A thin basement membrane separates the urothelium from the underlying lamina propria. The latter is formed of abundant connective tissue containing a rich vascular network, lymphatic channels, sensory nerve endings, and a few elastic fibers. The lamina propria varies in thickness in the empty versus the distended bladder but is generally thinner in the areas of the trigone and bladder neck. It is important to note that wisps or small fascicles of smooth muscle may be found within the superficial lamina propria, either isolated or forming a complete or incomplete muscularis mucosae (2,4). These superficial muscle fascicles must not be confused with the smooth muscle bundles of the muscularis propria because this might result in errors of tumor staging and treatment. More recently, studies have described a “hyperplastic” variant of muscularis mucosae, defined as muscle bundles within the lamina propria that are more than four layers in thickness, thus mimicking muscularis propria (5,6). These bundles may be arranged haphazardly in which individual fascicles are oriented in different directions or may have a compact arrangement with smooth outlines. When markedly thickened (hyperplastic), this pattern can easily be confused with or may be impossible to distinguish from muscularis propria. Although controversial, some authors have suggested that the smooth muscle of the muscularis mucosae and muscularis propria stain differently for smoothelin (7,8). It is also important to note that one may encounter fat within the lamina propria as well as in the muscularis propria (8,9). Fat may be found in bladders that have never been instrumented or in areas of prior transurethral resection. Whether its presence is as a result of a normal anatomic variant, a function of the patient’s body habitus, or a metaplastic phenomenon is not known. It is important to keep in mind that the normal anatomy of the bladder wall, particularly of the lamina propria and even the superficial half of the muscularis propria, may be disrupted at the site of prior biopsy. The normal muscularis propria has loosely anastomosing, ill-defined internal and external longitudinal layers and a more prominent middle circular layer of muscle. In the bladder neck of the male, the fascicles of the muscularis propria are continuous with the fibromuscular tissue of the prostate, whereas in the female they are continuous with the muscle fibers in the wall of the urethra (3). As mentioned, fat may be present between the muscle bundles of the muscularis propria. The outermost layer of the viscus is an adventitia of connective tissue; only the superior surface is covered by serosa of the pelvic peritoneum. The renal pelvis and ureters have a similar structure of urothelium, lamina propria, and, in the ureter, a relatively thick muscularis, which increases gradually from proximal to distal ureter.
The main blood supply to the bladder comes from branches of the internal iliac arteries; the superior vesical arteries supply the anterior and superior aspects of the bladder, whereas the inferior vesical arteries supply the bladder base (10). The veins of the urinary bladder form the vesical venous plexuses and drain mainly into the internal iliac veins. The lymphatic channels of the superior regions of the bladder drain into the external iliac lymph nodes. The lymphatic drainage of the ureters is complex. The upper portion drains into lateral aortic lymph nodes similar to the renal pelvis. The middle portion drains into the common iliac lymph nodes, whereas the inferior portion drains into the common, external, or internal iliac lymph nodes (10). The urethral lymphatics drain into sacral and internal iliac lymph nodes.
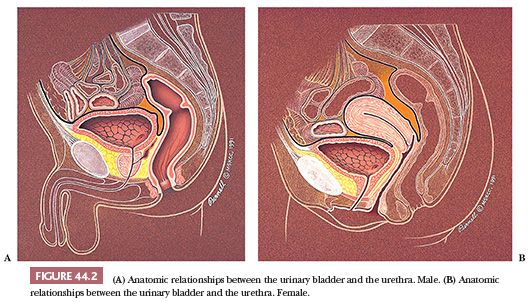
The adult empty bladder has the shape of a four-sided inverted pyramid and is enveloped by the vesical fascia (10). The superior surface faces superiorly and is covered by the pelvic parietal peritoneum. The posterior surface, also known as the base of the bladder, faces posteriorly and inferiorly. It is separated from the rectum by the uterine cervix and the proximal portions of the vagina in females and by the seminal vesicles and the ampulla of the vasa deferentia in males (Fig. 44.2A,B). These posterior anatomic relationships are very important clinically. Because most vesicle neoplasms arise in the posterior wall adjacent to the ureteral orifices, invasive tumor may extend into adjacent soft tissue and organs. The intimate anatomic relationships of these organs explain why hysterectomy and partial vaginectomy are routine at the time of radial cystectomy in women. Conversely, seminal vesicle and prostatic stromal involvement is a poor prognostic sign in male bladder carcinoma, a reflection of high pathologic stage.
The bladder bed (structures on which the bladder neck rests) is formed posteriorly by the rectum in males and the vagina in females. Anteriorly and laterally, it is formed by the internal obturator and levator ani muscles as well as the pubic bones. These structures may be involved in advanced tumors occupying the anterior, lateral, or bladder neck regions and render the patient inoperable.
The most anterosuperior point of the bladder is known as the apex and is located at the point of contact of the superior surfaces and the two inferolateral surfaces. The apex marks the point of insertion of the median umbilical ligament and, consequently, is the area where urachal carcinomas occur.
VARIANTS OF NORMAL HISTOLOGY
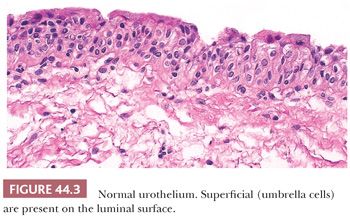
We have stated that urothelium lines the internal surface of the bladder wall. Assuming a perpendicular cut, it is flat and can be up to seven cells thick, depending on its state of distention. It is composed of a basal cell layer immediately above the basement membrane, several layers of polyhedral cells with their long axis perpendicular to the basement membrane, and a superficial layer of umbrella cells (Fig. 44.3). Nevertheless, a very important feature of this epithelium is its ability to vary its morphologic appearance, most likely as a reaction to a local stimulus (usually some sort of injury). The end result is that urothelium may take on a host of benign morphologic features, which are so common that they are considered variants of normal histology. The most common reactive proliferative change within the urothelium is the formation of Brunn nests (Fig. 44.4A), which represent invaginations of the surface urothelium into the underlying lamina propria (11–14). Basal and intermediate cells are readily identifiable. In some cases, these solid nests of benign-appearing urothelium may lose continuity with the surface, becoming isolated within the superficial lamina propria. The term cystitis cystica (Fig. 44.4B) has been coined to describe when the nests become cystically dilated, acquiring a luminal space. In this setting, the umbrella cell layer may take on a cuboidal or columnar appearance. In some cases, the epithelial lining undergoes glandular metaplasia, giving rise to what is called cystitis glandularis (15). The cells become cuboidal or columnar and mucin-secreting. If the epithelium acquires intestinal-type features characterized by the presence of goblet cells, this variant is called cystitis glandularis with intestinal metaplasia. Additional description of this lesion is given elsewhere in this text.
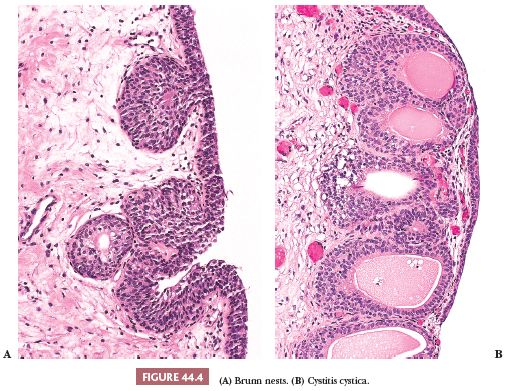
Brunn nests, cystitis cystica, and cystitis glandularis represent a continuum of proliferative or reactive changes seen along the entire urothelial tract, and it is common to see all three in the same specimen. Most investigators believe they occur as a result of local inflammatory insult (11,12). Nevertheless, these proliferative changes are seen in the urothelium of patients with no evidence of local inflammation; therefore, it is possible that they also represent normal histologic variants or the residual effects of an old inflammatory process (13,14).
Much has been written about the association of Brunn nests, cystitis cystica, and especially cystitis glandularis and urothelial carcinoma (11–18). The high incidence of these proliferative changes in normal bladder suggests they are not premalignant changes and that there is no cause–effect relationship between their presence and the appearance of bladder cancer. It is true that one or all of these changes are commonly present in biopsy specimens containing bladder cancer, but the coexistence may be coincidental. Alternatively, cancer itself may be producing the local inflammatory insult that causes them and not vice versa. The fact that exceptional cases may occur in which carcinoma clearly arises within the epithelium of these reactive lesions does not alter this argument (17,18).
METAPLASIA OF THE UROTHELIUM
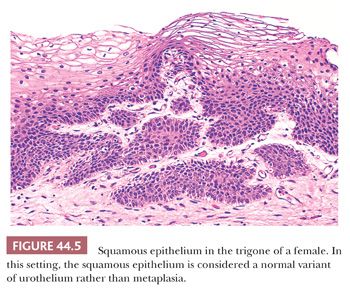
Metaplasia refers to a change in morphology of one cell type into another type, which is considered aberrant (divergent) for that location. The urothelium frequently undergoes either squamous or glandular metaplasia, presumably as a response to chronic inflammatory stimuli as urinary tract infection, calculi, diverticula, or frequent catheterization (12,14). Squamous differentiation, particularly in the area of the trigone, is a common finding in women and is responsive to estrogen levels in blood. This squamous epithelium is characterized by abundant intracytoplasmic glycogen and lack of keratinization, making it histologically similar to vaginal or cervical squamous epithelium (Fig. 44.5), and is likely to represent a normal histologic variant unassociated to local injury. More details regarding metaplastic changes within the urothelium are described in the sections dealing with glandular and squamous lesions.
