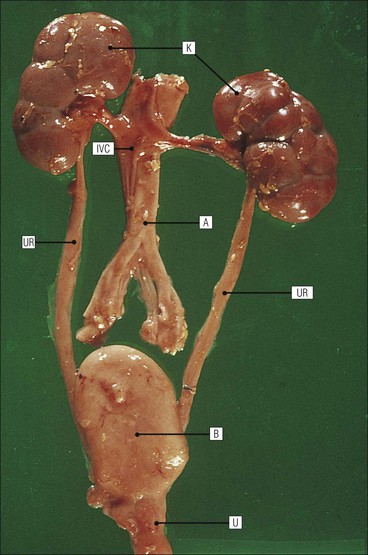
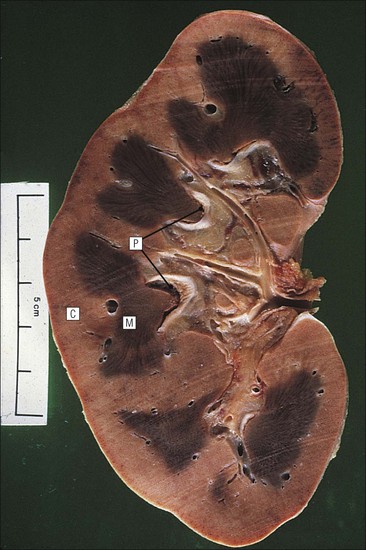
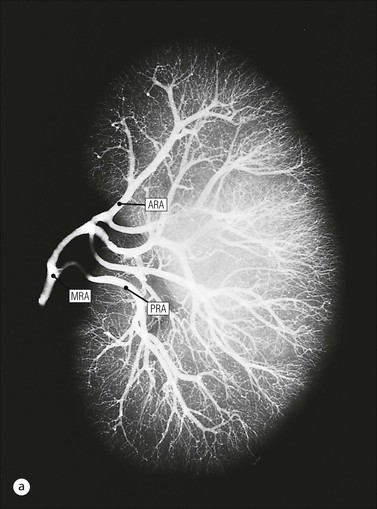
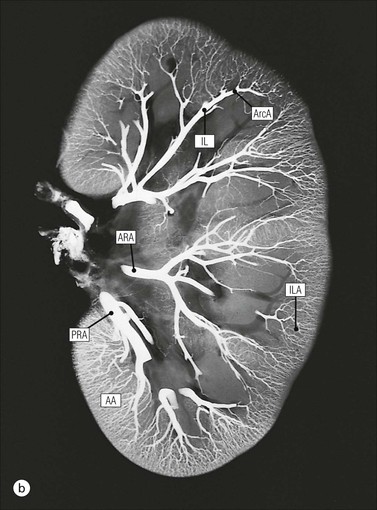
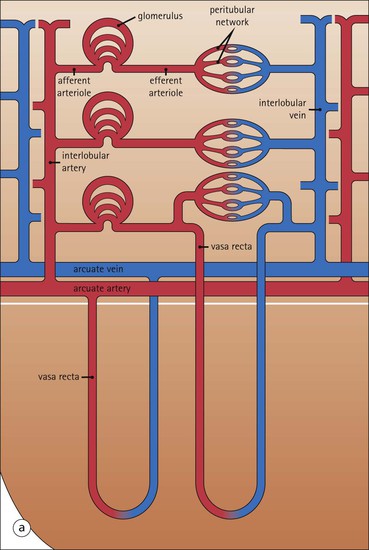
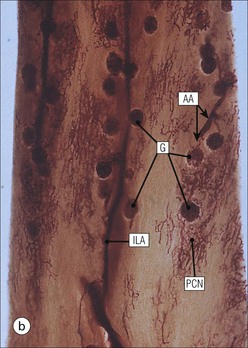
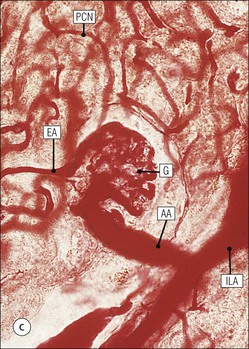
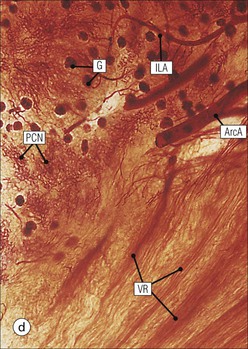
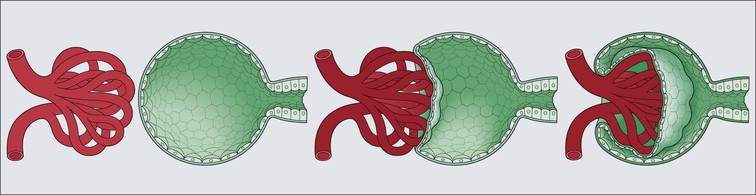
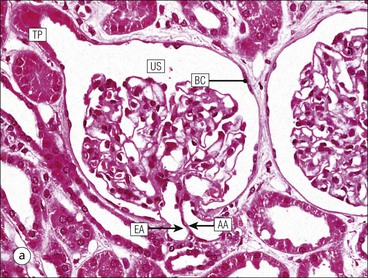
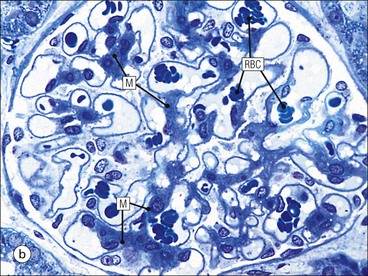
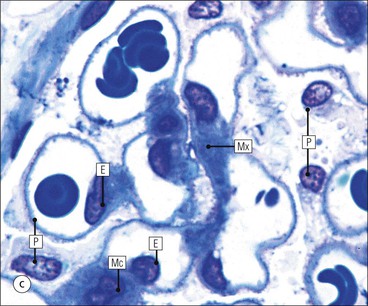
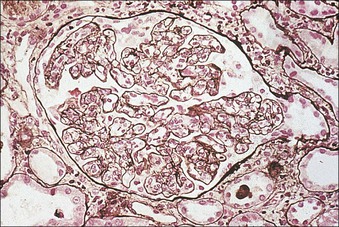
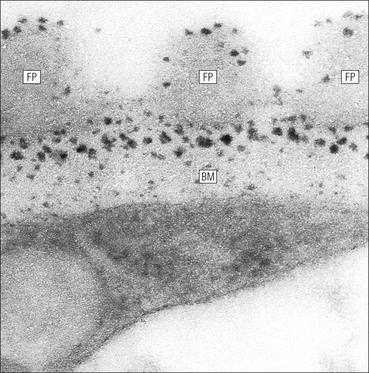
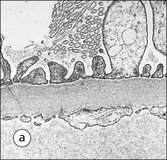
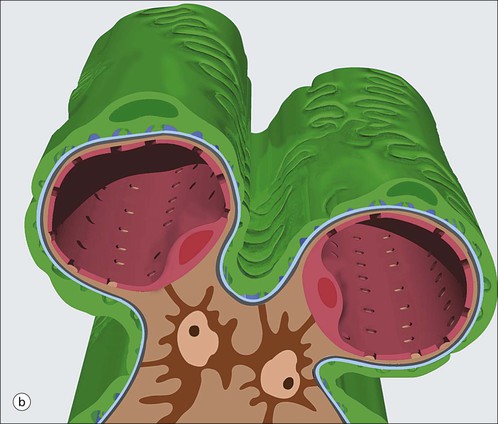
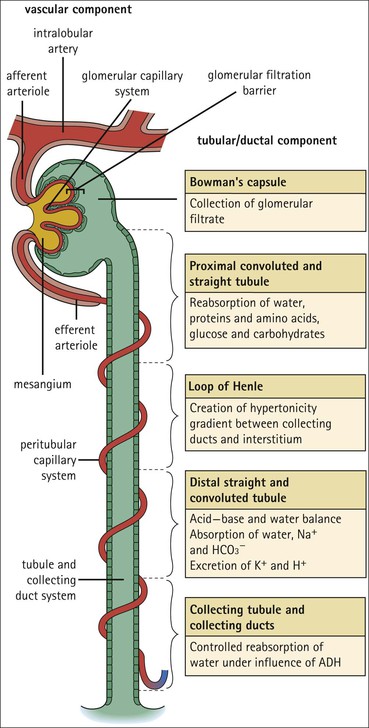
The main function of the urinary system is the production, storage and voiding of urine. Urine is an aqueous solution of excess anions and cations and many of the breakdown products of the body’s metabolic processes, particularly those that would be toxic if allowed to accumulate. The most important toxic metabolites are the nitrogen-containing products of protein breakdown, such as urea and creatinine. The composition and concentration of urine can be varied to maintain internal homeostasis. Urine production and the control of its composition are the responsibility of the kidneys, whereas storage and voiding are performed by the bladder. The pelvicalyceal systems and ureters transfer urine from the kidneys to the bladder, and the urethra is the channel through which stored urine is voided from the bladder. The pelvicalyceal systems, ureters, bladder and urethra have a relatively uncomplicated structure (see p. 311). The kidney, however, performs a wide range of biochemical and physiological tasks during the production of urine, and its structure is accordingly complex. The general arrangement of the urinary system is illustrated in Figure 15.1. The kidneys are solid, bean-shaped organs, located high on the posterior abdominal wall beneath the peritoneum The concave aspect of each kidney faces toward the midline, where the major channels of arterial supply (the aorta) and venous drainage (the inferior vena cava) run. This concave area is called the ‘hilum’. It is the site of entry of the renal arterial supply, and of the emergence of the renal venous drainage and urinary transport systems. The pelvicalyceal system and ureters are hollow muscular tubes lined by a specialized epithelium The epithelium is resistant to damage by the variable osmolarity of urine and by the concentration of toxic solutes within it. The walls of the tubes are composed of smooth muscle capable of pushing the fluid towards the bladder by alternate coordinated contraction and relaxation; this process is called ‘peristalsis’. The bladder acts as both a reservoir and a pump The bladder has basically the same structure as the pelvicalyceal system and ureters but its arrangement of muscle fibres is more sophisticated to allow it to act as both a capacious reservoir for urine and as a pump to force out the urine through the urethra under voluntary control. The kidney is divided into cortex and medulla Each kidney has two distinct zones: an outer cortex and an inner medulla (Fig. 15.2). The cortex forms an outer shell and also forms columns (the so-called ‘columns of Bertin’) that lie between the individual units of the medulla. The medulla is composed of a series of conical structures (medullary pyramids), the base of each cone being continuous with the inner limit of the cortex and the pointed peak of the pyramid protruding into part of the urine collecting