerably more challenging candidates than others. Whether or not such vaccines are developed and deployed is related to the severity and economic impact of the disease on the community as well as the effects upon the individual. Various factors governing the likelihood of an immunization programme being adopted are discussed below, while the principles of immunity, and of the production and quality control of immunological products are discussed in Chapters 9 and 24 respectively.
3.1 Disease severity
The severity of the disease in terms of its morbidity and mortality, the probability of permanent injury to its survivors and the likelihood of infection must be sufficient to warrant the costly development of a vaccine and its subsequent use. Thus, although influenza vaccines are constantly reviewed and stocks maintained, the control of influenza epidemics through vaccination is not recommended. Rather, those groups of individuals, such as elderly people, who are at special risk from the infection, are protected.
Vaccines to be included within national immunization and vaccination programmes should be chosen to reflect the infection risks within that country. Additional immunizations, appropriate for persons travelling abroad, are intended to protect the atrisk individual, but also to prevent importation of the disease into an unprotected home community.
3.2 Vaccine effectivness
Vaccination and immunization programmes seldom confer 100% protection against the target disease. More commonly the degree of protection is 60-95%. In such instances, although individuals receiving treatment have a high probability of becoming immune, virtually all members of a community must be treated in order to reduce the actual proportion of susceptible individuals to below the threshold for epidemic spread of the disease. Antidiphtheria and antitetanus prophylaxes, which utilize toxoids, are among the most efficient immunization programmes, whereas the performance of BCG is highly variable.
3.3 Safety
No medical or therapeutic procedure comes without some risk to the patient, but all possible steps are taken to ensure safety, quality and efficacy of vaccines and immunological products (see Chapter 24). The risks associated with immunization procedures are constantly reviewed and balanced against the risks associated with contracting the disease. In this respect, the incidence of paralytic poliomyelitis in the USA and UK in the late 1990s was low, with the majority of cases being related to vaccine use (vaccine-associated paralytic polio or VAPP). As the worldwide elimination of poliomyelitis approaches, there is debate as to the value of the live (Sabin) vaccine outside endemic areas, and the inactivated polio vaccine (IPV) is now the vaccine of choice in the UK for prophylaxis against paralytic polio.
3.4 Public perceptions
Public confidence in the safety of vaccines and immunization procedures is essential if compliance is to match the needs of the community. The correlation between actual risk and perception of risk is not always reliable, however. In this respect, public concern and anxiety in the mid 1970s over the perceived safety of pertussis vaccine led to a reduction in coverage of the target group from about 80% to 30%. Major epidemics of whooping cough, with over 100 000 notified cases, followed in the late 1970s and early 1980s. By 1992, public confidence had returned and coverage had increased to 92%, with a considerable associated decrease in disease incidence. Similarly, links have been claimed between the incidence of autism in children and the change in the UK from single measles and German measles vaccines to the combined measles, mumps and rubella (MMR) vaccine. Such claims have been proved to be unfounded beyond reasonable doubt but have nevertheless decreased the uptake of the MMR vaccine and thereby increased the likelihood and magnitude of measles epidemics.
3.5 Cost
Cheap, effective vaccines are an essential component of the global battle against infectious disease. It was estimated that the 1996 costs of the USA childhood vaccination programme, directed against polio, diphtheria, pertussis, tetanus, measles and tuberculosis, was $1 for the vaccines and $14 for the programme costs. The newer vaccines, particularly those that have been genetically engineered, are considerably more expensive, putting the costs beyond the budgets of many developing countries.
3.6 Longevity of immunity
The ideal of any vaccine is to provide lifelong protection of the individual against disease. Immunological memory (Chapter 9) depends on the survival of cloned populations of B-and T-lymphocytes (memory cells). Although these lymphocytes can persist in the body for many decades, the duration of protection varies from one individual to another and depends on the vaccine; commonly ranging between 10 and 20 years. Thus, if the immune system is not boosted, either by natural exposure to the organism or by reimmunization, protective immunity gained in childhood may be lost by the age of 30. Those vaccines that provide only poor protection against disease may have proportionately reduced timespans of effectiveness. Equally, vaccines may be less effective and have a shorter duration when administered to neonates. Yellow fever vaccination, which is highly effective, must there-fore be repeated at 10-year intervals, while the typhoid vaccine is only effective for up to 3 years. Whether or not immunization in childhood is boosted at adolescence or in adulthood depends on the relative risks associated with the infection as a function of age and the longevity of immunity conferred by the vaccine.
The theoretical background that underlies immunity to infection has been discussed in detail in Chapter 9.Immunity to infection may be passively acquired through the receipt of preformed, protective antibodies or it may be actively acquired through an immune response following deliberate or accidental exposure to microorganisms or their component parts. Active, acquired immunity might involve either or both of the humoral and cellmediated responses.