INFLAMMATORY DISORDERS
The many organisms that may cause cystitis include bacteria, viruses, fungi, and protozoa. Cystitis also may be secondary to calculus or other local trauma, radiation, or chemotherapy (19). The majority of cases of both acute and chronic cystitis show nonspecific histologic features of inflammation and do not require description here, but there are several inflammatory processes that can be distinguished by their clinical or pathologic features.
Tuberculous cystitis tends to involve the bladder adjacent to the ureteral orifices, consistent with the observation that the majority of cases are associated with—and presumably secondary to—renal tuberculosis. It is now a rare disease in most countries. Histologically, one sees caseating granulomas with Langerhans giant cells mostly in the lamina propria and often with mucosal ulceration. Appropriate stains should reveal the presence of acid-fast bacilli (20,21). Noncaseating granulomas are commonly seen after intravesical therapy bacillus Calmette-Guérin (BCG), but rarely do special stains reveal the presence of acid-fast organisms. A nonspecific granulomatous inflammation with foreign body giant cells can also be seen following transurethral biopsies (see “Granulomatous Cystitis Secondary to Bacillus Calmette-Guérin Therapy or Transurethral Biopsy” in the following section).
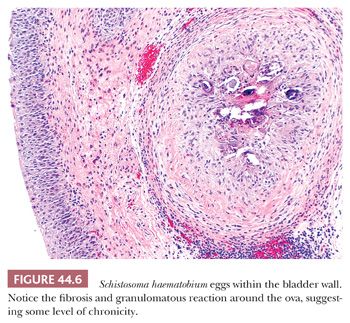
Schistosomal cystitis is common in the Nile Valley and sub-Saharan Africa but is rarely seen in the United States. It is usually caused by infestation with Schistosoma haematobium and rarely with Schistosoma mansoni. The ova are deposited in veins of the muscularis propria of the bladder where they degenerate and become permeable. Eosinophilic material (antigen–antibody complex) surrounds the ovum and incites a marked inflammatory response characterized initially by necrosis and a pleocellular inflammatory reaction rich in eosinophils (21,22). The overlying epithelium may become ulcerated. As the process becomes chronic, it is characterized by perioval fibrosis with surrounding lymphocytes, histiocytes, and giant cells forming a foreign body granulomatous reaction (Fig. 44.6). Dystrophic calcifications are frequent. The overlying epithelium may become hyperplastic or undergo proliferative changes of cystitis cystica, cystitis glandularis, or squamous metaplasia (18). Patients with chronic schistosomal cystitis have a high incidence of carcinoma of the bladder (see “Squamous Lesions Including Squamous Carcinoma” later in this chapter) (22–24).
Polypoid cystitis refers to an exophytic, inflammatory lesion for the most part causally related to the presence of indwelling catheters (25). It is seen mostly on the dome and posterior wall of the bladder and is characterized histologically by normal or mildly hyperplastic urothelium overlying a congested, chronically inflamed, and markedly edematous stroma (26,27). Discreet papillary structures are absent. Although these exophytic lesions may mimic a papillary neoplasm grossly, they can be easily distinguished histologically as an inflammatory pseudopolyp. Cold cup biopsies of the bladder wall may mechanically generate a pseudopolypoid appearance to the tissue sample. If the epithelium or underlying bladder wall is inflamed at this site, the sample may be erroneously interpreted as polypoid cystitis.
Eosinophilic cystitis is a rare process presenting clinically with episodes of marked urinary frequency, dysuria, and frequently with gross hematuria (28–31). The cystoscopic appearance in adults is often suspicious for carcinoma because of nodular or mass-like lesions that may be hyperemic, ulcerated, or frankly necrotic (32). Histologically, the inflammatory changes are acute, chronic, or both. The acute stage is characterized by edema, congestion, and an inflammatory infiltrate containing eosinophils and lymphocytes. The eosinophilic infiltrate is more prominent in the cases that have muscle necrosis. The chronic stage is characterized by fewer or absent eosinophils and by fibrosis in the lamina propria and interspersed among the superficial muscle layers. The overlying epithelium may undergo proliferative changes (Brunn nests or cystitis cystica) or squamous metaplasia (32).
Eosinophilic cystitis is most commonly encountered in children and women and is often associated with allergic disorders and peripheral blood eosinophilia (29,30,32). It also may affect elderly men and in these cases is often associated with the trauma of transurethral resection of the prostate or bladder, concomitant bladder cancer, and so on.
Interstitial cystitis (Hunner ulcer) also is a rare and poorly understood inflammatory process that is of possible autoimmune origin. It usually affects middle-aged women (33). The clinical symptoms are frequently severe and disabling and include urinary frequency, urgency, dysuria, and hematuria as well as perineal or suprapubic pain (34). Cystoscopy is required to establish the diagnosis. It may reveal a distensible bladder with characteristic pinpoint petechial hemorrhages and one or several ulcerations, which may be present anywhere in the bladder but are not a prerequisite for the diagnosis. In other cases, there may be a contracted, nondistensible bladder and a Hunner ulcer (35). Histologically, there is edema and congestion of the lamina propria and in a few cases either ulcerated or denuded mucosa. Along with a nonspecific inflammatory infiltrate, one should observe numerous mast cells, not only in the lamina propria but also throughout the muscularis propria (36,37). Patchy fibrosis of the lamina propria or superficial muscularis propria may be encountered. The diagnosis of interstitial cystitis can only be made after other causes have been ruled out and repeated urine cultures for bacteria, fungi, and viruses are negative.
Follicular cystitis is a term that has been used to describe the presence of lymphoid follicles with germinal centers in the wall of the urinary bladder (38). The term is a misnomer because it does not necessarily indicate inflammation. On the other hand, it does sometimes follow infection, repeated transurethral biopsies, or the instillation of intravesical chemotherapeutic agents or BCG.
Pyelitis and ureteritis are usually nonspecific and may occur as a consequence of renal lithiasis or lower urinary tract obstruction.
Urethritis is more commonly encountered in men than women and usually secondary to Neisseria gonorrhea or other sexually transmitted organisms including Chlamydia and Mycoplasma. Gonococcus, Chlamydia, and Trichomonas are common causes of urethritis in women (39).
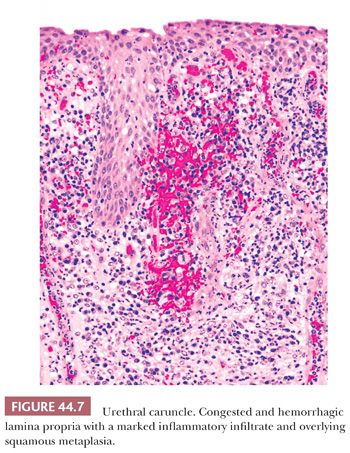
Urethral caruncles are small, highly vascular, usually polypoid inflammatory lesions commonly seen in elderly women. They may be painful and associated with hematuria. Histologically, caruncles usually resemble small hemangiomas, often with intense acute and chronic inflammatory cellular infiltrate. The overlying urothelium may be hyperplastic, ulcerated, or exhibit squamous or glandular metaplasia (Fig. 44.7) (40).
Urethral valves occur more frequently in male than in female patients. They may be asymptomatic but generally cause some degree of obstruction, inflammation, and hematuria. Posterior urethral valves are usually associated with bladder neck hypertrophy (41).
GRANULOMATOUS CYSTITIS SECONDARY TO BACILLUS CALMETTE-GUÉRIN THERAPY OR TRANSURETHRAL BIOPSY
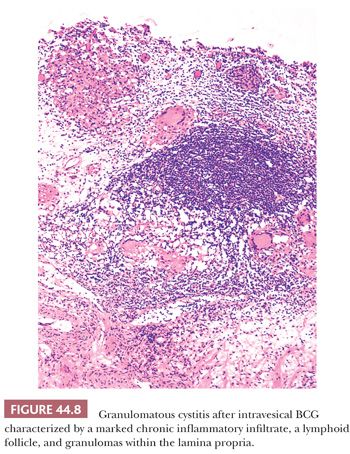
Urothelial carcinoma in situ (CIS) and high-risk papillary carcinomas are commonly treated with intravesical installations of BCG that usually induce an intense inflammatory reaction (BCG cystitis) (12,13,15,16). It is characterized by the presence of discrete, noncaseating granulomas containing epithelioid histiocytes and multinucleated giant cells. Rarely do special stains reveal the presence of acid-fast organisms (Fig. 44.8). The granulomas usually are situated in the superficial third of the lamina propria and are associated with an intense lymphocytic infiltrate. The overlying urothelium may show nonspecific reactive atypia or may be partially or entirely denuded. Urine cytology commonly reveals inflammatory cells, including epithelioid histiocytes and occasional giant cells (16). Many patients undergoing this type of therapy become quite symptomatic, developing dysuria and occasionally hematuria. In severe cases, they may develop regional or even systemic lymphadenopathy as well as fever and chills. In these cases, biopsy of the enlarged lymph nodes may demonstrate a granulomatous lymphadenitis.
A nonspecific granulomatous inflammation with foreign body giant cells can also be seen following transurethral resections (42,43). In severe cases, this procedure may lead to the development of necrotizing palisading granulomas. The necrotic center is surrounded by epithelioid histiocytes with occasional foreign body–type giant cells. Depending on the extent of the resection, the surrounding bladder wall is fibrotic with obliteration of the normal anatomic landmarks. If the epithelioid histiocytes and giant cells are distorted as a result of thermal artifact introduced by the transurethral resection, they may be mistaken for invasive carcinoma. Conversely, thermal artifact may mask microscopic foci of invasive disease. Immunohistochemistry (IHC) using epithelial markers may aid in the differential diagnosis.