system (the calyceal system) towards the hilum of the kidney. This pointed tip of the medullary pyramid is known as the ‘papilla’. Each human kidney contains 10–18 medullary pyramids, thus 10–18 papillae protrude into the collecting calyces. Each medullary pyramid, with its associated shell of cortex, comprises a functional and structural lobe of the kidney. This lobar architecture is clearly visible in the fetal kidney (see Fig. 15.1) but becomes less obvious as the kidney increases in size with increasing age. The kidney is essential for fluid, electrolyte and acid–base balance, and also has an endocrine function Urine is produced in the kidney by the selective removal of substances from the blood plasma. Subsequent controlled reabsorption of water, ions, salts, sugars and other carbohydrates, and small molecular weight proteins, allows the kidney to produce urine, the composition of which is appropriate to the body’s internal environment and requirements at the time. For example, if the plasma volume is expanded and diluted by a substantial ingestion of water, the kidney will then excrete the excess water by producing large quantities of dilute urine. Conversely, if fluid ingestion is restricted, the kidney will produce a small amount of highly concentrated urine. Whatever the quantity and concentration of the urine, it will contain the required amount of waste products and ions to maintain internal biochemical homeostasis. An inability to produce concentrated or dilute urine is an important feature of kidney failure, as it is evidence of inadequate excretion of nitrogenous waste products and other substances, for example potassium ions (see p. 309). It is important to realize that the kidneys (and the lungs) differ from most other organs in one important respect. In most organs, the vascular supply is the servant of the parenchymal tissues, providing them with oxygen and other raw materials required for the tissue’s metabolic processes, and carrying synthesized cell products and waste materials away from the organ. In the kidney, however, the parenchymatous components of the organ are the servants of the blood supply, as the function of the kidney, in broad terms, is the filtration and cleansing of the blood. Thus, the parenchymal unit of the kidney, the nephron, which is composed of the glomerulus and cortical and medullary tubular systems, can be regarded as an appendage of the renal blood vascular system, attached to it for servicing purposes. It is not surprising, therefore, that the blood vascular system of the kidney is both substantial and structurally unusual, reflecting its central role. The two kidneys receive 25% of the total cardiac output. Consequently, many of the important and common diseases of the kidney result from abnormality in the blood vascular component (see p. 309). The kidney also produces the hormones erythropoietin and renin. Arteries supplying the kidney branch within the renal substance to supply distinct regions In most cases, the arterial supply to each kidney comes from a single renal artery, which is a substantial, direct lateral branch of the abdominal aorta. A common variant is the presence of a separate artery arising directly from the aorta and supplying one or other pole. The renal artery runs towards the concave hilum of the kidney and divides into two main branches, one anterior, one posterior (Fig. 15.3), each of which divides into a number of interlobar arteries that run between the medullary pyramids, one branch to each developmental lobe. At about the midpoint of the