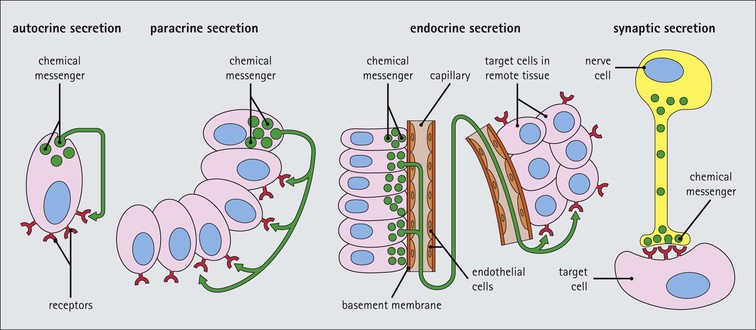
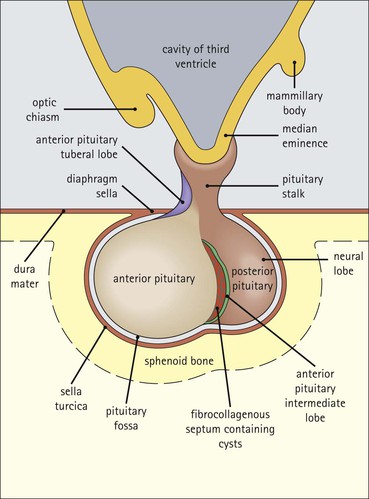
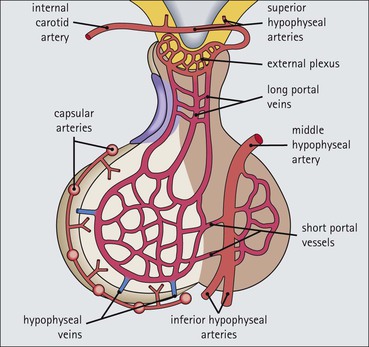
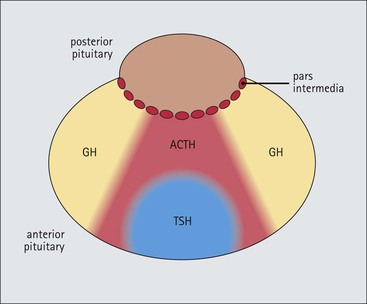
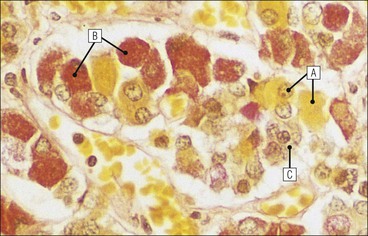
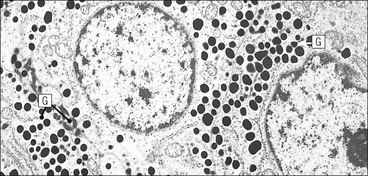
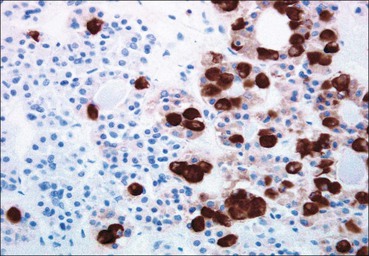
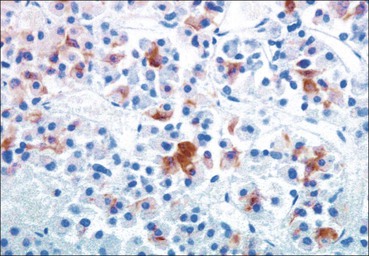
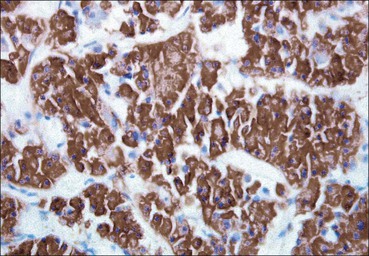
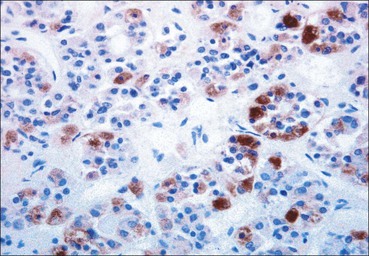
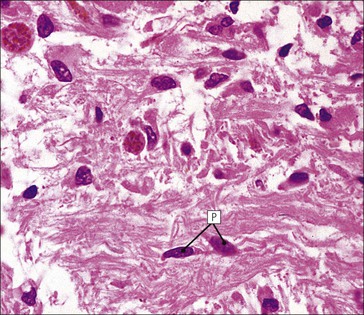
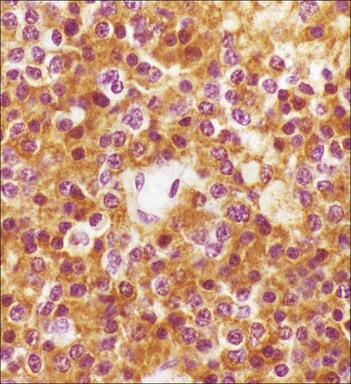
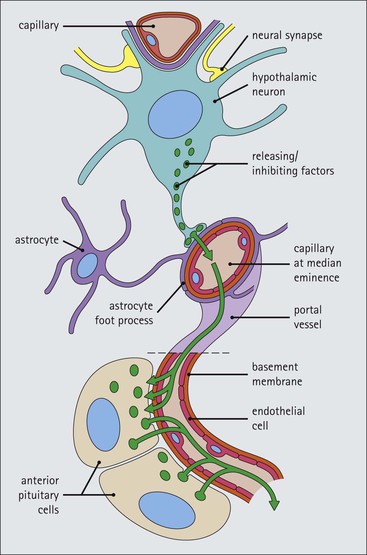
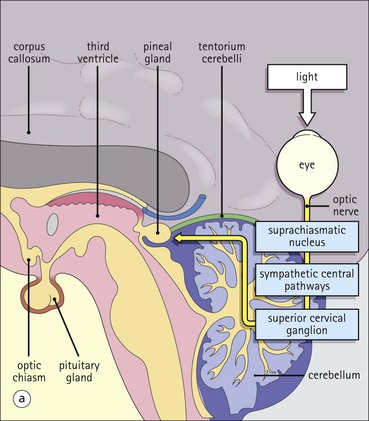
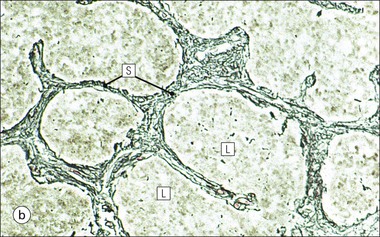
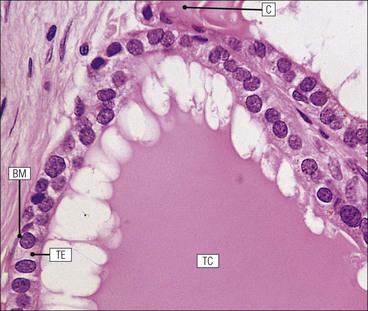
Cell communication is vital for any multicellular organism to function efficiently. At a local level, cells communicate via cell surface molecules and gap junctions, whereas remote communication is mediated by the secretion of chemical messengers which activate cells by interacting with specific receptors. Such secretion may be one of four main types: autocrine, paracrine, endocrine or synaptic (Fig. 14.1). Autocrine secretion occurs when a cell secretes a chemical messenger to act on its own receptors. This is particularly evident in the local control of cell growth by substances such as epidermal growth factor. Paracrine secretion describes the secretion of chemical messengers to act on adjacent cells. This is also mainly concerned with the local control of cell growth and is also a mode of action of many of the cells of the diffuse neuroendocrine system (see p. 280). Endocrine secretion is the secretion of chemical messengers (hormones) into the bloodstream to act on distant tissues. Synaptic secretion refers to communication by direct structural targeting from one cell to another via synapses, and is confined to the nervous system. The chemical messengers belong to four main molecular classes: • Amino acid derivatives (e.g. epinephrine (adrenaline), norepinephrine (noradrenaline), thyroxine) • Small peptides (e.g. encephalin, vasopressin, thyroid-releasing hormone) • Steroids (e.g. cortisol, progesterone, estradiol, testosterone). Most chemical messengers are water-soluble hydrophilic molecules that diffuse freely and usually interact with a cell surface receptor protein. However, steroids and thyroxine are hydrophobic: after carriage to a cell by special proteins in the blood, they pass through its membrane to interact with receptor proteins inside. Cells whose main role is to secrete messenger substances are termed endocrine cells Endocrine cells are found in three distinct anatomic distributions: Endocrine cells and tissues have special characteristics related to their secretory function Certain cells termed ‘neuroendocrine cells’ have membrane-bound vesicles, which are granules containing the chemical messenger. Secretion is achieved by exocytosis, in which the membrane of the vesicle fuses with the cell membrane, thereby discharging its contents outside the cell. Neuroendocrine cells transiently store the messenger as specific neuroendocrine granules, which can be identified in cells using immunohistochemical staining techniques. Endocrine tissues are usually highly vascular to facilitate rapid dissemination of secreted products into the bloodstream. In contrast to other types of secretory cell, the secretory pole of endocrine cells is adjacent to the capillary vessel wall and the nucleus is found at the opposite pole. Autocrine and paracrine messengers