RADIATION CYSTITIS
Radiation cystitis may be acute or chronic and can occur any time the bladder is included in the treatment field. The clinical severity and histologic features of radiation cystitis are both time- and dose-dependent; 5% of patients receiving 60 Gy to the bladder will develop late clinical symptoms of radiation cystitis, whereas 50% of patients suffer the same fate if the dose were 70 Gy (44). Clinically, the acute symptoms of radiation cystitis may appear as early as 4 to 6 weeks after initiation of therapy, whereas late symptoms appear as much as 10 years later (45). The toxic effects of irradiation are enhanced if administered in conjunction with cyclophosphamide (46).
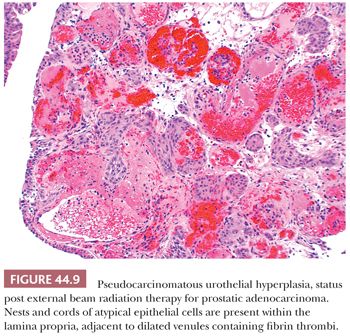
Microscopically, the early changes are characterized by marked edema and hyperemia. The edema produces thickened mucosal folds, resulting in a characteristic gross appearance termed radiation cystitis by Koss (47). These changes may be accompanied by desquamation and, rarely, superficial ulceration of the bladder epithelium (48). At this stage, the urothelium can take on very atypical cytologic features mimicking and sometimes indistinguishable from CIS, although mitotic activity is rare. The cells may become enlarged with prominent, hyperchromatic nuclei. However, the altered epithelial cells are typically more bizarre than cells of CIS, with giant cells and multinucleated cells that lack the crisp nuclear detail of nonirradiated cells. Squamous metaplasia may be evident. In many cases, there are epithelial cords and nests that extend into the lamina propria. They are seen adjacent to dilated vascular structures, which may contain fibrin. Given the presence of cytologic atypia, it would be easy to confuse these findings with invasive carcinoma (49–51). The term pseudocarcinomatous hyperplasia is used to describe these changes (Fig.44.9). It is said that these changes may disappear over time (52), although we have observed them many years after completion of therapy. Rarely, pseudocarcinomatous hyperplasia may be encountered in the absence of prior radiation therapy, likely due to another type of chronic local injury. Great care must be taken in making this diagnosis because radiation may be associated with an increased risk of developing urothelial carcinoma, particularly in patients previously treated for other pelvic tumors such as rectal adenocarcinoma and prostate cancer.
The late radiation-induced changes include collagenization of the lamina propria and muscular fibers, myointimal proliferation or hyalinization of the media of arterioles, and often ulceration with abundant fibrinous exudate. Atypical fibroblasts are invariably present in the scarred lamina propria. The urothelium may be atrophic or hyperplastic and still exhibit focal radiation-induced atypia (45).
HEMORRHAGIC CYSTITIS
In the late 1950s, systemic administration of cyclophosphamide was found to cause hemorrhagic cystitis. At times, hematuria could be massive and uncontrollable, sometimes requiring cystectomy (Fig.44.10A). Phillips et al. (53) showed that the tissue damage which led to hemorrhagic cystitis was caused by a topical effect of the metabolic byproducts of cyclophosphamide that were excreted through the kidney. The occurrence of hemorrhagic cystitis appears to be unrelated to dose and was reported in roughly 8% of patients receiving the drug (54,55). Currently, patients are treated with forced fluids and the incidence of hemorrhagic cystitis has been sharply reduced. More recently, another alkylating agent, busulfan, has been implicated as a rare cause of hemorrhagic cystitis (56).
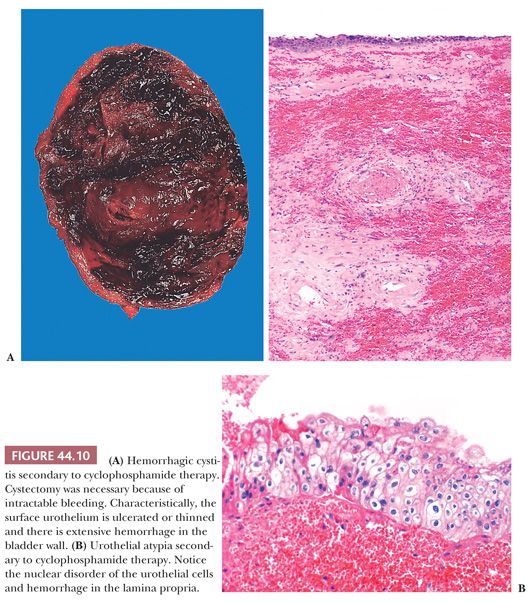
Microscopically, the bladder is characterized by marked edema and hemorrhage throughout the lamina propria, with extensive ulceration and an associated fibrinopurulent exudate. Where not ulcerated, the epithelium may be thinned and atypical. During the regenerative stage, macrophages and fibroblasts populate the lamina propria, whereas the overlying epithelium exhibits an increased mitotic rate, increased thickness, and marked atypia (53,55). Intravesical installation of chemotherapeutic agents such as thiotepa and mitomycin C are also able to induce cytologic atypia (which can be confused with CIS), including cytomegaly, pleomorphism, and hyperchromasia (Fig. 44.10B) (57). These changes usually are limited to the superficial cells and mitotic activity is not evident. Clinical trials evaluating the efficacy of gemcitabine, another intravesical chemotherapeutic agent, are ongoing. We have found that it induces similar morphologic changes that mimic carcinoma.
ENDOMETRIOSIS, ENDOCERVICOSIS, AND MÜLLERIANOSIS
Although most glandular lesions involving the urinary bladder arise through a process of metaplasia of the surface urothelium, that is not the case with these types of lesions. Genitourinary involvement by endometriosis occurs in 1% to 2% of cases. More than 200 cases of vesical endometriosis have been described, making the urinary bladder the most common site of involvement within the urinary tract (42,43,58). Classically, it affects women between the second and fifth decades of life but rarely may be seen in postmenopausal women receiving exogenous estrogen (59). Interestingly, rare cases of vesical endometriosis have been described in men with prostate carcinoma who were receiving exogenous estrogen therapy (60,61). The occurrence in men is intriguing; most likely, it represents activation of müllerian rests by exogenous estrogens.
Clinically, patients present with urgency, frequency, suprapubic pain, and rarely hematuria. A mass is frequently apparent either by palpation or cystoscopic examination (62,63). The lesion may reside in the superficial or deep layers of the vesical wall or in the adjacent perivesical soft tissue; therefore, a simple transurethral biopsy does not always provide diagnostic tissue. Microscopically, the lesion resembles endometriosis elsewhere; endometrium-like glandular epithelium is present in association with endometrial stromal cells and recent or old hemorrhage. Rarely, one finds only glands or stroma. In the former case, the differential diagnosis must include infiltrating adenocarcinoma from which it is distinguished by their recognizable growth pattern characteristics and by the uniform and orderly glands of endometriosis.
Endometriosis may involve the lower third of the ureter where it may produce obstructive symptoms unilaterally. Endometriosis involving the urethra is extremely rare.
Endocervicosis of the urinary bladder is a rare condition first described as a distinct entity by Clement and Young in 1992 (64). It is seen in women of childbearing age who present with a mass in the posterior wall or posterior dome, usually associated with suprapubic pain, frequency, and dysuria. It is thought to arise from müllerian rests and is characterized by extensive involvement of the bladder wall by benign or mildly atypical, columnar, mucin-secreting, endocervical-like glands. Extravasation of mucin is present in all cases secondary to gland rupture. Because endocervicosis of the bladder is commonly located deep within the viscus and associated with a mass, it must be distinguished from an invasive carcinoma clinically and from adenocarcinoma morphologically (65).
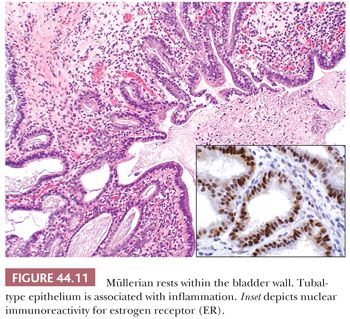
In 1996, Young and Clement (66) described müllerianosis of the urinary bladder. These rare lesions occur in the same clinical setting as endocervicosis, differing only in that the lesion may contain other benign müllerian-type components including endometrial or tubal epithelium (Fig.44.11). Once again, care must be taken not to confuse such lesions with adenocarcinoma (66–68).
MALACOPLAKIA
Malacoplakia is an unusual but distinctive type of inflammatory reaction of unknown etiology. The urinary bladder is the most common site of involvement, although it has been described in other genitourinary and nongenitourinary sites (69–71). On gross examination, malacoplakia presents as solitary or confluent yellow nodules or plaques. The larger nodules are centrally umbilicated or ulcerated and the adjacent mucosa is hyperemic. The lesions may be seen anywhere in the bladder and vary greatly in size and number. Cystoscopically, the nodules or plaques of malacoplakia may be confused with carcinoma. Histologically, the lesion involves primarily the lamina propria and is characterized by a well-demarcated, mixed inflammatory infiltrate in which epithelioid histiocytes predominate (Fig. 44.12A). These histiocytes have abundant eosinophilic or amphophilic cytoplasm within which are round or oval inclusions called Michaelis-Guttmann bodies (Fig. 44.12B). These inclusions may stain with hematoxylin and eosin (H&E) and represent concentrically laminated calcospherites, which stain for iron as well as calcium. The bodies are found within the interstitium and histiocytes. The typical nodule of epithelioid histiocytes often is surrounded by lymphoid aggregates with or without germinal centers. The overlying urothelium may be ulcerated, hyperplastic, or metaplastic. In long-standing lesions, the characteristic infiltrate is replaced by fibrosis and scarring.