thickness of the kidney parenchyma, where the cortex abuts on the broad base of the medullary pyramid (the corticomedullary junction), the interlobar artery divides into several arcuate arteries, which make a right angle turn and run laterally along the corticomedullary junction. The arcuate arteries then give rise to a series of side branches (interlobular arteries), which again make a right angle turn and run vertically upwards into the cortex. Interlobular arteries give rise laterally to a series of arterioles, called ‘afferent arterioles’, usually directly, but sometimes via a short intralobular artery. These arteries terminate at the periphery of the kidney, just beneath the capsule, supplying a stellate subcapsular arteriolar and capillary plexus. Thus far, the arterial supply has been anatomically unremarkable. From the afferent arterioles onward, however, the renal vascular system becomes unique. The renal microcirculation contains two capillary systems In almost every other organ, the capillary network lies between the terminal part of the arterial/arteriolar system and the proximal part of the venular/venous system, and is the major site of oxygen/carbon dioxide exchange. The renal vascular system, in contrast, has a highly specialized preliminary capillary network, the glomerular tuft, which receives blood from an afferent arteriole and which is the site of filtration of waste products from plasma, together with a second capillary system arising from the efferent arteriole, which varies in structure and function according to its location within the kidney (Fig. 15.4). In most cases, after leaving the glomerulus, the efferent arteriole divides into a complex capillary system, which runs in the interstitial spaces between the components of the system of cortical tubules. Each capillary is in intimate contact with these tubules, and is thus ideally placed to take up any substances reabsorbed from the glomerular filtrate by tubular epithelial cells (see p. 298). However, the capillary system originating from efferent arterioles leaving glomeruli situated deep in the cortex, close to the corticomedullary junction (juxtamedullary glomeruli), is different (see Fig. 15.4). These efferent arterioles divide into a series of long, thin-walled vessels, the vasa recta, which run straight down into the medulla alongside the medullary components of the tubular systems. These vessels play an important role in the ionic and fluid exchanges occurring in the medulla (see p. 301). Some vasa recta arise as direct, vertically running side branches of the arcuate artery. The first capillary system, the glomerular tuft, does not transfer its contained oxygen to the tissues, nor does it take up a significant amount of carbon dioxide. The major exchange of dissolved gases takes place in the second capillary system. Oxygen is supplied to the cortical and medullary parts of the renal parenchyma, which have the highest demand because of their high metabolic activity. The venous drainage of the kidney mirrors the arterial supply In general, the renal venous drainage mirrors the arterial supply, except that there is no venous equivalent of the glomerular capillary tuft. The subcapsular arteriolar and capillary plexuses drain into a subcapsular venular and venous plexus of stellate veins that forms the origin of the interlobular veins. As they proceed towards the corticomedullary junction, the interlobular veins receive venous tributaries from the peritubular capillary network and, as the juxtamedullary zone is approached, from some of