4.1 Passive (artificially acquired) immunity
Humoral antibodies of the IgG class are able to cross the placenta from mother to fetus. These antibodies will provide passive protection of the newborn against those diseases which involve humoral immunity and to which the mother is immune. In this manner, most newborn infants in the UK will have passive protection against tetanus, but not against tuberculosis. Protection against the latter relies to a large extent on cellmediated immunity. Secreted (IgA) antibodies are also passed to the gut of newborn, together with the first deliveries of breast milk (colostrum). Such antibodies provide some passive protection against infections of the gastrointestinal tract. Maternally acquired antibodies will react with antigens associated with an infection but also with antigens introduced to the body as part of an immunization programme. Premature immunization, i.e. before degradation of the maternal antibodies, may reduce the potency of an administered vaccine. This aspect of the timing of a course of vaccinations is discussed later.
Administration of preformed antibodies taken from animals, pooled human serum, or human cell lines is often used to treat existing infections (e.g. tetanus, diphtheria) or condition (e.g. venomous snake bite). Pooled serum may also be administered prophylactically, within a slow-release vehicle, for individuals travelling to parts of the world where diseases such as hepatitis A are endemic. Such administrations confer no long-term immunity and may interfere with concurrent vaccination procedures.
4.2 Active (artificially acquired) immunity
Active immunity (Chapter 9) relates to exposure of the immune system to antigenic materials and the subsequent response. Such exposure might be related to an infection or to the multiplication of an attenuated vaccine strain, or it might be associated with the direct introduction of non-viable antigenic material into the body e.g. a non-living or inactivated vaccine. The route of exposure to antigen will influence the nature of the subsequent immune response. Thus, injection of antigen will lead primarily to humoral (IgG, IgM) production, while exposure of epithelial tissues (gut, respiratory tract) will lead to the production of secretory antibodies (IgA, IgE) and to the stimulation of humoral antibody production.
The magnitude and specificity of an immune response depends upon the duration of the exposure to antigen and on its time-concentration profile. During a naturally occurring infection (or the administration of a live, atten-uated vaccine), the levels of antigen in the host may be low at the onset and localized to the portal of entry to the host. As the amounts of antigen are small, they will react only with a small, highly defined subgroup of small lymphocytes. These may undergo transformation to produce various antibody classes specific to the antigen and undergo clonal expansion. These immune responses and the progress of the infection may progress simultaneously. With time, microorganisms will produce greater amounts of antigenic materials that will, in turn, react with an increasing number of cloned lymphocytes to produce yet more antibodies. Eventually the antibody levels may be sufficient to eliminate the infecting organism from the host. Antibody levels will then decline, with the net result of this encounter being the clonal expansion of particular small lymphocytes relating to a highly specific ‘immunological memory ’ of the encounter.
This situation should be contrasted with the injection of a killed or nonliving vaccine where the amount of antigen introduced is relatively high when compared with the levels present during the initial stages of an infection. In a non-immune animal, the antigens may react not only with those lymphocytes that are capable of producing antibody of high specificity but also with those of a lower specificity. Antibody of both high and lower specificity may react with and remove the residual antigen. The immune response will cease after this initial (primary) challenge. On a subsequent (secondary) challenge (during a course of vaccinations), the antigen will react with residual preformed antibody relating to the first challenge, together with a more specific subgroup of the original cloned lymphocytes. As the number of challenges is increased, the proportion of stimulated lymphocytes that are specific to the antigen rises. After a sufficient number of consecutive challenges the magnitude and specificity of the immune response matches that which would occur during a natural infection with an organism bearing the antigen. This pattern of exposure brings with it certain problems. Firstly, as the introduced immunogen will react preferentially with preformed antibody rather than lymphocytes then sufficient time must elapse between exposures to allow the natural loss of antibody to occur. Secondly, immunity to infection will only be complete after the final challenge with immunogen. Thirdly, low specificity antibody produced during the early exposures to antigen might be capable of cross-reaction with host tissues to produce an adverse response to the vaccine.
Vaccines may comprise living, attenuated microorganisms, killed microorganisms, or purified bacterial and viral components (component vaccines). Recent innovations include the development of DNA vaccines that encode for the transcription of antigen when introduced directly into host tissues or vaccines that might be delivered nasally by non-pathogenic bacteria (e.g. Lactococcus lactis) or otherwise by viral vectors (e.g. adenovirus). Some aspects of these vaccine classes are discussed below.
5.1 Live vaccines
Live, infective microorganisms, attenuated with respect to their pathogenicity but retaining their ability to infect, can be used to confer protective immunity. Two major advantages stem from the use of live vaccines. First, the immunization mimics the course of a natural infection such that only a single exposure is required to render an individual immune. Secondly, the exposure may be mediated through the natural route of infection (e.g. oral), thereby stimulating an immune response that is appropriate to a particular disease (e.g. secretory antibody as a primary defence against poliomyelitis virus in the gut with oral polio vaccine (OPV); see below). Disadvantages associated with the use of live vaccines are also apparent. Live, attenuated vaccines, administered through the natural route of infection, will replicate in the patient and could be transmitted to others. If attenuation is reduced during the replication process, infections might result (see OPV below). A second, major disadvantage, of live vaccines is that the course of their action, and possible side effects, might be affected by the infection and immunological status of the patient.