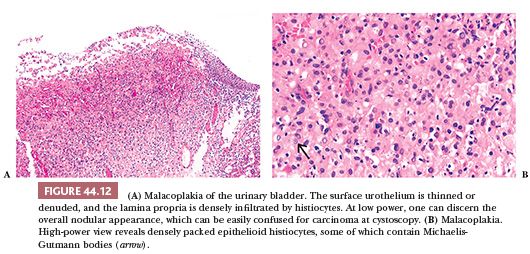
As mentioned, the etiology of malacoplakia remains unknown, although it is generally believed to represent a peculiar response to infection, perhaps the result of a disturbed immune response or abnormal macrophage or lysosomal activity in the host (72–75).
Malacoplakia has also been described in the renal pelvis, ureter, and urethra, the latter being the least frequent. Multifocal involvement along the entire urothelial tract also has been reported. In the ureter, it is often associated with obstruction (76,77).
AMYLOIDOSIS
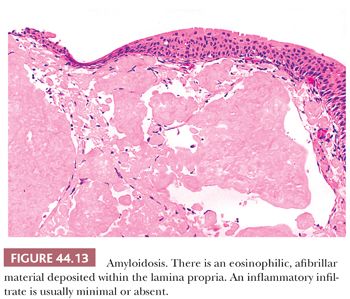
The bladder can be involved in cases of systemic amyloidosis but may be the primary site of this disease (78). The usual clinical presentation is that of hematuria. On cystoscopy, a localized amyloid tumor will be seen as an elevated mass, which can be confused with an invasive neoplasm. Less frequently, the disease may diffusely involve the bladder wall. Microscopically, the amyloid protein is an eosinophilic, afibrillar material deposited preferentially in the lamina propria and extending into the connective tissue surrounding muscle fascicles (Fig. 44.13) (79,80). Less frequently, and usually in systemic amyloidosis, there are perivascular amyloid deposits. Congestion and hemorrhage are common but inflammatory cells are scanty unless the overlying epithelium is ulcerated. A few fibroblast-like cells usually are interspersed within the eosinophilic material. Special stains such as Congo red, crystal violet, or van Gieson will help establish the diagnosis. Patients with localized lesions are usually successfully managed by transurethral resection, but patients with more diffuse involvement may require more radical surgery to control the bleeding (81).
In descending order of frequency, amyloid deposits have also been described in the ureter, renal pelvis, and urethra (82–85). At all sites, it is associated with hematuria and also may cause obstruction in the ureter and renal pelvis. It is important to remember that amyloid deposits may be associated with B-cell lymphoproliferative disorders.
DIVERTICULA
Bladder diverticula are relatively common, yet their etiology remains controversial. Most investigators agree that they occur secondary to increased intravesical pressure as a result of obstruction distal to the diverticulum (86–88). The obstruction brings about compensatory muscle hypertrophy and eventual mucosal herniation in areas of weakness (Fig. 44.14A). Others feel that at least some diverticula are a consequence of congenital defects in the bladder musculature; they cite as evidence cases of diverticula in young patients without evidence of obstruction (89,90). The most common sites of diverticula are adjacent to the ureteral orifices, the bladder dome (probably related to a urachal remnant), and the region of the internal urethral orifice. Grossly, one sees distortion of the external surface of the bladder. The diverticula may be widely patent but are usually narrow in symptomatic patients. The mucosa adjoining the diverticulum is usually hyperemic or ulcerated. There may be epithelial hyperplasia, and the muscularis propria is hypertrophic. Very commonly, there is inflammation involving the lamina propria and muscularis. The wall of the diverticulum itself consists of urothelium and underlying connective tissue, similar to the bladder mucosa with lamina propria. The lamina propria may contain muscularis mucosae which, on occasion, may be thickened and hyperplastic (Fig. 44.14B). Otherwise, few if any muscle fascicles will be identified in the majority of cases of acquired diverticula. The true “congenital” diverticula contain a thinned outer muscle layer. Infrequently, the epithelium lining the sac undergoes squamous or glandular metaplasia because of local irritation associated with urine stasis, infection, or stone. In these cases, it is not unusual for the diverticular wall to become extensively fibrotic.
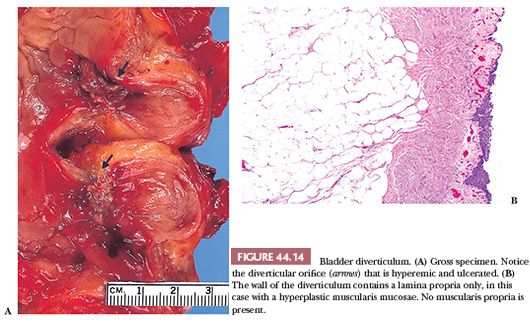
Major complications of bladder diverticula include infection, lithiasis with subsequent obstruction, and carcinoma (91–93). It is believed that 2% to 7% of patients with bladder diverticula will develop an associated neoplasm, presumed secondary to the chronic inflammatory stimuli mentioned. The neoplasms may develop at the os or may occur within the diverticula, making endoscopic evaluation difficult. Although morphologically “usual” urothelial carcinomas predominate, there is a relative increase in the incidence of divergent differentiation or pure squamous carcinoma and adenocarcinoma associated with squamous or glandular metaplasia in the lining urothelium. Sarcomas and at least one carcinosarcoma also have been reported in association with vesical diverticula, but we feel that most of these cases represent metaplasia spindle cell differentiation of high-grade carcinomas (sarcomatoid carcinoma) (93).
Diverticula may also occur in the ureter (94) and urethra. The overwhelming majority of urethral diverticula occur in women (95,96). They may be asymptomatic but usually present with irritative symptoms or dribbling. Complications of urethral diverticula are similar to those of the bladder (95). It is not uncommon to see the diverticular sac lined by metaplastic glandular or squamous epithelium and, if neoplasms occur, these tend to be either glandular or squamous (97,98).
UROTHELIAL NEOPLASIA
EPIDEMIOLOGY
More than 72,000 new cases of bladder cancer occur in the United States each year, with more than 15,000 people dying of the disease (99,100). Seventy-five percent of patients present with either noninvasive disease or invasion limited to the lamina propria, whereas 20% and 5% present with muscularis propria invasive and metastatic disease, respectively. Carcinoma of the bladder affects men more than women at a ratio of 3 to 4:1. This difference is probably accounted by differences in smoking habits and occupational exposure in the two sexes. The incidence of bladder cancer differs between counties, different states and counties in this country, and adjacent urban and rural areas, also suggesting that social, occupational, environmental, or dietary factors might promote the development of bladder cancer (99).
In 1895, Rehn demonstrated an increased risk of bladder cancer in dye industry employees. Since then, a substantial number of industrially used aromatic amines such as naphthylamines, benzidine, and biphenyls have been identified as human bladder carcinogens (99–101). Occupational exposure to these organic amines has been associated with a relatively high incidence of bladder cancer. These occupations include chemical, dyestuff, rubber, paint, and textile manufacturing as well as laboratory work, leather work, and printing (101–103).
It is generally accepted that cigarette smokers have an increased relative risk of developing bladder cancer up to four times the risk of the general population (99). It has also become clear that the relative risk of developing cancer increases with cigarette smoking but not with cigar or pipe smoking, suggesting that inhalation is an important factor. Although it is uncertain which components in cigarette smoke are carcinogenic, prime suspects include 2-naphthylamine, nitrosamines, and tryptophan metabolites (104,105).
An elevated risk in coffee drinkers has also been reported but this association remains much more controversial (106). No direct or causal relationship between coffee consumption and human bladder cancer has been established nor has there been convincing evidence for a carcinogenic effect of other foodstuffs such as artificial sweeteners (107–109). Despite the established importance of occupational and environmental factors in the development of bladder cancer, a great proportion of bladder tumors occur among nonsmokers and persons without occupational exposure to bladder carcinogens. This suggests that other factors, perhaps related to diet, could promote the development of bladder cancer. Armstrong and Doll (110) showed a higher incidence of bladder cancer in association with a high-fat diet, and the low incidence of bladder cancer in the Japanese population may be related to a low dietary fat intake.
Another factor that must be considered among the causes of bladder cancer is prolonged local irritation of the mucosa, which may play a role in patients with chronic bladder infection, diverticula, calculi, exstrophy, and schistosomiasis. Urothelial carcinoma has also been reported following long use of certain phenacetin-containing analgesics (111,112) and after treatment with the alkylating drug cyclophosphamide (113), as have rare cases of sarcoma (114).
CLASSIFICATION OF UROTHELIAL TUMORS
Approximately 90% of malignant bladder tumors are usual or “not otherwise specified (NOS)” urothelial carcinomas. The remaining 10% comprise all other types of carcinoma, a small number of sarcomas, and miscellaneous tumors. As defined by the most recent World Health Organization classification, the types of carcinoma arising in the urothelium are listed in Table 44.1, but this list will certainly expand as pathologists describe additional variants (115).

