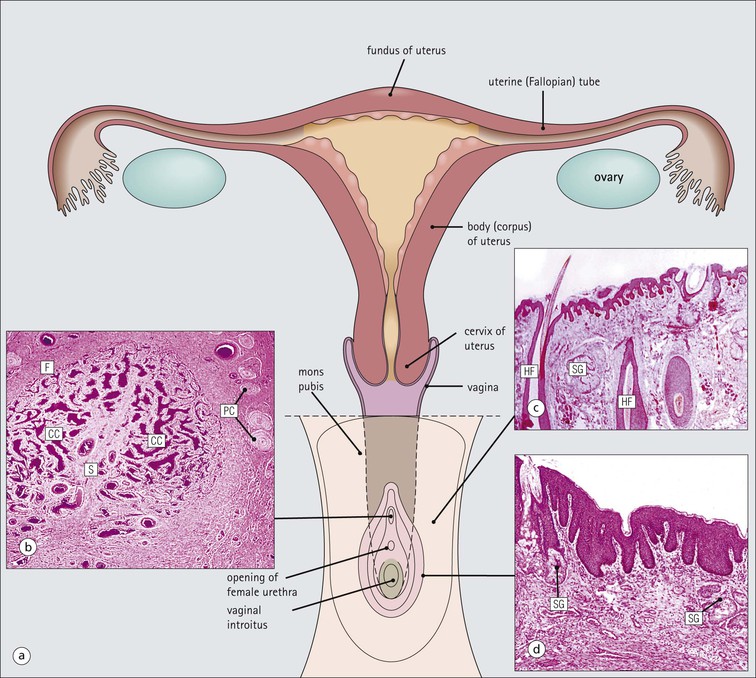
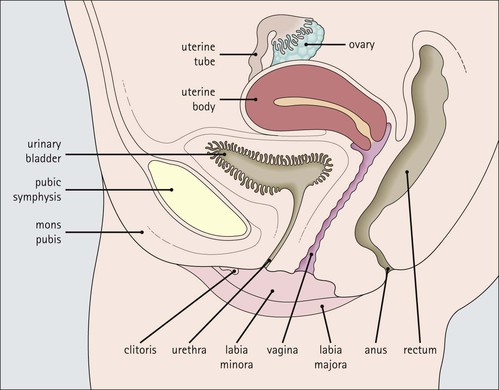
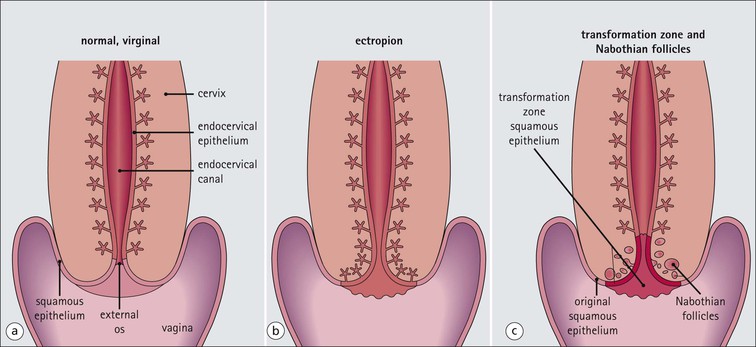
The female reproductive system: • Produces haploid female gametes (ova) • Receives haploid male gametes (spermatozoa) prior to fertilization • Provides a suitable environment for fertilization of ova by spermatozoa • Provides a suitable physical and hormonal environment for implantation of the embryo • Accommodates and nourishes the embryo and fetus during pregnancy The structure of the human female reproductive system changes considerably from childhood into reproductive maturity, and later the menopause, under the control of tropic hormones. Furthermore, the various components undergo structural and functional modification at different stages in the monthly cycle. The system (Figs 17.1, 17.2) comprises the ovaries, uterine tubes, uterus and vagina (collectively referred to as the internal genitalia), together with the mons pubis, vulva (labia majora and labia minora) and clitoris (referred to as the external genitalia). Unlike many other mammals, the human female ovulates at regular intervals (approximately every 28 days) throughout the year. As tissues from other animals do not accurately reflect the changes seen in the human, only human tissues are described and shown in this chapter. The mons pubis, labia majora and labia minora all consist of modified skin (see Chapter 18) as given below. The mons pubis (mons veneris) is skin superimposed on a substantial pad of subcutaneous fat The mons pubis is the area overlying the symphysis pubis. It is characterized by the presence of unusually oblique hair follicles, which produce the coarse, curly pubic hair common to most races. Underneath the skin is a pad of fat. The labia majora are posterolateral extensions of the mons pubis on either side of the vaginal introitus The labia majora are similarly richly endowed with subcutaneous fat and oblique hair follicles (see Fig. 17.1c). There are smooth muscle fibres in the subcutaneous fat. The accumulation of subcutaneous fat and the development of the oblique hair follicles and pubic hair is hormone dependent and begins with the onset of sexual maturity, usually between the ages of 10 and 13 years. This area is also rich in apocrine glands and prominent sebaceous glands, both of which mature and become active at the onset of sexual maturity, but eccrine sweat glands, which are present from birth, show no change. The labia minora are thin flaps of skin devoid of adipose tissue but with abundant blood vessels and elastic fibres Although hair follicles are absent, there are many sebaceous glands that open directly on to the epidermal surface (see Fig. 17.1d). The epidermis of both the labia majora and minora becomes pigmented by melanin with the onset of puberty. The outer, lateral aspect of the labia minora is usually more pigmented than the inner medial aspect, and the epidermis has a well-developed rete ridge system. On the inner aspect, the melanin pigmentation becomes progressively reduced as the vaginal introitus is approached and the keratinized, stratified, squamous epithelium becomes thinner, with flattening of the rete ridges and thinning of the keratin layer. This keratinized epithelium extends into the vaginal vestibule as far as the hymen, which is a thin fibrous membrane that is rarely completely intact and usually appears as an irregular ‘frill’ in the lining of the lower vagina, the hymeneal tegmentum. On its exterior (vulval) surface the hymen is covered with the keratinized stratified squamous epithelium, whereas the inner (vaginal) surface is covered with non-keratinized stratified squamous epithelium, rich in glycogen, which is similar to that lining the vagina (Fig. 17.3). The hymen can be regarded as the junction between the internal and external genitalia. The clitoris is located below the mons pubis and is the female equivalent of the penis The clitoris is composed of two corpora cavernosa of erectile vascular tissue which lie side by side surrounded by a fibrocollagenous sheath; an incomplete central septum partly separates the two corpora (see Fig. 17.1b). The clitoris is covered by thin epidermis that is devoid of hair follicles, sebaceous glands, eccrine and apocrine glands, but is richly equipped with sensory nerves and a variety of receptors. Over the superior