the venous tributaries from the medulla, which are the venous equivalent of the arterial vasa recta. Many of the medullary venous vessels drain directly into the arcuate veins, which run laterally with the equivalent artery at the corticomedullary junction. These in turn drain into large interlobular veins lying between adjacent medullary pyramids, and then into the major vein tributaries at the renal hilum. The major renal vein opens end-to-side into the inferior vena cava. The functional unit of the kidney parenchyma that serves the blood supply is called the nephron The nephron has two main components: • The glomerulus, which is associated with the first capillary system • The cortical and medullary tubular systems, which are associated with the second capillary system. The glomerulus is the site of initial blood filtration and the tubular systems are the site where the concentration and chemical content of blood returned to the general systemic circulation, and hence the concentration and content of the urine to be voided from the body, are controlled. Figure 15.5 shows the structure of a nephron and its relationship to its microcirculation. The simplified overview of the various homeostatic functions of the tubular component of the nephron will be dealt with in greater detail later in the chapter. Within the renal cortex, the nephrons are organized into a distinct repeated lobular pattern The glomeruli, and proximal and distal tubules, are aligned mainly on either side of the interlobular arteries that supply them with blood (see Fig. 15.4). At the midpoint between adjacent interlobular arteries is a vertically running arrangement of tubules and ducts known as the ‘medullary ray’ (see Fig. 15.36c), at the centre of which is a main collecting duct that collects all of the largely unconcentrated urine from the nephrons on either side. The other tubular components of the medullary ray are the straight collecting tubules carrying urine from the end of the distal tubule to the main cortical collecting duct. The duct systems of the medullary ray run vertically downward into the medulla. The subunit of the cortex, comprising a centrally placed medullary ray and the nephrons on either side of it, is called the ‘renal lobule’ and each interlobular artery runs upward in the cortex between adjacent lobules. This lobular arrangement and the medullary ray system can be seen in Figure 15.36. The first functional component of the nephron encountered by the microcirculation is the glomerulus, which is the site of initial filtration of the blood arriving by the afferent arterioles. The afferent arteriole enters the glomerulus at the vascular pole The afferent arteriole, which enters the glomerulus immediately, splits up into about five main branches. Each branch then subdivides into its own capillary network (see Fig. 15.4), the short main branch and its capillaries being supported by its own strip or stalk of mesangium (see p. 297). The division of the glomerular capillary network into about five independent segments gives the glomerular tuft an implicit lobulation, which is rarely apparent by routine light microscopy in health, but which becomes evident in some forms of primary glomerular disease, particularly when the mesangial component of each segment is enlarged (see Fig. 15.15). The independence of each glomerular segment is also demonstrated by disease affecting only one segment (e.g. segmental glomerulonephritis). The glomerular capillaries converge to form a single efferent arteriole, which leaves the glomerulus at