5.2 Killed and component vaccines
Since these vaccines are unable to evoke a natural infection profile with respect to the release of antigen, they must be administered on a number of occasions. Immunity may not reach optimal levels until the course of immunization is complete and, with the exception of toxin-dominated diseases such as diphtheria and tetanus where the immunogen is a toxoid, are unlikely to match the performance of a live vaccine. The specificity of the immune response generated in the patient may initially be low. This is particularly the case when the vaccine is composed of a relatively crude cocktail of killed cells, where the immune response is directed only partially towards antigenic components of the pathogen. This increases the possibility of adverse reactions in the patient. Release profiles of these immunogens can be improved through their formulation with adjuvants (Chapters 9 and 24), and the immunogenicity of certain purified bacterial components such as polysaccharides can be improved by their conjugation to a carrier.
5.3 DNA vaccines
A development associated with research into gene therapy has been the use of DNA encoding specific virulence factors of defined pathogens to evoke an immune response. The DNA is introduced directly into tissue cells by means of a transdermal ‘gene gun’ and is transcribed by the recipient cells. Accordingly, the host responds to the antigenic material produced as though it were an infection. The course of release of the antigen reflects that of a natural infection and, therefore, a highly specific response is invoked. Eventually the introduced DNA is lost from the recipient cells and antigen release ceases. To date, few experimental trials have demon-strated convincing protection in humans, but this remains a promising approach. Protective immunity has been reported in a trial of a human vaccine for bird flu and a West Nile virus vaccine for horses has been approved.
6 Routine immunization against in fectious disease
6.1 Poliomyelitis vaccination
Poliovirus, a picornavirus, has three immunologically distinct serotypes (I, II and III) and there may be three phases of the disease. The first is an acute infection of lymphoid tissues associated with the gastrointestinal tract (Peyer’s patches), during which time the virus can be found in the throat and in faeces. The second phase is characterized by an invasion of the bloodstream, and in the third phase the virus migrates from the bloodstream into the meninges. Infections range in severity from asymptomatic (the majority of cases) to paralytic poliomyelitis which may cause permanent neurological damage and muscle paralysis. Paralytic poliomyelitis is a major illness, but occurs in only 0.1-2% of cases. It is characterized by the destruction of large nerve cells in the anterior horn of the brain, resulting in varying degrees of paralysis, and unvaccinated adults are at greater risk of paralytic infection than children. The infection is transmitted by the faecal-oral route.
Polio is the only disease, at present, for which both live and killed vaccines compete. Since the introduction of the killed virus (Salk) in 1956 and the live, attenuated virus (Sabin) in 1962 there has been a remarkable decline in the incidence of poliomyelitis (Figure 10.1). The inactivated polio (Salk) vaccine (IPV) contains formalin-killed poliovirus of all three serotypes. On injection, the vaccine stimulates the production of antibodies of the IgM and IgG class that neutralize the virus in the second stage of infection. A course of three injections at monthly intervals produces long-lasting immunity to all three poliovirus types. The live, oral polio (Sabin) vaccine (OPV) is now less commonly used. Advantages over the IPV vaccine include lower costs and easier administration. OPV contains attenuated poliovirus of each of the three types and is administered, as a liquid, onto the tongue. The vaccine strains infect the gastrointestinal mucosa and oropharynx, promoting the common immune response, and involving both humoral and secretory antibodies. IgA, secreted within the gut epithelium, provides local resistance to the first stages of poliomyelitis infection. OPV therefore provides protection at an earlier stage of the infection than does IPV. Infection of epithelial cells with one strain of entero-virus, however, may inhibit simultaneous infection by related strains. At least three administrations of OPV are therefore given, with each dose conferring immunity to one of the vaccine serotypes. These doses must be separated by a period of at least 1 month in order to allow the previous infection to lapse. Booster vaccinations are also provided to cover the eventuality that some other enterovirus infection, present at the time of vaccination, had reduced the response to the vaccine strains. Faecal excretion of vaccine virus will occur and may last for up to 6 weeks after administration. Such released virus may spread to close contacts and infect or (re)immunize them. Vaccine-associated poliomyelitis may occur through reversion of the attenuated strains to the virulent wild type, particularly with types II and III and has been estimated to occur once per 4 million doses. Since the introduction of OPV, notifications of paralytic poliomyelitis in the UK have markedly dropped. However, from 1985 to 1995, 19 of the 28 notified cases of paralytic poliomyelitis were associated with reverted vaccine strains (14 recipients, 5 contacts). As the risk of natural infections with poliomyelitis within developed countries has now diminished markedly, the live vaccine strains present the greatest risk. Consequently, OPV has now been replaced with IPV as the polio vaccine of choice in the UK and USA.
