Fig. 30.1
Acneiform eruption on the face arising shortly after initiation of EGFR inhibitor therapy
Treatment of the folliculitis depends on the severity and any associated symptoms. In asymptomatic, relatively mild cases, patients may prefer no treatment at all. Topical steroids and, occasionally, antihistamines are employed for cases of limited severity. More widespread and intense eruptions warrant consideration of doxycycline or minocycline therapy (at doses of 100 mg daily or 100 mg twice daily), or tetracycline 500 mg twice daily. Finally, for the most severe cases, dose discontinuation or reduction should be considered, in addition to topical steroids and systemic antibiotics.
Paronychia and pyogenic granuloma-like lesions around the nail plate are also frequently observed in association with EGFR inhibitor therapy, and typically arise within 4 weeks after beginning treatment. Paronychia is thought to result from retention of nail plate fragment and abnormal desquamation of periungual tissue. While this reaction is non-infectious, secondary bacterial infection may occur. Treatment strategies include avoidance of trauma and use of silver nitrate sticks for pyogenic granuloma-like lesions. Topical steroids, tetracycline antibiotics, and antiseptic soaks may also be helpful to reduce inflammation. This reaction resolves upon discontinuation of the medication.
Several hair changes have been observed to affect EGFR inhibitor-treated patients, usually 2–3 months after beginning therapy. This includes trichomegaly of the eyelashes, which is thought to result from EGFR inhibitor-mediated disruption of the hair cycle, preventing exit from anagen to the catagen phase. Many patients report changes in the hair texture, specifically finer hair and curlier hair, than they had prior to therapy. Alopecia, sometimes with decreased frontal hairline growth, is observed in approximately 20 % of those treated with EGFR inhibitors.
VEGF and VEGFR Inhibitors
Vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) inhibitors are designed to block the process of angiogenesis, which is a critical process during tumorigenesis that provides sufficient blood supply to allow a tumor to grow beyond a certain size. Bevacizumab is a monoclonal antibody that targets VEGF-A, and was approved by the FDA in 2004 for the treatment of metastatic colon cancer; it is now indicated for a number of solid malignancies. Regorafenib has dual activity against VEGFR2 as well as the angiopoeitin receptor TIE2, and was approved in 2012 for the treatment of advanced colorectal cancer.
Hand–foot skin reaction (Fig. 30.2), which is focal hyperkeratosis of the palms and soles with associated mild to moderate erythema, is a commonly seen cutaneous adverse effect of VEGF and VEGFR inhibitors. Typically, this reaction occurs within the first 2–6 weeks after starting the causative medication. It should be noted that this reaction is distinct from the similar sounding hand–foot syndrome, or palmar-plantar erythrodysesthesia, which occurs in patients treated with traditional chemotherapeutic medications such as 5-fluorouracil or capecitabine and manifests with more diffuse and severe erythema and edema. Minimization of friction and trauma is recommended for hand–foot skin reaction, as are keratolytics and emollients. Ice packs and cool water immersion may also be beneficial.
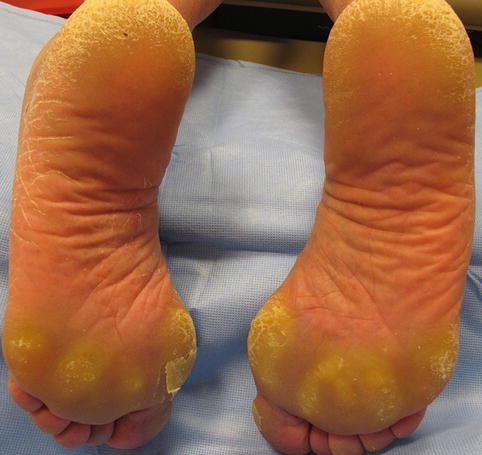

Fig. 30.2
Hand–foot skin reaction of the feet, with prominent hyperkeratosis noted over pressure points
An expected adverse effect of anti-angiogenesis therapy is impaired wound healing, and indeed it has been reported that 1.3–13 % of patients treated with bevacizumab display poor wound healing. Ulcerated striae distensae have also been observed in those taking bevacizumab. Splinter hemorrhages of the nail beds occur commonly. Finally, stomatitis and morbilliform eruptions have been well-documented to occur secondary to anti-VEGF therapy.