the same vascular pole as the afferent arteriole enters. The glomerulus is a complex structure in which capillaries are in intimate contact with a specialized epithelium In simple terms, the structure of the glomerulus is best conceived as a globular capillary network intruding into a hollow sphere of epithelial cells called ‘Bowman’s capsule’, which represents the bulbous, distended closed end of a long hollow tubular system (Fig. 15.6). This means that the glomerular capillary system, which is lined internally by endothelial cells, acquires an outer layer of epithelial cells that is continuous at the vascular pole with the cells lining Bowman’s capsule. The epithelial cells of Bowman’s capsule are flat and simple, except near the opening of the tubular system, where they become more cuboidal and acquire some of the cytoplasmic organelles of the proximal convoluted tubule epithelial cells (see pp. 298-299). In contrast, the epithelial cells coating the glomerular capillary tuft are larger and have a highly specialized and unusual structure, which has led to their being named ‘podocytes’ (see p. 295). The epithelium-lined space between the coated glomerular capillary network and the parietal shell of Bowman’s capsule is called the ‘urinary space’, and is continuous with the lumen of the long tubular system of the nephron. As well as the outer epithelial (podocyte) coating, the glomerular capillary network has other unusual features, including the possession of an unusually thick basement membrane (the glomerular basement membrane) and the presence of a supporting strip or stalk, analogous to the mesentery of the small bowel, called the ‘mesangium’ (Fig. 15.7 and see p. 296). Blood enters the glomerular capillary network from the afferent arteriole. Ultrafiltration of the blood then occurs in the glomerular capillary network and the filtrate passes into the urinary space before passing down the tubular system. The partly filtered blood leaves the glomerulus via the efferent arteriole and flows onward to provide an oxygenated blood supply to the tubular systems. The barrier between circulating blood and the urinary space is the glomerular filtration barrier (Fig. 15.8). It is composed of: • The capillary endothelial inner layer • The unusually thick glomerular capillary basement membrane An additional component of the functional barrier is a high polyanionic charge on some of the above structural components. Glomerular capillary endothelial cells are attenuated and fenestrated The endothelial cells in the renal glomeruli are adapted for their specialized filtration role. The cytoplasm forms a thin sheet broken by numerous small circular pores or fenestrations, each about 70 nm in diameter. Their nuclei are usually located near the mesangium. Normal glomerular capillary endothelium is shown ultrastructurally in Figure 15.8. A diagrammatic representation, illustrating the structure of the endothelium and its relationships with other glomerular components, is shown in Figure 15.14b. The glomerular basement membrane is divided into three distinct zones Glomerular basement membrane is much thicker than normal capillary basement membranes elsewhere. It measures approximately 310–350 nm in healthy young adults, being slightly thicker in males. Both the inner endothelial and outer epithelial cell populations contribute to its production. It has three layers: • A central electron-dense lamina densa • Electron-lucent lamina rara interna on the endothelial or capillary lumen side • Electron-lucent lamina rara externa on the