6.2 Measles, mumps and rubella vaccination (MMR)
Measles, mumps and rubella (German measles) are infectious diseases with respiratory routes of transmission and infection and each is caused by distinct members of the paramyxovirus group. Each virus only has one serotype. Although the primary multiplication sites of these viruses are within the respiratory tract, the diseases are associated with viral multiplication elsewhere in the host.
6.2.1 Measles
Measles is a severe, acute, highly contagious infection that frequently occurs in epidemic form. After multiplication within the respiratory tract, the virus is tran-sported throughout the body, particularly to the skin where a characteristic maculopapular rash develops. Complications of the disease can occur, including measles encephalitis, which can cause permanent neurological injury and death, and subacute, sclerosing panencephalitis (SSPE), which is a rare form of progressive encephalitis associated with persistence of the measles virus that primarily affects children and young adults. It is incura-ble, although early treatment can slow progression or improve the remission rate.
A live, attenuated vaccine strain of measles was introduced in the USA in 1962 and to the UK in 1968. A single injection produces high-level immunity in over 95% of recipients. Moreover, as the vaccine induces immunity more rapidly than the natural infection, it may be used to control the impact of measles outbreaks. The measles virus cannot survive outside an infected host. Widespread use of the vaccine therefore has the potential, as with smallpox, of eliminating the disease worldwide. Mass immunization has markedly reduced the incidence of measles, although a 15-fold increase in the incidence was noted in the USA between 1989 and 1991 because of poor compliance with the vaccine.
6.2.2 Mumps
Mumps virus infects the parotid glands to cause swelling and a general viraemia. Complications include pancreatitis, meningitis and orchitis, the last occasionally leading to male sterility. Infections can also cause permanent unilateral deafness. In the absence of vaccination, infection occurs in more than 90% of individuals by age 15 years. A live, attenuated mumps vaccine has been available since 1967 and has been part of the childhood vaccination programme in the UK since 1988 when it was included as part of the MMR triple vaccine (see below).
6.2.3 Rubella
Rubella is a mild, often subclinical infection that is common among children aged between 4 and 9 years. Infection during the first trimester of pregnancy brings with it a major risk of abortion or congenital deformity in the fetus (congenital rubella syndrome, CRS). Rubella immunization was introduced to the UK in 1970 for pre-pubertal females and nonimmune women intending to start families. The vaccine utilizes a live, cold-adapted strain of the virus. The major disadvantage of the vaccine is that, as with the wild type, the fetus can be infected. While there have been no reports of CRS associated with use of the vaccine, the possible risk makes it imperative that women do not become pregnant within 1 month of vaccination. Prepubertal females were immunized to extend the period of immunity through the childbearing years. Until 1988 boys were not routinely protected against rubella. Their susceptibility to the virus was thought to maintain the natural prevalence of the disease in the community and thereby reinforce the vaccine-induced immunity in vaccinated, adult females. This proved not to be the case and in fact cases of CRS could be related to incidence of the disease in younger children within the family. Rubella vaccine is now given to both sexes at the age of about 13 months as part of the MMR programme (below).
6.2.4 MMR vaccine
MMR vaccine was introduced to the UK in 1988 for young children of both sexes, replacing the single measles vaccine. It consists of a single dose of a lyophilized preparation of live attenuated strains of the measles, mumps and rubella viruses. The MMR vaccine had previously been deployed in the USA and Scandinavia for a significant number of years without any indication of increased adverse reaction or of decreased seroconversion over separate administration of the component parts. Immunization results in seroconversion to all three viruses in > 95% of recipients. For maximum effect, MMR vaccine is recommended for children of both sexes aged 12-15 months but can also be given to non-immune adults. From October 1996 a second dose of MMR was recommended for children aged approximately 4 years in order to prevent the reaccumulation of sufficient susceptible children to sustain future epidemics.
In 1998 a research paper attempted to associate an increase in autism to the introduction of the triplevaccine. This led to a decreased public confidence in the vaccine. Detailed examination of the data and also the results of several clinical studies have indicated that there is no association between use of the triple vaccine and autism. This is backed up by over 20 years of successful deployment of the vaccine outside of the UK. Currently, much effort is being made to restore confidence in the vaccine in order to avoid the lack of compliance leading to the occurrence of measles epidemics.