Multikinase Inhibitors: Sorafenib, Sunitinib, and Vandetanib
Multikinase inhibitors harbor activity against multiple tyrosine kinases. Two prototypic medications in this class are sorafenib and sunitinib. Sorafenib targets VEGFR2, 3, PDGFR, c-kit, FLT3, RET, and RAF; while sunitinib targets VEGFR1, 2, and 3, PDGFR, c-kit, FLT3, RET, and CSF-1R. Both are utilized to treat advanced renal cell carcinomas, among other tumor types.
Given its anti-c-kit activity, it is not surprising that sunitinib is associated with hair depigmentation and hypopigmentation of the skin (similar to imatinib and dasatinib). Hand–foot skin reaction and alopecia are also observed in patients treated with sunitinib. Yellow pigmentation is a distinctive finding attributed to sunitinib, and is likely related to the color of the drug itself.
Sorafenib is associated with a diverse group of skin adverse effects. Facial and scalp erythema and dysesthesias occur in approximately 60 % of patients treated with sorafenib. Hand–foot skin reaction and splinter hemorrhages also occur frequently. The so-called sorafenib dermatitis presents as a dusky erythema, and may mimic erythema multiforme clinically, but typically has an indolent course. Keratosis pilaris-like eruptions and keratoacanthomas are observed, similar to patients treated with selective BRAF inhibitors.
The multikinase inhibitors offer an intriguing opportunity to correlate specific skin adverse effects with inhibition of a specific receptor tyrosine kinase. Vandetanib is a multikinase inhibitor which targets EGFR, VEGFR2, and the RET tyrosine kinase. It holds FDA orphan drug status for the treatment of medullary thyroid cancer, which commonly harbors RET mutations. Patients treated with vandetanib developed several cutaneous adverse effects, including photosensitivity and diffuse xerosis and eczematous dermatitis. In addition, acneiform eruptions are observed frequently, which is attributable to vandetanib’s activity against EFGR. Those treated with vandetanib also commonly harbor splinter hemorrhages, related to the anti-VEGFR activity of the medication.
A unique side effect of vandetanib is the development of darkly pigmented macules, which are typically folliculocentric in distribution and are often preceded by photosensitivity as well as the characteristic EGFR-related acneiform eruption (Fig. 30.3). They typically occur on the face and neck, trunk, and upper extremities. The macules are typically blue-gray in appearance, and may appear within scars as well. On histopathology, there are many pigmented macrophages in the dermis. The pigmentation is highlighted by Fontana Masson stains, and variably by Prussian blue iron stains. The dyspigmentation may resolve spontaneously while the patient continues therapy, with use of sunscreen and sun avoidance measures. Improvement is also seen slowly after the medication is discontinued or reduced in dose. Finally, it has been shown that Q-switched alexandrite laser therapy yields significant benefit.
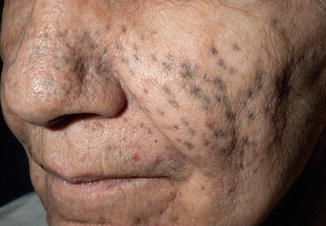

Fig. 30.3
Gray folliculocentric macules on the face, which developed after use of vandetanib. Several acneiform papules and pustules are also seen
Selective BRAF Inhibitors
The selective BRAF serine/threonine kinase inhibitor vemurafenib was approved by the FDA in 2011 for the treatment of patients with advanced, unresectable, V600E-mutated melanoma. A similar agent, dabrafenib, was approved as a single-agent treatment for late-stage V600E melanoma in 2013. The selective BRAF inhibitors are also being utilized for several other types of advanced malignancies harboring V600E mutations, including papillary thyroid cancer and colon cancer. In melanoma patients, use of vemurafenib or dabrafenib has been documented to result in rapid tumor involution, within weeks of initiation of therapy. However, development of resistance to BRAF inhibitors several months after beginning therapy is a common problem. To circumvent tumor resistance, combination regimens such as those using concurrent BRAF and MEK inhibitor therapy have been developed, with the goal of prolonging survival in advanced melanoma patients.