It is not uncommon to see focal areas of squamous or glandular differentiation in urothelial carcinomas, especially in intermediate- and high-grade tumors. We believe these should be classified as urothelial carcinomas, adding the phrase “ . . . with ____ differentiation.” The term carcinoma with mixed epithelial features should be reserved for tumors that exhibit prominent areas of two or more histologic types, in which case the histologic tumor types present should be reported. The diagnosis of adenocarcinoma, squamous cell carcinoma, or small cell carcinoma should be reserved for tumors in which those are essentially pure patterns or, at most, there are only focal areas of concomitant usual urothelial carcinoma. Sarcomatoid carcinoma refers to those tumors exhibiting tumor cells with spindle cell differentiation or recognizable mesenchymal elements, be it leiomyosarcoma, rhabdomyosarcoma, chondrosarcoma, or osteosarcoma. It is advisable to state the presence and nature of the heterologous elements that are present. Some pathologists use the term carcinosarcoma to describe carcinomas with mesenchymal differentiation. In our opinion, sarcomatoid carcinoma and carcinosarcoma are a morphologic continuum representing varying degrees of differentiation within an epithelial tumor. However, if overt mesenchymal differentiation is evident, a prudent way to handle these cases is to classify them as “sarcomatoid carcinoma with heterologous differentiation in the form of . . . .” These issues are described with greater depth in the “Glandular Lesions Including Adenocarcinoma” and “Squamous Lesions Including Squamous Carcinoma” sections later in this chapter.
STAGING OF BLADDER CANCER
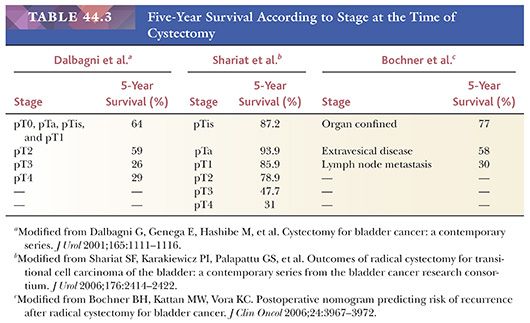
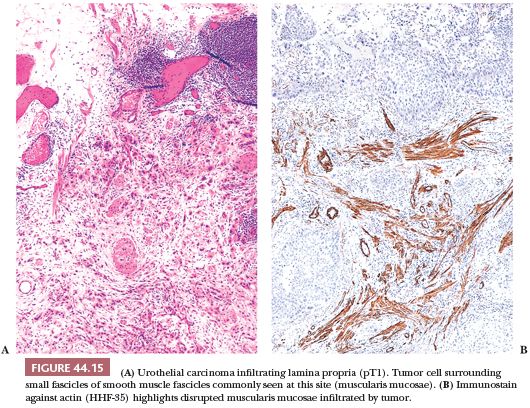
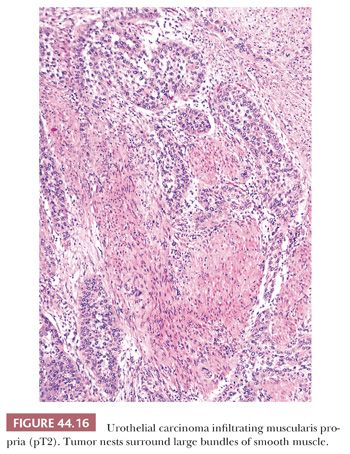
The usefulness of any staging scheme is directly related to its ability to discriminate among prognostic groups. In 1946, Jewett and Strong (116,117) first demonstrated the importance of the depth of infiltration as a prognostic variable in bladder cancer and proposed a staging scheme, which later was modified by Marshall (118). A similar classification was later adopted by the American Joint Committee on Cancer (AJCC) (119) and is presently used throughout the world. As evidenced in Table 44.2, it is quite simple to convert from one staging classification to another. The ability of these staging schemes to discriminate among clinically distinct prognostic groups is well established in the literature (Table 44.3) (120–122). Many investigators have shown a statistically disease progression and significant survival difference between patients with noninvasive tumors, tumor confined to lamina propria, and those with muscle infiltration (123–126). The former are treated by transurethral resection and possibly intravesical chemotherapy, unless they have other pathologic or clinical features, which place them in a high-risk category for progression. Patients with tumors infiltrating the muscularis propria or beyond (at least T2 lesions) are immediate candidates for more aggressive local therapy, most commonly radical cystectomy although neoadjuvant chemotherapy is being given more often. For this reason, the pathologist must be very careful in assessing depth of invasion on a biopsy specimen. The few wisps or small fascicles of smooth muscle that are commonly seen within the lamina propria (refer to “Normal Anatomy and Histology” earlier) must not be mistaken for fascicles of the muscularis propria (Fig. 44.15A,B) (4,6). To be certain the tumor is infiltrating muscularis propria, one must see tumor cells within or among broad bundles of smooth muscle and only then should it be reported (Fig. 44.16). The value of the antibody smoothelin to differentiate muscularis propria from muscularis mucosae remains controversial (7,8).
In invasive disease, the most powerful prognostic factor is stage. Pathologic staging takes on greater importance because we know that clinical overstaging or understaging may occur in up to 40% of cases. Unfortunately, pathologic staging also has its problems, specifically as it pertains to invasion of the lamina propria (pT1) (126–130). Identification of lamina propria invasion usually, but certainly not always, is straightforward. Some may be difficult to classify, and a decision as to the presence of invasion depends on the criteria used by the pathologist. This fact has resulted in wide variability in the reported incidence of T1 in individual series, accompanied by equally disparate rates in progression.
Interobserver variability in pathology is a well-known fact. Aside from the inherent subjectivity of our field, the major cause of this variability is the lack of widely accepted, reproducible criteria for identifying lamina propria invasion in biopsy material. Compounding the problem are technical issues, including our inability to properly orient biopsy specimens resulting in tangential sectioning, “crush,” and cautery artifact brought about by the biopsy procedure itself, as well as distortion of anatomic landmarks caused by prior biopsy or intravesical therapy. During the past few years, we have developed better morphologic criteria to identify T1 disease. It is apparent that noninvasive disease (Ta and CIS) behaves quite differently from superficially invasive (T1) tumors and, as such, probably should not be classified together for prognostic and treatment purposes. Although urologists routinely use the term superficial bladder cancer for tumors that do not invade muscularis propria, we should make every effort to discourage its use, given its lack of specificity.
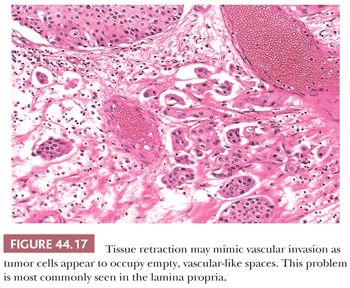
There are several morphologic criteria useful in the determination of lamina propria invasion (Table 44.4). Growth pattern characteristics of the epithelium are very important (131). One of the common difficulties in determining superficial invasion is the fact that several benign, proliferative urothelial lesions, such as Brunn nests, inverted papilloma, nephrogenic metaplasia, and so on, manifest as pseudoinvasive nests of urothelium within the lamina propria. The problem often is compounded by the unoriented nature of the specimen. Tangentially sectioned, densely packed, noninvasive papillary tumors exhibit a stroma–epithelial interface, which is smooth and regular. In cases of true invasion, one is likely to see variably sized and irregularly shaped nests or individual tumor cells percolating through the stroma. The morphologic appearance of the basement membrane also may supply useful information. When dealing with tangential sections through noninvasive disease or when urothelial carcinoma involves Brunn nests, the basement membrane preserves a regular contour, whereas it is frequently absent in the cases of true invasion. This feature may be assessed on H&E, although in many cases, additional clues are needed, including the presence of a parallel array of thin-walled vessels that evenly line the basement membrane of noninvasive nests but that are lacking in cases of invasive tumors. Another useful parameter is the assessment of stromal reaction. The stroma of the lamina propria adjacent to an invasive tumor is commonly altered when compared to uninvolved bladder wall. This stromal reaction usually consists of a fibroblastic stromal proliferation, sometimes with myxoid change. The stromal reaction may be quite cellular, adopting a pseudosarcomatous myofibroblastic appearance, or it may consist of a minimal increase in fibroblasts. The invasive nests may be accompanied by a peritumoral inflammatory response. If this inflammatory response is brisk, it may obscure the stromal hypercellularity. On occasion, invasive nests may be surrounded by a collagenous stroma, which is not seen in the uninvolved lamina propria. Although the majority of bladder tumors with unquestionable lamina propria invasion exhibit some sort of stromal reaction, microinvasive disease usually does not, making its identification even more difficult. Another important feature of invasion is the presence of retraction artifact around individual tumor cells or nests of cells (Fig. 44.17). This feature may be of great use in identifying microinvasive disease and is often confused for lymphatic invasion. Nevertheless, it is important to remember that this feature may be very difficult to assess in areas exhibiting cautery or crush artifact. The final clue to identifying lamina propria invasion is the presence of paradoxical differentiation. This feature is particularly helpful in microinvasive disease, where the invasive tumor cells may acquire abundant, eosinophilic cytoplasm, similar to that seen in microinvasive uterine cervical cancer. At low and medium magnification, these microinvasive cells appear to be more differentiated than the overlying noninvasive disease.

With few exceptions, such as the nested variant of urothelial carcinoma, the enumerated criteria allow us to consistently and reproducibly separate “superficial” bladder tumors into noninvasive (Ta and CIS) tumors and those that have invaded the lamina propria (T1), the latter having a significantly worse prognosis. In a cohort of 180 patients with superficial bladder cancer seen at Memorial Hospital and treated with transurethral resection alone, the progression rate of noninvasive tumors was 2.85 per 100 persons/year, whereas for T1 lesions, it was more than double, 6.86 per 100 persons/year. Other investigators have reached similar findings, and urologists are now debating whether T1 tumors should be treated more aggressively. If this is the case, pathologists will experience additional pressure to accurately stage these tumors.