6.3 Tuberculosis
Tuberculosis (TB) is a major cause of death and morbidity worldwide, particularly where poverty, malnutrition and poor housing prevail. Human infection is acquired by inhalation of Mycobacterium tuberculosis or M. bovis. Tuberculosis is primarily a disease of the lungs, causing chronic infection of the lower respiratory tract, but may spread to other sites or proceed to a generalized infection (miliary tuberculosis). Active disease can result either from a primary infection or from a subsequent reactivation of aquiescent infection. Following inhalation, the mycobacteria are taken up by alveolar macrophages where they survive and multiply. Circulating macro-phages and lymphocytes, attracted to the site, carry the organism to local lymph nodes where a cell-mediated immune response is triggered. The host, unable to eliminate the pathogen, contains the bacteria within small granulomas or tubercles. If high numbers of mycobacteria are present then the cellular responses can result in tissue necrosis. The tubercles contain viable pathogens that may persist for the remaining life of the host. Reactivation of the healed primary lesions is thought to account for over two-thirds of all newly reported cases of the disease.
The incidence of TB in the UK declined 10-fold between 1948 and 1992, to just over 5000 new cases being notified each year. Those most atrisk include pubescent children, health service staff and individuals intending to stay for more than 1 month in countries where TB is endemic. However, since 1994 there has been a gradual increase in the number of notified cases in England and Wales to approximately 7000 new cases per year which can be associated with age group (20-45-year-olds more susceptible), sex (males slightly more susceptible), geographical region and ethnicity. The rise in notifications can be attributed to a number of different factors, includ-ing increased immigration from countries where TB is more prevalent, crowded conditions (e.g. prisons, hostels, wards, etc.), a greater number of susceptible individuals now in society (e.g. substance abusers, immunocompromised individuals) and limited training for GPs in recognizing the symptoms of TB. Fortunately this latter issue has now been addressed, along with the reemergence of TB research programmes.
A live vaccine is required to elicit protection against TB and both antibody and cell-mediated immunity are required for protective immunity. Vaccination with BCG (bacille Calmette-Gu érin), derived from an attenuated M. bovis strain, is commonly used in countries where TB is endemic. The vaccine was introduced in the UK in 1953. Efficacy in the UK has been shown to be over 70%, with protection lasting at least 15 years. In other countries, where the general state of health and well-being of the population is less than in the developed world, the efficacy of the vaccine has been shown to be markedly lower than this.
Because of the risks of adverse reaction to the vaccine by individuals who have already been exposed to the disease, a sensitivity test must be carried out before immunization with BCG. A Mantoux skin test assesses an individual’s sensitivity to a purified protein derivative (PPD) prepared from heat-treated antigens (tuberculin) extracted from M. tuberculosis. A positive test implies past infection or past, successful immunization. Those with strongly positive tests may have active disease and should be referred to a chest clinic. However, many people with active TB, especially disseminated TB, seroconvert from a positive to a negative skin test. Results of the skin test must therefore be interpreted with care.
Much debate surrounds the use of BCG vaccine, a matter of some importance, considering that TB kills around 3 million people annually and that drug-resistant strains have emerged. Although the vaccine has demonstrated some efficacy in preventing childhood TB, it has little prophylactic effect against postprimary TB in those already infected. One solution is to bring forward the BCG immunization to include neonates. Immunization at 2-4 weeks of age will ensure that immunization precedes infection, and also negates the requirement for a skin test. Passive, acquired maternal antibody to TB is unlikely to interfere with the effectiveness of the immunization as immunity relates primarily to a cell-mediated response. Alternative strategies involve improvement of the vaccine, possibly through the introduction into the BCG strain of genes that encode protective antigens of M. tuberculosis.
The current UK policy (introduced in September 2005) is to reserve the BCG vaccine for those considered to be at highest risk based on location or familial links to TB endemic areas. As such, this targeted approach will seek to immunize all infants in areas where the incidence of TB is 40 or more per 100 000; infants with at least one parent or grandparent who was born in a country with a TB incidence of 40 or more per 100 000; previously unvaccinated new immigrants from high-prevalence countries for TB; those who have lived in the same household or had prolonged, close contact with someone with TB; or atrisk workers (doctors, nurses, social workers, etc.).
6.4 Diphtheria, tetanus and a cellular pertussis (DTaP) immunization
Immunization against these three unrelated diseases is considered together because the vaccines are nonliving and are often co-administered as a triple vaccine as part of the childhood vaccination programme.
6.4.1 Diphtheria
This is an acute, non-invasive infectious disease associated with the upper respiratory tract (Chapter 7). The incubation period is 2-5 days although the disease remains communicable for up to 4 weeks. A low molecular weight toxin is produced which affects the myocardium, nervous and adrenal tissues. Death results in 3-5% of infected children. Diphtheria immunization stimulates the production of an antitoxin which protects against the disease but not against infection/colonization of the respiratory tract. The immunogen is a toxoid, prepared by formaldehyde treatment of the purified toxin (Chapter 24) and administered while adsorbed to an adjuvant, usually aluminium phosphate or aluminium hydroxide. The primary course of diphtheria prophylaxis consists of three doses starting at 2 months of age and separated by an interval of at least 1 month. The immune status of adults may be determined by administration of Schick test toxin, which is essentially a diluted form of the vaccine.