surface of the clitoris, the skin forms an incomplete hood (the clitoral prepuce), and, on the inferior surface, a thin midline frenulum. At the base of the clitoris, the corpora cavernosa diverge to lie along the pubic rami, where they contain fibres of the ischiocavernosus muscle. The clitoris, which is small before puberty, enlarges to a greater or lesser extent with the onset of sexual maturity. During sexual arousal, it becomes engorged in a manner similar to that of the penis. The urethral meatus opens to the exterior in the midline below the clitoris On either side of the meatus are the openings of the paraurethral glands (of Skene). These glands are located around the urethra, mainly posteriorly and laterally, and are lined by pseudostratified columnar epithelium. The vagina is a fibromuscular tube extending from the vestibule to the uterus In the mature female, the vagina is 7–9 cm long, but is capable of both marked distension and elongation (see Fig. 17.1). It forms an angle of more than 90° with the normal anteverted uterus (see Fig. 17.2). At its inner end, the vagina forms a cuff around the protruding cervix of the uterus, forming anterior, posterior and lateral pouches known as the ‘fornices’. The vagina has four layers (see Fig. 17.3) as follows: The rich meshwork of elastic fibres in the vaginal wall is responsible for its elasticity and permits the great temporary distension required during parturition. The extensive submucosal plexus of thin-walled blood vessels is thought to permit the diffusion of watery fluid across the epithelium and to contribute to vaginal fluid. Although structurally a tube, at rest, the vagina is collapsed so that the anterior wall is in contact with the posterior wall, and for much of its length there are shallow longitudinal grooves in the midline of both these walls. In addition, the vaginal mucosa is rugose, being thrown up into a series of closely packed transverse mucosal folds or ridges. The structure of the vagina varies with age and hormonal activity Changes occur in the non-keratinizing, stratified squamous epithelium which lines the vagina. Before puberty the epithelium is thin, a state to which it reverts after the menopause, but during the reproductive years the epithelium responds to the activity of oestrogens and thickens. The basal cells and the distinct parabasal layer show increased mitotic activity, and the more superficial cells increase not only in number but also in size as a result of the accumulation of stored glycogen and some lipid within the cytoplasm. Glycogen content is maximal at the time of ovulation, and some glycogen-rich surface squames are shed into the vaginal cavity after ovulation, during the secretory phase of the menstrual cycle. Breakdown of the glycogen by commensal lactobacilli in the vaginal cavity produces lactic acid, resulting in an acid pH. This restricts the vaginal bacterial flora to acid-loving commensals and deters invasion by bacterial pathogens and fungi, such as Candida albicans, which is the cause of vaginal ‘thrush’. Bartholin’s (vulvovaginal) glands are located around the lower vagina The Bartholin’s glands are composed of acini lined by tall columnar mucus-secreting cells with pale cytoplasm and small basal nuclei. These glands open into the vagina posterolaterally at the level of the hymeneal remnants through a duct lined by a transitional epithelium with a surface mucin-secreting layer. The uterus is a muscular organ and receives the right and left uterine (fallopian) tubes. It is lined by columnar epithelium and at its lower end, it opens into the vagina. The uterus can be divided into three parts: the fundus, the body and the cervix (Fig. 17.4). Whereas the fundus and body have the same histological structure (see p. 344), that of the cervix is different. The cervix is the lower part of the uterus, part of which protrudes into the vagina The junction between the cervix and the uterine body is the internal os, and at this point the nature of the lining epithelium and the uterine wall changes (Fig. 17.5). The cervical lumen opens into the vaginal cavity at the external os, where again there is an important change in the nature of the lining epithelium. This zone is the site of many important pathological changes (see Figs 17.8, 17.9). The cervix is cylindrical and symmetrical, being about 3 cm long and 