epithelial podocyte, or urinary space, side. This layered structure is clearly seen in rodents and children but becomes less apparent in most adults (see Fig. 15.8b). The lamina densa is partly composed of type IV collagen (see p. 56), and the fibril network acts as a physical barrier to the passage of large molecules from the blood into the urinary space. The lamina rara layers, and the surfaces of some podocyte secondary foot processes, contain fixed negatively charged (polyanionic) sites composed of glycosaminoglycan (see p. 55). In the basement membrane, this is heparan sulfate, and on the foot process surfaces it is a sialic acid-rich substance named ‘podocalyxin’. When demonstrated ultrastructurally using a cationic substance, such as ruthenium red or polyethylenimine (Fig. 15.10), such sites appear to be organized to form fairly regular lattice with a spacing of approximately 60 nm. It is thought that the polyanionic sites act as a charge barrier, preventing the passage of cationic molecules. The combination of layers in the glomerular basement membrane produces both a physical and an electrical barrier to the passage of large molecules (i.e. over 70 kDa) and highly cationic molecules of many sizes. Nevertheless, certain large molecules, including some proteins, may pass through into the urinary space and require reabsorption in the tubular system. The full thickness of the glomerular basement membrane does not completely surround the circumference of the capillary wall, but instead occupies about three-quarters of it, being partly deficient at the site of the attachment of the capillary to the mesangium. The lamina rara interna continues as an ill-defined layer between the endothelial cytoplasm and the cytoplasmic and matrix components of the mesangium (see Fig. 15.14b). The podocyte layer lies on the outer surface of the glomerular capillaries The podocyte layer is composed of specialized epithelium which is continuous at the vascular hilum with the flat, relatively inert epithelium lining Bowman’s capsule. It is highly specialized in structure, and presumably in function as well, though much of its functional detail is not known. The podocyte is so named because the main body of the cell hovers above the external surface of the glomerular capillary and sends down cytoplasmic extensions (foot processes) that make contact with the basement membrane (see Figs 15.8 and 15.11). Between adjacent foot processes on the glomerular basement membrane is a fairly consistent gap of 30–60 nm, the filtration slit. A thin membrane, the filtration slit membrane, bridges the gap between adjacent foot processes. This filtration slit membrane contains a cell adhesion molecule called ‘nephrin’, which connects with actin filaments within the adjacent foot processes. The functional aspects of this complex cytoplasmic arrangement are not understood, but it obviously plays an important role in preventing certain molecules from passing into the urinary space. Loss of the podocyte foot process pattern in some renal diseases is associated with such excessive protein loss (mainly albumin) that the patient develops the nephrotic syndrome (see below). The mesangial support to the glomerular capillary network has two components, mesangial cells and extracellular mesangial matrix. The role of the mesangium as a support for the glomerular capillary system can be seen in Figure 15.7, and Figure 15.14 illustrates the details of the structure of the mesangium and its relationship to the glomerular capillary and the glomerular basement membrane.