6.4.2 Tetanus
Tetanus results from the production of a toxin by germi-nating spores and vegetative cells of Clostridium tetani that can potentially infect deep wounds. The organism, which may be introduced into the wound, grows anaero-bically at such sites. The toxin is adsorbed into nerve cells and has a profound effect on nerve synapses resulting in spastic paralysis in affected individuals (Chapter 7). Mortality rates are highest in individuals over 60 years of age and the unvaccinated. Tetanus immunization employs a toxoid and protects by stimulating the production of antitoxin. This antitoxin will neutralize the toxin as the organisms release it and before it can be adsorbed into nerves. As the toxin is produced only slowly after infection, the vaccine, which acts rapidly, may be used prophylactically in non-immunized individuals who have recently suffered a high-risk injury. The toxoid, as with diphtheria toxoid, is formed by reaction with formaldehyde and is adsorbed onto an inorganic adjuvant. The primary course of tetanus vaccination consists of three doses starting at 2 months of age and is separated by an interval of at least 1 month.
6.4.3 Pertussis (whooping cough)
Whooping cough is caused by the non-invasive respiratory pathogen Bordetella pertussis (Chapter 7). This disease may be complicated by bronchopneumonia, or by repeated post-tussis vomiting leading to weight loss and to cerebral hypoxia associated with a risk of brain damage. Until the mid 1970s the mortality from whooping cough was about 1 per 1000 notified cases, with a higher rate for infants under 1 year of age. A full course of vaccine now consists of highly purified selected components of the Bordetella pertussis organism (i.e. acellular pertussis) which are treated with formaldehyde or glutaraldehyde and absorbed on to aluminium phosphate or aluminium hydroxide adjuvants. This vaccine gives considerably lower incidence of local and systemic reactions in comparison to the whole-cell pertussis vaccine that preceded it and gives comparable protection (> 80%). The primary course of pertussis prophylaxis consists of three doses starting at 2 months of age and separated by an interval of at least 1 month.
6.4.4 DTaP vaccine combinations and administration
The primary course of DTaP protection consists of three doses of a combined vaccine, each dose separated by at least 1 month and commencing not earlier than 2 months of age. In such combinations, the pertussis component of the vaccine acts as an additional adjuvant for the toxoid elements. Tetanus and diphtheria vaccines are also available in a combined tetanus/diphtheria/inactivated polio vaccine (Td/IPV) vaccine.
The primary course of pertussis vaccination is considered sufficient to confer lifelong protection, especially as the mortality associated with disease declines markedly after infancy. The risks associated with tetanus and diphtheria infection however persist throughout life. The Td/IPV vaccination is therefore repeated before school entry, at 4-5 years of age, and once again at puberty.
6.5 Immunization against bacteria associated with meningitis
6.5.1 Meningococcal immunization
Meningococcus (Neisseria meningitidis) is a bacterium that exclusively colonizes and/or infects humans; there is no animal reservoir. It is present as part of the normal microbiota of the pharynx in approximately 10% of individuals but can rarely spread through the bloodstream and to the brain through poorly understood mechanisms, causing meningitis and septicaemia. These are life-threatening, systemic infections; overall mortality from meningococcal disease is approximately 10%, assuming symptoms are recognized and treatment is commenced without delay, but rises considerably if there are delays. Diagnosis is therefore considered a medical emergency. At least 12 subtypes or serogroups of meningococcus have been identified, but groups B and C account for the majority of cases in Europe and the Americas. In the UK, group B accounts for approximately two-thirds of reported cases with group C accounting for the remaining third. Neisseria meningitidis group A does not normally cause disease in the UK but is an endemic cause of meningitis in other parts of the world, particularly sub-Saharan Africa in an area between Senegal (West Africa) to Ethiopia (East) that has been termed the ‘meningitis belt’. It is believed that reasons for the hyperendemic inci-dence of group A meningococcal meningitis in this part of the world include high incidence of upper respiratory tract infections during the dry (dusty) season, combined with overcrowding, migration and pilgrimages.
Whilst group C meningitis is most common in the under 1-year-old group in the UK, mortality is highest in adolescents. Although meningitis accounts for the majority of invasive meningococcal disease, in 15-20% of cases septicaemia predominates and is associated with significantly higher mortality. There is currently no vaccine available for group B meningococcus but vaccines are available for groups A and C. As with the Hib vaccine (see below), the preparations are intended to invoke protective immunity towards the polysaccharide component of the bacterium. Early vaccines, composed of purified polysaccharide, worked in adults but had poor efficacy in infants —the most at-risk group. The new MenC conjugate vaccine comprises capsular polysaccharide components conjugated to a carrier protein (usually diphtheria or tetanus toxoid). This vaccine is effective in the very young and is therefore suitable for protecting infants. The vaccine is normally administered along with DTaP and Hib at 3, 4 and 12 months and a single dose is sufficient to immunize individuals over 12 months of age; it has also been used to provide prophylaxis for teenagers, adolescents and young adults. Group A vaccine is available for those travelling to areas of the world where the infection is epidemic.