Several investigators have attempted to subclassify T1 tumors based on their depth of invasion. Younes et al. (132) subclassified T1 tumors into three groups based on whether invasion was limited to above the muscularis mucosa (T1a), reached the level of the muscularis mucosa (T1b), or extended beyond the muscularis mucosa (T1c). In their series of 50 cases, T1a and T1b tumors had a 5-year survival rate of 75% compared to 11% in the T1c group (132). Hasui et al. (133) used a slightly different subclassification scheme but arrived at similar results, namely, that T1 tumors that infiltrate to or near the muscularis mucosa have a higher progression rate than those that do not (53.5% vs. 6.7%, p <.01). In a larger study, Angulo et al. (134) confirmed these findings but also reached the important conclusion that, using the presence of muscularis mucosae or large blood vessels as determining factors, subdividing the superficial bladder wall into lamina propria (T1a) and submucosa (T1b) could be done in only 58% of transurethral resection specimens. If we are to subdivide these tumors, it is clear that we need additional criteria which we can apply to all specimens. Much work remains to be done before we can incorporate this information into the pathology report, although the 2004 World Health Organization (WHO) classification leaves it up to the individual pathologist to do so (115). The role of smoothelin in discriminating muscularis propria from muscularis mucosa is yet to be determined (7).
UROTHELIAL CARCINOMA
Approximately 98% of malignant tumors arising in the urinary bladder are of epithelial origin, and of these, 90% are usual urothelial carcinomas (transitional cell carcinoma [TCC]). The highest incidence is in the sixth and seventh decades of life; men are affected more often than women at a ratio of 3 to 4:1. Most urothelial carcinomas at initial diagnosis are papillary and superficial and as many as 70% are characterized by a prolonged clinical course over which the patient experiences multiple recurrences following local resection without tumor progression (135,136). In contrast, a smaller but significant percentage of patients present with tumors, which have an aggressive clinical course over a short period of time. A number of pathologic features have been identified, which are accurate predictors of the clinical course of bladder cancer. The most important are depth of invasion, if any, at presentation; multiplicity (multifocality); a history of prior urothelial tumors; tumor size; and grade. Tumor size and grade are not as important an influence on the recurrence rate as tumor multiplicity (multifocality), a prior history of tumors, and depth of invasion at diagnosis (135–140). As a rule, only high-grade tumors will develop regional lymph node metastasis. Cytologically benign papillary tumors may and do recur but do not invade. Tumors are as likely to recur at a different site in the bladder, indicating multifocal occurrence rather than recurrence of the original tumor.
Pathologic stage is the most potent predictor of survival in urothelial carcinoma. Although a 5-year survival rate of approximately 75% is to be expected in patients with no more than lamina propria invasion at the time of cystectomy, 5-year survival rates for tumors infiltrating muscularis propria or perivesical fat are 50% and 20%, respectively (141) (Table 44.3). Similarly, regional lymph node metastasis at the time of cystectomy is more frequent in tumors infiltrating muscularis propria or perivesical fat. Some urothelial tumors exhibit an endophytic pattern of growth, at times mimicking inverted papilloma (131,142). In such cases, it may be quite difficult to determine the presence of stromal invasion, especially in biopsy material.
Tumor configuration is another important prognostic variable. Papillary tumors tend to be of lower grade, earlier stage, and of less aggressive behavior than nonpapillary tumors (137,138,143,144). Kakizoe et al. (145) studied 186 cystectomies and found that tumors with a nodular (sessile) configuration presented at a higher stage when compared with exclusively papillary tumors. Survival was adversely (p <.01) affected in the former group. Small vessel infiltration also affects prognosis. Fossa et al. (139) found that in a group of patients with superficial bladder carcinoma, those who had lamina propria small vessel invasion had a significantly higher risk of subsequent tumor progression. Larsen et al. (146) disagree that lymphatic invasion is of clinical significance in pT1 disease, but they raise the valid issue that the presence of lymphatic invasion may be very difficult to evaluate by light microscopy alone because of retraction artifact (Fig. 44.17). Heney et al. (138) reported on 86 patients who underwent radical cystectomy for invasive carcinoma infiltrating at least into muscularis propria. Those who exhibited small vessel invasion had regional lymph node metastasis in 38% of cases and a 5-year survival rate of 30%, whereas those patients who did not have small vessel invasion had a regional lymph node metastatic rate and 5-year survival rate of 16% and 52%, respectively. Additional contemporary studies have confirmed the clinical importance of finding vascular invasion at the time of cystectomy (147,148).
These data clearly illustrate that a number of histologic features are useful predictors of the clinical course of bladder cancer. Pathologists should make it a point to comment on these features at the time of evaluation and reporting of bladder biopsy and cystectomy specimens (Table 44.5). In evaluating biopsy specimens, the pathologist should state what components of the bladder wall are represented (i.e., mucosa, lamina propria, or muscularis propria). If the epithelial surface is ulcerated or denuded, it should be so stated; if a tumor is present, one should establish the histologic type (e.g., urothelial), pattern of growth (e.g., papillary or nodular), tumor grade, and depth of invasion. The presence or absence of small vessel involvement should be noted. The status of the adjacent mucosa should be evaluated, especially with reference to CIS. In cystectomy specimens, one should comment on the location and size of the lesion(s), status of mucosal and soft tissue margins, status of adjacent organs (e.g., urethra, ureters, prostate), as well as location, number, and status of regional lymph nodes. This information allows the clinician to offer a more exact opinion as to the prognosis of the patient and to better choose among available therapeutic options.

IMMUNOHISTOCHEMISTRY OF UROTHELIAL NEOPLASIA
Most usual urothelial carcinomas have recognizable morphologic and cytologic features, making the use of IHC unnecessary to establish a proper diagnosis. However, high-grade tumors, particularly when located at metastasis sites, may be difficult to classify. These tumors commonly exhibit divergent differentiation, which further complicates the issue. In addition, high-grade urothelial carcinoma has what I refer to as a “patternless pattern” not unlike any poorly differentiated non–small cell carcinoma irrespective of primary site. For example, it may be entirely indistinguishable from a nonkeratinizing squamous cell carcinoma of the cervix or head and neck. At times, it may be very difficult to distinguish urothelial cancer from recurrent prostate cancer after radiation or hormonal therapy. Several investigators have addressed this issue with IHC, taking advantage of the fact that urothelial carcinoma commonly expresses a constellation of epithelial markers which, when evaluated as a unit, may be very helpful in establishing the proper diagnosis (149–152). Although there is some variability with relation to the degree of differentiation of the tumor, urothelial carcinomas are likely to be immunoreactive for CK7 and CK20 as well as 34βE12 and 4A4 (p63). Uroplakin is the most specific marker but is commonly lost in high-grade tumors. Thrombomodulin is very sensitive but entirely nonspecific. GATA3 is another marker that has been recently introduced and is being promoted as sensitive and relatively specific for urothelial phenotype, but a few other tumors are known to overexpress it, most notably mammary carcinomas (153–155). Our overall experience with this marker, however, is still limited and it will require more studies of larger cohorts to determine its usefulness in the differential diagnosis of urothelial tumors.
GRADING OF UROTHELIAL TUMORS
Grading is a method by which pathologists evaluate the cytologic or growth pattern characteristics of a tumor in an attempt to predict its biologic potential. To this effect, multiple grading schemes have been developed and employed throughout the years. In all classifications, those lesions that cytologically resemble normal urothelium are low-grade (well differentiated), whereas cytologic anaplasia characterize high-grade or poorly differentiated carcinomas. Nevertheless, classifications differ in their recognition of a benign tumor counterpart and in the number of categories into which urothelial carcinoma should be graded.
GRADING OF PAPILLARY UROTHELIAL LESIONS
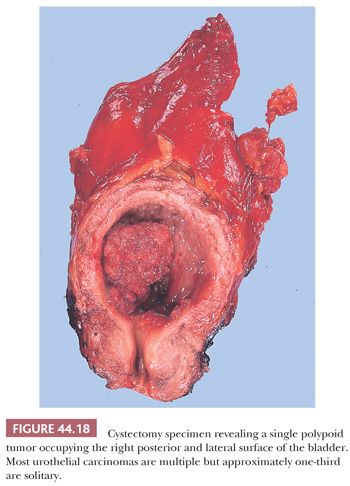
The most common tumors of the urinary tract (excluding prostate) are papillary (exophytic) tumors of urothelial origin (Fig. 44.18). They frequently are multiple and often recur after being removed, yet most of these tumors are easily managed by local resection. Patients who have had papillary tumors of the bladder are at risk of developing invasive carcinoma, and some very experienced pathologists regard nearly all papillary tumors as carcinoma (47,156). During the past decade, the community of academic urologic pathologists vigorously debated this issue; the end result is the 2004 WHO classification (115) (Table 44.6). The evidence to support the latter classification is compelling. The key to the classification of papillary urothelial neoplasms lies in the rigorous evaluation, at medium magnification, for the presence of cytologic and architectural disorder. By cytologic disorder we mean, using common terms, the extent of variability in size, shape, and color (pleochromasia) of the neoplastic urothelial cells. By architectural disorder we mean the relation of the neoplastic urothelial cells to the underlying basement membrane (polarization) and to themselves. Secondary criteria that may or may not be helpful include mitoses and apoptotic bodies.