Urinary System
Introduction
Outline of the Urinary System
Kidney Structure
Kidney Function
Kidney Vasculature
Renal Microcirculation
Nephron
Glomerulus
Glomerular Filtration Barrier
Mesangium
Urinary System
Chapter 15
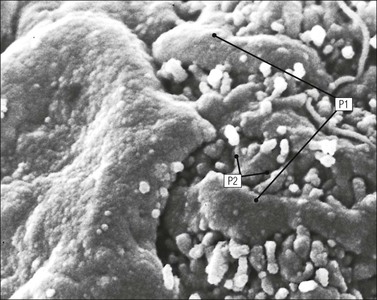
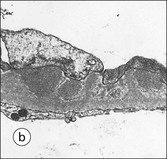
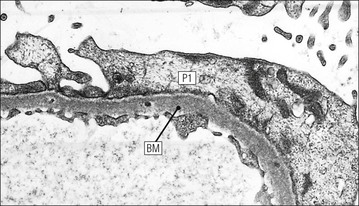
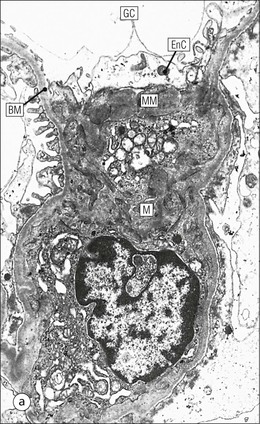
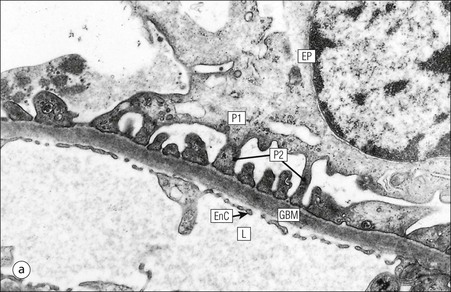
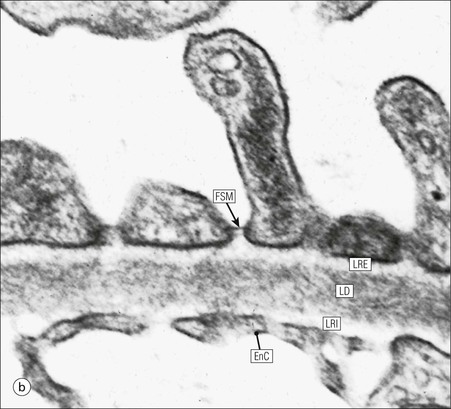
FIGURE 15.8 Glomerular capillary wall. (a) Electron micrograph showing the relationship between the glomerular basement membrane (GBM), capillary lumen (L), endothelial cytoplasm (EnC) and the epithelial podocyte (EP), with its primary (P1) and secondary (P2) foot process system. (b) High-power electron micrograph of the glomerular filtration barrier, comprising fenestrated endothelial cytoplasm (EnC), glomerular basement membrane and podocyte foot processes. Note the filtration slit membrane (FSM, see p. 295). In this high-magnification electron micrograph, the three layers of the basement membrane can be seen, although they are indistinct in adult humans (as here). The layers are lamina densa (LD) and, on either side of it, lamina rara interna (LRI) and lamina rara externa (LRE).