6.5.2 Haemophilus influenzae type b (Hib) immunization
Haemophilus influenzae can cause infections ranging from bronchitis and otitis media to life-threatening, invasive disease (meningitis and bacteraemia). Invasive infections, which are most common in young children, are normally caused by encapsulated strains of the bacterium that can be serologically differentiated into six typeable capsular serotypes (a-f). Before the introduction of vaccination, H. influenzae type b (Hib) was the most prevalent of these in invasive disease. Non-invasive haemophilus disease is most often caused by non-encapsulated strains that are not amenable to typing based on capsular serology. Although the most common form of invasive Hib disease is meningitis, accounting for 60% of cases, Hib can also cause other infections, including pneumonia and pericarditis.
The fatality rate for treated Hib meningitis infections is approximately 5% and complications include deafness and intellectual impairment (in c. 10% of cases). Hib often forms part of the normal microbiota of the nasopharynx in healthy individuals and the frequency of carriage before the Hib vaccine was introduced was approximately 4 in every 100 for preschool children. Carriage of this bacterium is now rare because of the effectiveness of the Hib vaccination. Hib meningitis is rare in children under 3 months and peaks in its incidence at around 10-11 months of age; infection is uncommon after 4 years of age. Before the introduction of Hib vaccination the incidence of the disease in the UK was estimated at 34 per 100 000.
The vaccine utilizes purified preparations of the polysaccharide capsule of the major serotypes of the bacterium associated with disease. Polysaccharides are poorly immunogenic and must be conjugated onto a protein carrier (diphtheria or tetanus toxoids) to enhance their efficacy. The Hib vaccine is given as part of a combined product of H. influenzae type b, diphtheria, tetanus, acel-lular pertussis and inactivated polio vaccine (DTaP/IPV/ Hib), or as the Hib/MenC conjugate vaccine.
6.5.3 Pneumococcal vaccination
Streptococcus pneumoniae (pneumococcus) is an encapsulated Gram-positive coccus. As with meningococcus and Hib, the capsule is an important virulence factor for this bacterium (non-capsulated strains are normally avirulent). Many different capsular types have been charac-terized but approximately 66% of the serious infections in adults and 80% of invasive pneumococcal infections in children are caused by about 10 capsular types. Pneumococci are often part of the normal microbiota of the nasal cavity and associated tissues but commensal strains are often avirulent. Conversely, invasive infections (i.e. meningitis and septicaemia) are often caused by strains not considered to be normal commensals. S. pneumoniae is a versatile pathogen which can cause sinusitis or otitis media (infections of the sinuses or middle ear). The bacterium may also cause deep lung infections (pneumonia), which accounts for its species name, and is also capable of causing systemic infections including bacteraemic pneumonia, bacteraemia and meningitis. The incidence of infection by pneumococci is highest in the winter, and transmission by aerosols or direct contact with respiratory secretions is believed to require either frequent or prolonged close contact. Two distinct vac-cines have been developed to provide protection against this bacterium: the pneumococcal polysaccharide vaccine (PPV) which contains purified capsular polysaccharide from 23 capsular types of bacterium and the pneumococcal conjugate vaccine (PCV) which comprises capsular polysaccharides from 7 common capsular types. Importantly, PCV is conjugated to protein in a manner similar to the Haemophilus influenzae type b (Hib) and MenC vaccines. While PPV is an effective vaccine in adults, the effectiveness of pneumococcal prophylaxis is considerably improved in children by protein conjugation; PCV is immunogenic in children and the childhood vaccination schedule recommends that doses be given at 2 and 4 months of age with a booster at 13 months. The PPV vaccine is recommended for adults over 65 years and atrisk groups aged 2 years or over. It will provide additional prophylaxis to individuals who have already received the PCV vaccine because it protects from additional serotypes.
6.6 Human papillomavirus (HPV) vaccination
Human papillomaviruses (HPV) are a group of viruses that infect squamous epithelia, including the skin and mucosal surfaces of the upper respiratory and anogenital tracts. Approximately 100 types of HPV have been identified, of which around 40 infect the genital tract. The majority of genital infections are asymptomatic but in some cases may be associated with genital warts and (the reason for the development of the HPV vaccine), cervical cancer. The time between infection and development of cancers may range from 12 months to over 10 years. It is important to note that HPV is associated with genital and anal cancers in both men and women and also with cancers of the mouth and throat. Importantly, not all types of HPV are carcinogenic; the risk of cancer development associated with different strains is variable and HPV viruses that cause warts (a common sexually transmitted infection) may be low risk for carcinogenicity and in some cases strains are not considered carcinogenic. Genital HPV infections are transmitted primarily through sexual intercourse with infected individuals and the use of condoms reduces the risk of sexual transmission. HPV may also be transmitted vertically from mother to child. Persistent HPV infection with HPV 16 and HPV 18 (high-risk strains) is associated with the majority of cer-vical cancers. There are however, strains other than HPV 16 and HPV 18 that are carcinogenic.