act relatively slowly, having to diffuse into the bloodstream. They act on local cell receptors and are rapidly destroyed once secreted, thereby limiting their activity. Endocrine messengers act relatively slowly, having to diffuse into the bloodstream, circulate to a target organ and then enter a target cell. Many neuroendocrine cells secrete amines or peptides and have common metabolic features involving the uptake of amines, which then undergo decarboxylation in the process of hormone synthesis. This has led to the term ‘APUD cells’ (amine precursor uptake and decarboxylation). Cells containing neuroendocrine granules and of neuroendocrine lineage have a restricted set of specific metabolic isoenzymes and structural proteins, which can be detected histochemically. γ-Enolase is an isoenzyme in the glycolytic pathway that is present in high concentration in neuroendocrine cells together with PGP 9.5 (ubiquitin-C-terminal hydrolase). In the neuroendocrine granules, chromogranin is a component of the core protein and synaptophysin is a glycoprotein in their membrane. Histochemical detection of these substances enables their use as markers of neuroendocrine differentiation. The pituitary is a multifunctional endocrine gland The pituitary gland secretes a large number of hormones to activate many peripheral endocrine cells, for example those in the adrenal glands, thyroid, testes and ovaries. The pituitary is a bean-shaped gland approximately 12 × 10 × 9 mm in size and weighing 0.4–0.9 g in the adult. It is situated beneath the brain, to which it is linked by the pituitary stalk, and is surrounded by the bone of the base of the skull in a depression of the sphenoid bone termed the ‘sella turcica’ (Fig. 14.2). Anatomically the pituitary is divided into two parts The anterior pituitary (adenohypophysis) is an epithelial-derived tissue with three distinct components: the distal lobe (pars distalis) forms the major portion of the gland; the intermediate lobe (pars intermedia), which is a rudimentary zone in man but prominent in other mammals; the tuberal lobe (pars tuberalis), which is a layer of cells running up the pituitary stalk. The posterior pituitary (neurohypophysis) is composed of neuronal processes and glia and has three components: the neural lobe (pars nervosa, infundibular process), lying behind the anterior pituitary in the sella turcica; the pituitary stalk (infundibular stem), in which axons run from the brain above; the median eminence (infundibulum), a funnel-shaped extension of the hypothalamus. Embryologically, the anterior pituitary is thought to be derived from an outgrowth of the foregut ectoderm called ‘Rathke’s pouch’. This connects with a downgrowth of the developing hypothalamus, which forms the posterior pituitary and comes to lie in the base of the skull. The vascular supply of the pituitary integrates the functions of the endocrine and nervous systems A special network of blood vessels (pituitary portal system, Fig. 14.3) carries hormones from the hypothalamus of the brain to stimulate or inhibit the hormone secretion of the anterior pituitary. The major blood supply to the anterior pituitary is derived from vessels that run down the pituitary stalk. These are easily damaged in severe head injury, resulting in death of the anterior pituitary and consequent ablation of its endocrine function. The anterior pituitary secretes several hormones into the bloodstream The anterior pituitary