2–2.5 cm in diameter, but becomes more barrel-shaped after pregnancy and parturition. After childbirth, the external os becomes a transverse slit dividing the distal end of the cervix into anterior and posterior lips, whereas the external os of the nulliparous cervix is circular. The cervical stroma is important in childbirth The cervical stroma is composed of smooth muscle fibres embedded in collagen, the proportions of the two components varying with age and parity. Normally, the cervix is firm and rubbery and the cervical lumen is a narrow channel, but the cervix can be dilated under some circumstances, a fact which is used in investigative gynaecological practice to obtain samples of the uterine lining epithelium for histological examination. A measure of the capacity of the cervix for dilatation is the fact that a cervical lumen, which is normally a narrow canal incapable of taking a pencil, becomes, at childbirth, capable of allowing the passage of a baby. Furthermore, after delivery, the cervix reverts to normal in a very short time. This capacity depends on radical alterations in the nature of the cervical stroma, attended by a change in texture (‘cervical softening’). The external surface of the part of the cervix that protrudes into the vagina is the ectocervix and the lining of the lumen is the endocervix. The ectocervix is covered by epithelium continuous with that of the vagina at the vaginal fornices Like vaginal squamous epithelium, the ectocervical epithelium is non-keratinizing, stratified, squamous and rich in glycogen in the sexually mature period (Fig. 17.6). It undergoes cyclical changes during the menstrual cycle under the influence of oestrogens and progesterone. Before menarche and after menopause, the epithelium is much thinner, with fewer layers and smaller cells containing less glycogen. The endocervical canal runs between the uterine and vaginal cavities The canal is lined by a single layer of tall columnar mucus-secreting epithelium, the endocervical epithelium (Fig. 17.7a). Longitudinal or transverse histological sections of the cervix have given the impression that there are glandular structures (endocervical mucous glands) extending into the underlying stroma. Three-dimensional studies, however, indicate that the structures are in fact deep, slit-like invaginations of the surface epithelium, with blind-ended tubules arising from the clefts (Fig. 17.7b). Thus, there is a large surface area for the production of cervical mucus, which fills the endocervical canal. Before puberty and after the menopause, the amount of cervical mucus is greatly reduced. As well as contributing to vaginal lubrication during sexual intercourse, the mucin in the endocervical canal acts as a protective barrier, preventing bacterial ascent into the endometrial cavity. Movement of endocervical mucus into the vaginal canal is facilitated by a few ciliated columnar epithelial cells scattered among the mucus-secreting endocervical cells, particularly at the upper end of the canal, close to the junction with the endometrium. Both the mucin-secreting and ciliated cells have fine microvilli, which are only visible ultrastructurally. The columnar epithelium of the endocervical canal and the squamous epithelium of the ectocervix meet at the squamocolumnar junction of the cervix This is the most likely area of the cervical epithelium to be affected by disease. The original squamous columnar junction is usually located in the region of the external os, but its precise location at birth is influenced by maternal hormone exposure in utero. At about puberty, hormonal influences lead to extension of the columnar epithelium on to the ectocervix, forming an ectropion or cervical erosion (Fig. 17.8), which is augmented by a first pregnancy, particularly when this occurs shortly after menarche. Before puberty, the pH of the vagina and cervix is alkaline, but afterwards, bacterial breakdown of the glycogen in the vaginal and cervical squamous epithelium renders an acidic environment, with a pH of about 3. Exposure of the sensitive columnar epithelium of the ectropion to the postpubertal acidic environment of the vagina induces squamous metaplasia and a transformation zone between the endocervical columnar epithelium and the ectocervical squamous epithelium. Thus, the squamous columnar junction is of variable size but its