Use of BRAF inhibitors leads to development of several inflammatory eruptions, including generalized morbilliform reactions. Such reactions are particularly prominent in patients treated sequentially with ipilimumab and then BRAF inhibitors, likely because ipilimumab enhances patients’ immune responses to vemurafenib or dabrafenib. Harding et al. reported a series of 13 patients, of whom 4 patients developed grade 3 reactions 6–8 days after starting vemurafenib, after previously having received ipilimumab therapy. On histology, the rash shows a spongiotic dermatitis with perivascular inflammation composed of lymphocytes and eosinophils, typical of a medication hypersensitivity reaction. The rash can be successfully treated by dose interruption and/or reduction. It is notably relatively resistant to treatment with systemic steroids.
Photosensitivity occurs commonly in patients treated with vemurafenib, which results in severe sunburn reactions, often with blistering, in sun-exposed areas of the skin. Erythema may occur with limited sun exposure. Dummer and colleagues found that patients with this type of photosensitivity demonstrated reduced minimal erythema dose (MED) testing to UVA spectrum irradiation, while the UVB MED remained within normal limits. More recently, it has been suggested that a metabolite of vemurafenib, rather than the medication itself, is the photosensitizing agent. As a result of these observations, it is necessary to counsel patients treated with BRAF inhibitors about the high incidence of photosensitivity, and advise them to use sunscreens with broad-spectrum UVA and UVB activity and sun-protective clothing, in addition to practicing sun avoidance when possible.
Panniculitis is a well-documented adverse effect of BRAF inhibitor treatment, occurring in patients treated with vemurafenib or dabrafenib monotherapy and BRAF inhibitor and MEK inhibitor combination therapy (vemurafenib and cobimetinib, dabrafenib and trametinib), but not affecting those treated with MEK inhibitors alone. Painful red nodules develop on the trunk and extremities, often associated with fever, chills, and arthralgias. On histopathology, there is a lobular and sometimes septal inflammatory infiltrate in the subcutaneous fat. This infiltrate may be neutrophil predominant, lymphocyte predominant, or mixed in nature. Occasionally, leukocytoclastic vasculitis is observed on biopsy specimens. Dose interruption and/or reduction of BRAF inhibitors, systemic steroids, and non-steroidal anti-inflammatory medications have been used to effectively treat panniculitis.
Sweet’s syndrome has also been described in patients treated with BRAF inhibitors. On biopsy, the lesions feature a dense neutrophilic infiltrate in the dermis associated with papillary dermal edema. Sweet’s syndrome and the neutrophilic panniculitis described above may represent slightly different manifestations on the same spectrum of BRAF inhibitor adverse effects.
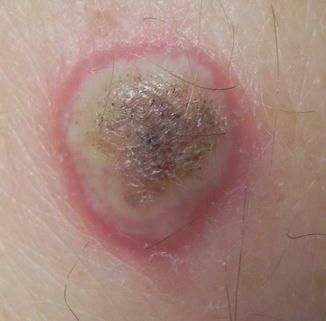
Similar to the multikinase inhibitor sorafenib (which is a non-selective RAF inhibitor), vemurafenib and dabrafenib induce keratoacanthomas and keratoacanthoma-type squamous cell carcinomas (referred to here collectively as cuSCCs, Fig. 30.4). Approximately 20–30 % of patients treated with BRAF inhibitors develop one or more cuSCCs. Lesions tend to develop in patients with a history of extensive sun exposure, and/or clinical evidence of sun damage. Moreover, there is typically histologic evidence of at least moderate solar elastosis in biopsy specimens of cuSCCs. BRAF inhibitor-induced cuSCCs may arise within 1–2 weeks of initiation of therapy. The squamous cell carcinomas are typically well differentiated, often with keratoacanthoma-like features histologically including cup-shaped architecture with a keratin-filled central crater. They often lack another typical feature of classic keratoacanthomas, that of microabscesses composed of neutrophils and/or eosinophils within keratin-filled nests. To our knowledge, to date, there have been no reports of cuSCCs arising from BRAF inhibitors that have gone on to metastasize. Cohen et al. however reported a cuSCC with spindle cell morphology associated with BRAF inhibitor therapy.