Historical Review
First, it is worth noting that papillary tumors of the bladder left untreated may grow profusely within the bladder without invading through the wall or metastasizing—a fact noted by pathologists in the nineteenth century, who did not regard these growths as malignant tumors. More than 30 years ago, Nichols and Marshall reported that the 5-year survival of patients with “papilloma” (low-grade papillary tumors) was exactly that of the age-matched general population. However, this was not true of cytologically high-grade tumors. Ash (157) had recognized this as early as 1940 in his adaption of Broder’s cytologic grading system for the classification of papillary transitional cell tumors, grades 1 to 4. Mostofi et al. (156) later modified Ash’s classification for the WHO, distinguishing a class of papillomas from TCC, grades 1, 2, and 3. Their definition of papilloma was very conservative, restricted to the approximately 1% of patients who had a usually solitary, delicate papillary tumor with no more than eight cell layers of normal-appearing transitional epithelium on a fibrovascular core. Koss (47) later accepted this definition in his influential monograph on tumors of the urinary bladder, published for the Armed Forces Institute of Pathology (AFIP), although he permitted no more than seven cell layers.
The accumulating data from an increasing number of clinicopathologic studies indicate that these definitions are too narrowly drawn—that many of the grade 1 papillary “carcinomas” so defined are in fact biologically benign and should be classified as papillomas. Detailed histologic studies, including some that mapped the entire bladder, have shown that the low-grade papillary tumors, including those with hyperplastic epithelium and minimal atypia, are noninvasive. It is the flat, nodular, or papillary tumors with cytologically malignant epithelium that give rise to the great majority of invasive carcinomas (158–162). Long-term clinical follow-up studies also confirm the benign nature of the low-grade papillary tumors. Bergkvist et al. (163) followed 300 patients with bladder tumors for 9 years. Of 64 (21%) who had low-grade tumors, only one died of bladder cancer, and that one was believed to have died of a new carcinoma, which developed 5 years later (163). Of the 236 patients with higher grade tumors, 172 died of their bladder cancer. Lerman et al. (164), in a study from Memorial Sloan-Kettering Cancer Center, reported on 125 patients with papilloma (now called papillary urothelial neoplasm of low malignant potential) defined by the broader cytologic criteria we favor and followed for 10 to 20 years. Although nearly half recurred, only 5% later developed CIS and another 5% developed invasive carcinoma; in no case was there invasion at the site of histologically benign papilloma, suggesting both by location and late occurrence that these were new tumors. In other studies, Heney et al. (140) reported only 2% of 92 patients with grade 1 TCC developed a more anaplastic tumor over a period of 36 months; Gilbert et al. (165) reported that 9 of 155 patients (5%) with grade 1 TCC developed high-grade and muscle-invading tumors, whereas 41% did not have a single recurrence after no less than 5 years. In 1987, Jordan et al. (166) studied 400 patients with TCC classified by WHO criteria and followed for 10 to 20 years; of 91 with grade 1 TCC, only 7 (8%) subsequently developed more advanced tumors. The remaining 84 had no progression of disease and had normal life expectancy, although some had recurrent tumors of the same type. Fifty-five patients (60%) had no recurrences.
Some of the most interesting and informative data have come from the National Bladder Cancer Collaborative Group A program (NBCCGA) (135). That multi-institutional study found that when recurrences presented in patients with low-grade TCC, they were as likely as not to be at sites different from the original. This, of course, suggests that the clinical recurrences were actually new tumors and that their formation was due to the neoplastic diathesis of an unstable urothelium. They are manifestations of the multifocal origin of tumor in these patients. Althausen et al. (162) studied the normal-appearing mucosa adjoining low-grade papillary tumors and found that most of the invasive carcinomas developing later were in patients who had CIS or atypia in the adjacent flat mucosa. Presumably, most invasive carcinomas develop from mucosa which has CIS or develops it over a period of time following resection of the papillary tumor. That CIS frequently is present elsewhere in the bladder mucosa of patients with low-grade papillary tumors is strongly suggested by other data from the NBCCGA. Positive exfoliative cytology was found in 43% of the patients with grade 1 tumors (papillomas) (167,168). Because benign papillary lesions (WHO grade 1 tumors) do not shed cytologically malignant cells, they must come from other areas of occult carcinoma or CIS.
The information presented in the preceding as well as the experience of many urologic pathologists has led to changes in the classification of bladder tumors. This fact is evidenced in the grading scheme proposed by Murphy et al. (168) in the latest Atlas of Tumor Pathology: Tumors of the Kidney, Bladder and Related Urinary Structures.
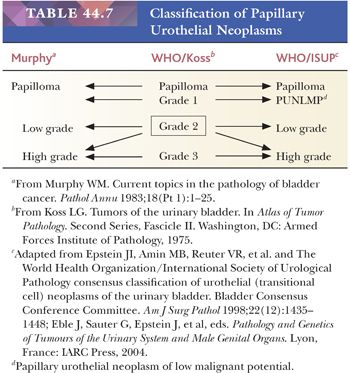
In October 1997, Mostofi assembled a group of pathologists, urologists, urologic oncologists, and basic scientists with an interest in bladder neoplasia at a meeting in Washington, DC, with the purpose of discussing the terminology of bladder lesions and making recommendations to the WHO Committee on Urothelial Tumors. All in attendance agreed that it was imperative to develop a universally acceptable and clinically relevant classification system for bladder neoplasia that could be used effectively by pathologists, urologists, and oncologists. Following this meeting, a group of urologic pathologists who had attended decided to broaden the representation of the group and arranged a meeting primarily of the members of the International Society of Urological Pathology (ISUP) at the 1998 United States and Canadian Academy of Pathology Meeting held in Boston. At this meeting, issues regarding terminology of bladder lesions—primarily neoplastic and putative preneoplastic lesions—were discussed, resulting in a consensus statement. In December 1998, the WHO/ISUP consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder was published (Table 44.7) (169,170). It was not universally accepted, with pockets of resistance coming from within the pathology as well as urology communities (171). One of the major sources of criticism was the fact that the classification was not based on hard data but, rather, the “experience” of seasoned pathologists. Others questioned lumping of a variety of tumors into a high-grade category. The 1999 edition of the World Health Organization International Classification of Tumors subclassified tumors into low malignant potential, grades 1, 2, and 3 disease (172). Nevertheless, since the publication of the 1998 WHO/ISUP classification, several well-designed studies, including those from the Malström group, have validated its clinical use (173–175).
Late in 2002, under the auspices of the WHO, a group of urologic pathologists met in Lyon, France to discuss and finalize the new Blue Book on the pathology and genetics of tumors of the urinary system. The 1998 WHO/ISUP classification was accepted with few changes and few dissenting votes and now has become the 2004 WHO classification of urothelial tumors (115).
The potential advantages of the WHO (2004)/ISUP system are (a) input from a broad spectrum of urologic pathologists, allowing for uniform terminology and common definitions; (b) detailed morphologic definitions of various preneoplastic conditions and various grades of tumor, hopefully leading to greater interobserver reproducibility; (c) the terminology used in the WHO (2004)/ISUP system is more or less consistent with what is used in urine cytology, creating a consensus classification between cytology and histopathology; (d) creation of a category of tumor that identifies a papillary urothelial neoplasm of low malignant potential (PUNLMP) with a negligible risk of progression, whereby patients avoid the label of having cancer with its psychosocial and financial (i.e., insurance) implications. These patients are also not diagnosed as having a non-neoplastic lesion (i.e., papilloma), whereby they might not be followed as closely; and (e) identification of a larger group of patients at high risk for progression for urologists to more closely follow.
It is imperative that we now turn our attention to more important issues if we are to move ahead as a field. Rather than arguing about classification, we should be participating in multidisciplinary studies evaluating multiple risk factors, including molecular markers, to better stratify patients into prognostic groups.
PAPILLARY UROTHELIAL HYPERPLASIA
At least one study suggests that papillary urothelial hyperplasia is a precursor lesion to low-grade papillary urothelial neoplasms (176). Typically, they are discovered on routine follow-up cystoscopy for papillary urothelial neoplasms and very frequently in the workup for microhematuria or urinary obstructive symptoms. Histologically, papillary hyperplasia consists of undulating urothelium, which is often thicker than normal. In addition to the diagnostic mucosal folds, in some cases, there are also tent-shaped, somewhat broader folds that lack the edema and inflammation typical of polypoid cystitis. The cytology and architectural order is similar to normal urothelium but cases show increased vascularity in the stroma at the base of the folds. It is distinguished from papillary urothelial neoplasms by a lack of arborization and absence of true papillary fronds. Although these changes may be seen in urothelial cancer–bearing patients, studies with sufficient follow-up that evaluate the clinical significance of de novo papillary hyperplasia are lacking. The term hyperplasia may also be appropriate when the urothelium takes on a pseudopapillary architecture as a result of local injury. The papillations are small, often with variable intervening stroma, and the underlying lamina propria is inflamed. The urothelium may be somewhat hyperchromatic and disorganized but otherwise unremarkable. In such situations, I think it is justified to use the descriptive term of reactive papillary hyperplasia.
UROTHELIAL PAPILLOMA
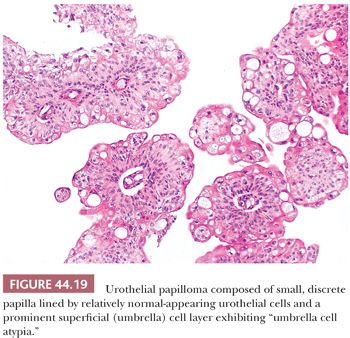
This is a rare, benign condition typically occurring as a small, isolated growth seen primarily (but not exclusively) in younger patients. Morphologically, it is a discrete, exophytic papillary growth with a central fibrovascular core lined by urothelium of normal thickness and cytology (Fig. 44.19). Recurrences are rare and progression to carcinoma even more so (177–179). Although most investigators still embrace the concept that these lesions are totally distinct from “true” papillary urothelial neoplasm, the fact that they may be seen in patients who harbor papillary urothelial carcinoma and that a small percentage go on to develop carcinoma makes this statement at best controversial. The one thing we all agree on is the fact that they are benign.