is served by a fine capillary network (Fig. 14.3) which, bringing blood from the hypothalamus, contains both stimulatory and inhibitory hormones (Fig. 14.4). These hormones control the neuroendocrine cells of the anterior pituitary. In turn, their secretions diffuse back into the capillary network, which subsequently drains into the pituitary veins and thence into the carotid venous sinus and systemic circulation. The anterior pituitary contains five distinct types of endocrine cell, which vary in their distribution within the gland (Fig. 14.5). Cells were originally named by their staining characteristics, but it is more precise to name the cells according to their specific hormone product (Fig. 14.6); each cell type has the same basic ultrastructure (Fig. 14.7). These cells are: • Somatotrophs (Fig. 14.8), which secrete growth hormone (GH) • Lactotrophs (Fig. 14.9), which secrete prolactin (PRL) • Corticotrophs (Fig. 14.10), which secrete adrenocorticotropic hormone (ACTH), β-lipotropin (β-LPH), α-melanocyte stimulating hormone (α-MSH), and β-endorphin • Thyrotrophs, which secrete thyroid-stimulating hormone (TSH) • Gonadotrophs (Fig. 14.11), which secrete the gonadotropic hormones, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The intermediate lobe of the anterior pituitary is relatively small in man The intermediate lobe of the anterior pituitary is located between the posterior pituitary and the distal lobe (see Fig. 14.2). It is poorly developed in humans compared with other mammals, consisting of a series of gland-like acini lined by a cuboidal epithelium. These cells are usually immunoreactive for corticotropic hormones, and it has been suggested that they may be producing one of the minor subunits of the pre-pro-opiomelanocortic peptides, such as β-LPH, α-MSH or β-endorphin, rather than ACTH. The tuberal lobe is an upward extension of the anterior pituitary around the pituitary stalk The tuberal lobe consists of a thin layer of cuboidal epithelial cells. Immunohistochemically, most of the cells are gonadotrophs. Occasionally, nests of squamous cells can be seen, from which cysts and even tumours may develop. It has been assumed that these are embryological remnants of the ectodermal Rathke’s pouch. The posterior pituitary is a continuation of the hypothalamic region of the brain The posterior pituitary extends down into the pituitary stalk and sella turcica (see Fig. 14.2) and secretes oxytocin and antidiuretic hormone (vasopressin). It is composed of the axons of neuronal cells lying in the supraoptic and paraventricular nuclei of the hypothalamus, together with supporting glial cells termed ‘pituicytes’ (Fig. 14.13). The axons terminate in the posterior pituitary adjacent to a rich network of capillary vessels. The actions of the endocrine and nervous systems are coordinated by the hypothalamus The hypothalamus is a region of brain composed of several clusters of neurons that secrete hormones. These hormones act as either releasing or inhibiting factors for the hormones secreted by the anterior pituitary, and are transported to the pituitary gland by two routes as follows: • A specialized system of blood vessels transports hypothalamic hormones to act locally on neuroendocrine cells in the anterior pituitary (Fig. 14.14) The pineal gland secretes melatonin The pineal gland is located just below the posterior end of the corpus callosum of the brain. It is a flattened conical structure 