site always approximates to the external os. In older women, it may retreat into the endocervical canal. The transformation zone is composed of new squamous epithelium in an area previously occupied by columnar epithelium. One almost invariable consequence of squamous metaplasia near the external os is that the openings of some of the deep crypts of previously everted endocervical mucous glands become obliterated so that mucin produced deep in the crypts cannot be excreted into the endocervical lumen or vagina. Instead, it accumulates within the blocked clefts and produces spherical cystic masses of inspissated mucus lined by flattened endocervical mucus-secreting epithelium; these are called Nabothian follicles. Other consequences of this constant change of epithelium include the development of abnormal epithelium, which may progress to cancer. The body and fundus of the uterus have thick walls composed of smooth muscle (myometrium) The myometrial smooth muscle is arranged into three ill-defined layers. Myometrium is hormone sensitive and undergoes both hypertrophy (an increase in cell size) and hyperplasia (an increase in cell numbers) during pregnancy (Fig. 17.10), progressively returning to its normal size (involution) in the weeks after delivery. Within the myometrium are prominent blood vessels, both arterial and venous, which undergo marked dilation and thickening of their walls during pregnancy. With cessation of hormonal stimulation after the menopause, the myometrial cells atrophy and the uterus shrinks. The fibrocollagenous tissue between the muscle bundles, which is comparatively insignificant when the myometrial smooth muscle is prominent during sexual maturity, then becomes obvious. The body of the uterus is lined by the endometrium, which is composed of glands and supporting stroma Before puberty, the endometrium is simple and composed of low cuboidal epithelium supported by a scanty spindle-celled stroma. Downgrowths of epithelium into the stroma produce a small number of rudimentary tubular glands. Between menarche (the first menstrual period) and menopause, the endometrium can be differentiated into two layers, a deep basal layer at the junction with the myometrium, and a superficial functional layer lining the lumen. It is the functional layer that is hormone responsive and undergoes the monthly cycle of proliferation, secretion, necrosis and shedding. The endometrium is sensitive to the fluctuating levels of oestrogen and progesterone secreted by the ovary, and the changes are known as the menstrual cycle. The relationships between the histological changes in the endometrium and the ovarian and pituitary hormone secretions are discussed below and illustrated in Figure 17.24, p. 355. If fertilization and successful implantation of an ovum occur, the endometrium remains unshed and forms the decidua (see p. 360). The basal layer, which is not shed at menstruation, provides a cellular reserve from which a new functional layer develops after menstrual shedding. The endometrium does not respond evenly to ovarian hormonal stimulation: functional endometrium in the lower segment close to the junction with the cervix, and patches around the entrances of the uterine tubes, may show little proliferative or secretory activity, and often resembles the basal layer. As menopause approaches, more and more of the endometrium may fail to respond fully to the hormonal stimulus. At menopause, when hormonal stimulation ceases, the endometrium reverts to the simple prepubertal pattern, although the tubular downgrowths may undergo cystic distension and the stroma may become compact (atrophic cystic endometrium). The uterine (fallopian) tubes convey ova from the ovary to the lumen of the body of the uterus (endometrial cavity) They are also the site of fertilization of the ovum by spermatozoa. After fertilization, the tube transmits the fertilized ovum to the endometrial cavity where implantation can take place. Each uterine tube is 10–12 cm long and extends from a dilated open end close to the ovary to a narrow portion which passes through the myometrial wall of the uterus before opening into the uterine cavity. There are four recognizable tubal segments (Fig. 17.12), each differing histologically, particularly in their proportions of muscle and epithelium and the degree of convolution of their epithelium.