INVERTED UROTHELIAL PAPILLOMA
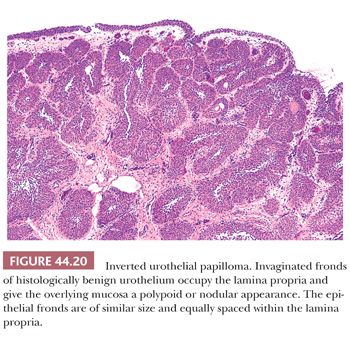
Inverted papillomas are relatively rare lesions that may occur anywhere along the urothelial tract; they may be confused clinically and pathologically with urothelial carcinoma (180,181). In order of decreasing frequency, they occur in the bladder, renal pelvis, ureter, or urethra (182–188). Patients usually present with hematuria. Cystoscopically, the lesions are polypoid and either sessile or pedunculated. The mucosal surface is smooth or nodular, without villous or papillary fronds. Microscopically, the surface transitional epithelium is compressed but otherwise unremarkable (Fig. 44.20). It is undermined by invaginated cords and nests of transitional epithelium, which occupy the lamina propria. The accumulation of these endophytic growths gives the lesion its characteristic nodular or polypoid gross appearance. The urothelial cells forming the cords are benign, exhibiting normal maturation and few mitoses. They are similar to the cells of bladder papillomas, differing only in that the epithelial cords are endophytic and consequently more closely packed. Frequently, the cells are oval or spindle-shaped. Epithelial nests may become centrally cystic, dilated, and even lined by cuboidal epithelium. The cords of urothelium in the lamina propria represent invagination, not invasion. As such, there are no fibrous reactive changes within the stroma. Although mitotic figures can be seen, they are rare, regular, and located at or near the basal layer of the epithelium. Inverted papillomas are discrete lesions that do not exhibit an infiltrative border (182,183).
Some cases of urothelial carcinoma exhibit an endophytic pattern of growth and mimic inverted papilloma (131,142). The presence of cytologic disorder similar to that seen in papillary carcinoma (low or high grade), irregularity in the thickness of the epithelial nests, irregularity in the size and distribution of the nests within the lamina propria, and the presence of a stromal reaction offer us clues as to the correct diagnosis. Broussard et al. (189) described a group of cases they called inverted urothelial papillomas with atypia. Although this topic remains controversial, it highlights the fact that great care should be taken in making the diagnosis of inverted papilloma. Jones et al. (190) recently compared the IHC and molecular features of inverted papillomas and inverted urothelial carcinomas, and found significant differences which may aid in arriving at the proper diagnosis.
The etiology of these rare lesions is unclear. Some investigators support a reactive or proliferative etiology, whereas others suggest they are neoplastic. Although I favor the latter hypothesis, I admit that only rare cases have been associated with carcinoma (184–186,191).
PAPILLARY UROTHELIAL NEOPLASM OF LOW MALIGNANT POTENTIAL
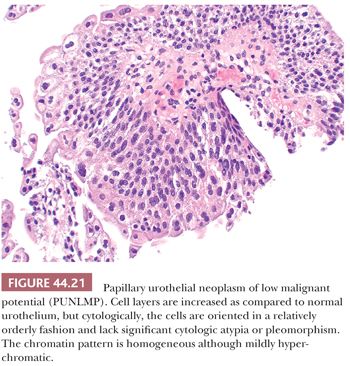
This term, papillary urothelial neoplasm of low malignant potential, describes a papillary urothelial neoplasm with an orderly arrangement of cells within papillae with minimal architectural abnormalities and minimal nuclear atypia, irrespective of cell thickness (Fig. 44.21). Although the neoplastic urothelium is usually somewhat thickened and mildly hyperchromatic, overall cytologic and architectural order is retained: Tumor cells are relatively similar in size, shape, and color; are evenly spaced among themselves; and are oriented in a similar plane in relation to the basement membrane. Mitoses are infrequent and usually limited to the basal layer. Apoptotic bodies are rarely, if ever, seen. When strictly defined, PUNLMP is not associated with invasion or metastasis. Nevertheless, these patients are at risk of developing new bladder tumors (recurrence), usually of similar histology (174,175,192). Occasionally (7% to 10%), these subsequent lesions manifest as urothelial carcinoma, such that close follow-up of the patient is obligatory.
LOW-GRADE NONINVASIVE PAPILLARY UROTHELIAL CARCINOMA
Low-grade carcinomas exhibit an overall orderly appearance but have variability in architecture or cytologic features, which are easily recognizable at scanning magnification (Figs. 44.22 and 44.23). Variability in polarity, nuclear size, shape, and chromatin texture (cytologic disorder) are modest but comprise the hallmarks of the cytologic atypia seen in low-grade papillary urothelial carcinoma. Mitotic figures are infrequent. Although mitoses may be seen at any level of the urothelium, they are not atypical. Apoptotic bodies are rarely seen.
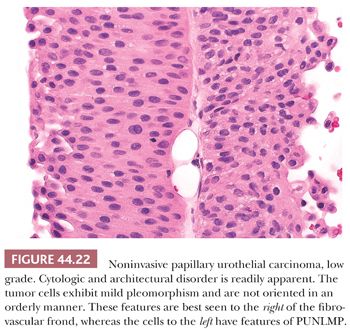
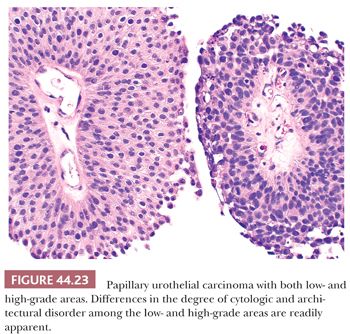
It is common to see fusion of adjacent papillae, a feature that may lead to overgrading because of an appearance of disorder. They frequently recur and may rarely (<5%) be associated with invasive disease, even without increasing in grade. The overall risk of progression is higher than for PUNLMP (10%–15%).
HIGH-GRADE NONINVASIVE PAPILLARY UROTHELIAL CARCINOMA
High-grade carcinomas are characterized by a disorderly appearance because of marked architectural and cytologic abnormalities, recognizable at scanning magnification (Figs. 44.23 and 44.24). Cellular pleomorphism ranges from moderate to marked, and mitotic figures, including atypical forms, frequently are seen at all levels of the urothelium, as are apoptotic bodies. They are commonly associated with invasive disease at the time of initial presentation, and the adjacent mucosa may show evidence of CIS. Because of the degree of architectural and cytologic abnormalities (disorder), the pathologist is free to comment on the presence of “anaplasia.” Progression rate varies from 15% to 40%, much higher than low-grade lesions.
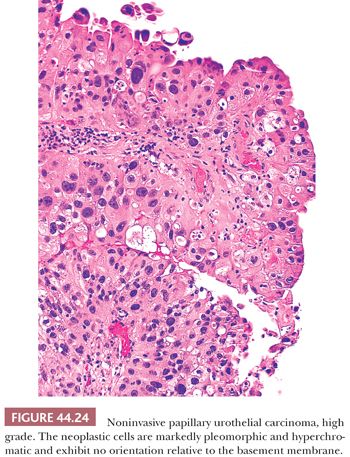
It is important to remember that a single papillary urothelial neoplasm may contain a spectrum of cytologic and architectural abnormalities (Fig. 44.20). In tumors with variable histology, the tumor should be graded according to the highest grade, although current practice is to ignore minuscule areas of higher grade tumor. Studies are needed to determine how prevalent a minor component must be in order to have an impact on prognosis.
FLAT UROTHELIAL LESIONS INCLUDING CARCINOMA IN SITU
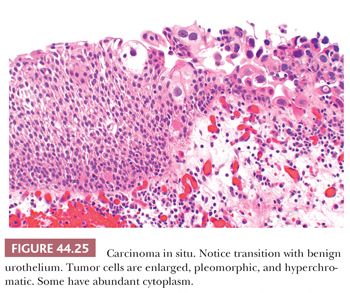
Unless it is specifically described within a papillary tumor, CIS usually refers to a nonpapillary (flat) mucosa in which the normal urothelium has been transformed into or replaced by cancer cells that have not invaded through the basement membrane (Figs. 44.25 to 44.28). CIS is a high-grade lesion and easily recognized because the cytologic abnormalities are obvious, similar to those seen in high-grade papillary tumors. We cannot and do not make the diagnosis of low-grade CIS. To cover the spectrum of minimal cytologic atypias, some pathologists have adopted the term dysplasia (167,168,193–197). It is a term that suggests specificity where none exists and suggests clinical significance where none has been demonstrated, especially in cells with mild to moderate cytologic abnormalities where these changes overlap significantly with those seen in reactive conditions. Reactive atypias or atypias of unknown significance are best reported as such. These concepts were reflected in the 1998 WHO/ISUP classification and adopted in the 2004 WHO classification. The term dysplasia should be limited to those cases where we are certain that the cytologic abnormalities seen are neoplastic yet short of CIS. We understand that this approach differs somewhat with what is described in the 2004 WHO classification. Distinction from what has been called severe dysplasia and CIS suffers from great interobserver variability (197). As such, these should be called carcinoma in situ.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