The HPV vaccine comprises recombinant subunits expressed in yeast or in cells of insect origin such that the vaccine contains non-infectious, virus-like particles. The current UK vaccine, Cervarix, affords protection against HPV 16 and HPV 18 and is very effective at preventing precancerous lesions associated with these virus strains for at least 6 years, probably longer. Since the main aim of the HPV vaccine programme is to reduce the incidence of cervical cancer, the vaccine is currently administered to females (not males) between the age of 12 and 13 with a catch-up programme for older females up to the age of 18.
7 The UK routine childhood immunization programme
The timing of the various components of the childhood vaccination programme is subject to continual review. In the 1960s, the primary course of DTP vaccination consisted of three doses given at 3, 6 and 12 months of age, together with OPV. This separation gave adequate time for the levels of induced antibody to decline between successive doses of the vaccines. Current recommendations (Table 10.1) accelerate the vaccination programme with no reductions in its efficacy. Thus, the MMR vaccination has replaced separate measles and rubella prophylaxis and BCG vaccination may now be given at birth, but only for infants living in areas of the UK where the annual incidence of TB is 40 per 100 000 or greater and those with familial or other links to high-risk countries. DTaP vaccination occurs at 2, 3 and 4 months to coincide with administration of Hib and IPV. It is imperative that as many individuals as possible benefit from the vaccination programme. Fewer visits to the doctor’s surgery translate into improved patient compliance and less like-lihood of epidemic spread of the diseases in question. The current recommendations minimize the number of separate visits to the clinic while attempting to maximize the protection generated.
Table 10.1 Schedule for the UK’s routine childhood immunizations (2009)
Adapted from Salisburyetal. (2006).
The BCG vaccine (for TB prophylaxis) is now administered only to children considered to be at increased risk of developing severe disease and/or of exposure to TB infection.
The table is intended as a general guide to the vaccines used. Policies can and do change. For definitive information, see the UK Department of Health website: (http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_120973.pdf) Accessed 5 November 2010.
| Vaccines given | Administration | Timing |
| Diphtheria, tetanus, pertussis, polio and Hib (DTaP/IPV/Hib) | One injection | 2 months old |
| Pneumococcal conjugate vaccine (PCV) | One injection | |
| Diphtheria, tetanus, pertussis, polio and Hib (DTaP/IPV/Hib) | One injection | 3 months old |
| Meningococcal MenC | One injection | |
| Diphtheria, tetanus, pertussis, polio and Hib (DTaP/IPV/Hib) | One injection | 4 months old |
| Meningococcal (MenC) | One injection | |
| Pneumococcal conjugate vaccine (PCV) | One injection | |
| Hib/MenC | One injection | 12 months old |
| Measles, mumps and rubella (MMR) | One injection | c. 13 months old |
| Pneumococcal conjugate vaccine (PCV) | One injection | |
| Diphtheria, tetanus, pertussis and polio (DTaP/IPV or (Td/IPV) | One injection | 3 years 4 months to 5 years old |
| Measles, mumps and rubella (MMR) | One injection | |
| Human papilloma virus (HPV) | Three injections; over a c. 6 month period | 12–13 years old (females only) |
| Tetanus, diphtheria (Td/IPV) | One injection | 13–18 years old |
8 Immunization of special risk groups
While not recommended for routine administration, vaccines additional to those represented in the childhood programme are available for individuals in special risk categories. These categories relate to occupational risks or risks associated with travel abroad. Such immunization protocols include those directed against cholera, typhoid, meningitis (group A), anthrax, hepatitis A and B, influenza, Japanese encephalitis, rabies, tick-borne encephalitis and yellow fever.
The authors acknowledge the contributions of Peter Gilbert (1951-2008) who was a coauthor on previous versions of this chapter. Much of the information in this chapter was originally obtained from Salisburyetal. (2006 and earlier versions); an authoritative source of information about the composition and schedules for current UK vaccines and vaccination policies.
10 References and further reading
Department of Health. Immunisation. (regularly updated). http://www.dh.gov.uk/en/Publichealth/Healthprotection/Immunisation/index.htm
Mims, C.A., Nash, A. & Stephen, J. (2001) Mims ’ Pathogenesis of Infectious Disease, 5th edn. Academic Press, London.
Salisbury, D.M., Ramsay, M. & Noakes K. (2006) Immunization Against Infectious Disease. The Stationery Office, London (frequently updated).
Salyers, A.A. & Whitt, D.D. (1994) Bacterial Pathogenesis: A Molecular Approach. ASM Press, Washington.
< div class='tao-gold-member'>