Female Reproductive System
Introduction
Mons Pubis, Labia Majora and Labia Minora
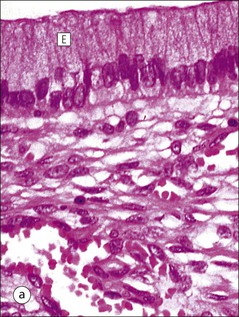
Clitoris
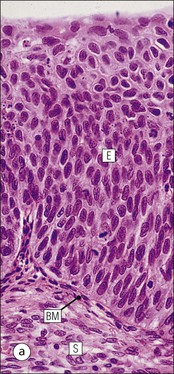
Vagina
Uterus
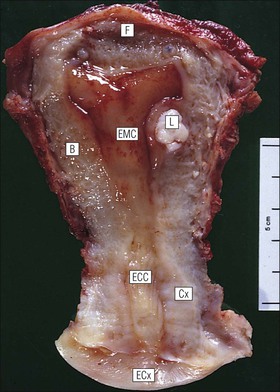
Cervix
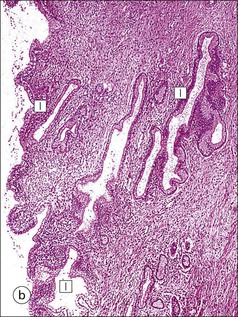
The Epithelial Content of the Cervix
Uterine Body
Uterine Tubes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Female Reproductive System
Chapter 17
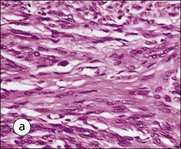
FIGURE 17.1 Internal and external genitalia. (a) The relationship between the external and internal genitalia. (b) Micrograph of clitoris; note the two corpora cavernosa (CC), arranged side by side and engorged with blood, the incomplete central septum (S) and the fibrocollagenous sheath (F), outside which are prominent nerve endings (mainly Pacinian touch corpuscles (PC), see also Chapter 18). (c) Micrograph of labia majora. The skin contains many hair follicles (HF), sebaceous glands (SG) and eccrine sweat glands and ducts. (d) Micrograph of labia minora. The skin is devoid of hair follicles but is rich in sebaceous glands (SG), which open directly on to the surface. Note that the dermis is highly vascular.
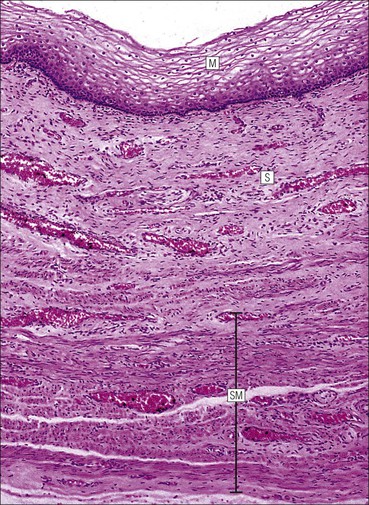
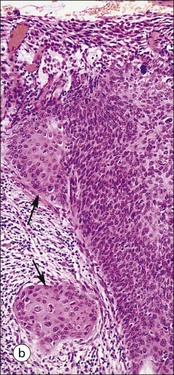
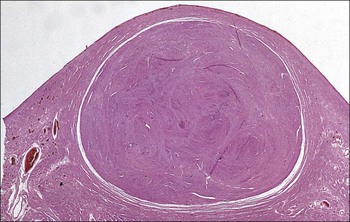
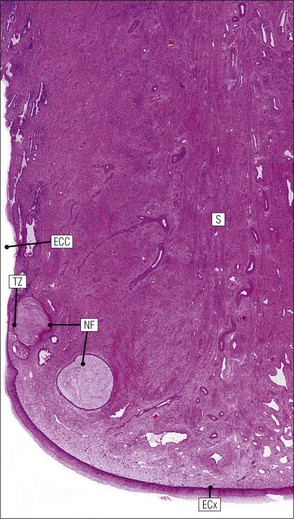
FIGURE 17.5 Cervix. Low-power micrograph of the cervix. The stroma (S) is composed of smooth muscle fibres embedded in collagen, the proportions of muscle and fibrous tissue varying according to age and parity; blood and lymphatic vessels are prominent and numerous. The ectocervix (ECx) is covered with stratified squamous epithelium, and the endocervical canal (ECC) is lined by tall columnar epithelium. The junction between the squamous and columnar epithelium is located in the region of the external os. In this example there is a transformation zone (TZ) (see p. 343) of squamous epithelium, which has extended into the endocervical canal; note the Nabothian follicles (NF) (see Fig. 17.8c).
FIGURE 17.8 Squamous/columnar junction of the cervix. The mobility of the squamous/columnar junction, the development of ectropion and the formation of the transformation zone. (a) The squamous/columnar junction is originally situated in the region of the external os. (b) At puberty, the endocervical epithelium extends distally into the acid environment of the vagina and forms an ectropion. (c) A transformation zone forms as squamous epithelium regrows over the ectropion. The openings of the crypts may be obliterated in the process, resulting in the formation of mucus-filled Nabothian follicles (see Fig. 17.5).