6–10 mm long and 5–6 mm wide; it is covered by leptomeninges and composed of lobules of specialized cells, which are separated by septa containing unmyelinated nerves and blood vessels (Fig. 14.15). The gland consists of two major cell types: pinealocytes and glial cells. Pinealocytes are neuron-like cells that produce melatonin, which induces rhythmic changes in the secretions of the hypothalamus, pituitary and gonads, and is said to act as an endocrine transducer. They have pink-staining cytoplasm and dark-staining rounded nuclei, and are often arranged in rosettes, where several cells surround a central fibrillary area composed of cell processes directed towards a small capillary vessel. Glial cells tend to be bipolar elongated cells that run between nests of pinealocytes. They are indistinct unless specially stained. A common age-related change in the pineal is the accumulation of calcium particles, which are visible on a skull radiograph; thus, the gland can be used as a radiological midline landmark. The pineal gland is innervated by sympathetic and parasympathetic nervous systems. In addition, signals from the retina arrive indirectly, as shown in Figure 14.15a. The thyroid gland secretes two hormones, thyroxine and calcitonin. Thyroxine has the major role in the regulation of the basal metabolic rate, whereas calcitonin is involved in calcium homeostasis. The thyroid gland consists of two lateral lobes and an isthmus The lateral lobes of the thyroid are arranged on either side of the thyroid cartilage and upper trachea in the anterior portion of the neck. These lobes are joined near their lower poles by the isthmus crossing in front of the lower larynx; occasionally, a small triangular pyramidal lobe projects upwards from the midpoint of the isthmus. Each lateral lobe is about 5 cm long, 3–4 cm wide and 2–3 cm deep. In the healthy adult, the thyroid weighs 15–20 g and is slightly heavier in males, though many factors influence its weight at any one time, for example pathological abnormalities. The thyroid is enclosed by a thin collagenous capsule from which internal septa penetrate the parenchyma, dividing it into irregular lobules. Embryologically, the thyroid develops from a downgrowth of endoderm arising near the root of the tongue and called the ‘thyroglossal duct’, which atrophies and leaves a nodule of thyroid tissue at its correct anatomic site. Occasionally, the thyroglossal duct fails to atrophy completely, and thyroglossal duct tissue persists in the midline of the neck, usually as a cyst or a sinus track (thyroglossal duct cyst). The thyroid gland contains colloid, which is rich in thyroglobulin The glandular component of the thyroid is composed of epithelium arranged as tightly packed spherical units called ‘acini’ (Fig. 14.16). Each acinus is lined by a single layer of specialized thyroid epithelium, which rests on a basement membrane and encloses a lumen filled with thyroid colloid, a pink-staining (eosinophilic) homogeneous proteinaceous material rich in thyroglobulin. Thyroglobulin is the storage form of thyroxine and is an iodinated glycoprotein (Fig. 14.17). The size of an acinus depends on whether it is in a secretory or a storage phase.
Endocrine System
Introduction
Endocrine Cell and Tissue Specialization
Pituitary
Anterior Pituitary
Posterior Pituitary
Hypothalamus
Pineal Gland
Thyroid Gland
Introduction
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine System
Chapter 14