Chapter 21 Joseph (Yossi) Schwartz; Yvette C. Tanhehco; Suzanne A. Arinsburg; Jeffrey S. Jhang; Eldad A. Hod; Richard O. Francis; Steven L. Spitalnik A. Immature platelet fraction. B. Dye exclusion viability assay. C. Acanthamoeba culture. D. Mac-1 enumeration. E. Dimethyl sulfoxide (DMSO) concentration. 2. Which one of the following is an advantage of using peripheral blood–derived HPCs instead of bone marrow–derived or cord blood–derived HPCs for an allogeneic transplant? A. Lower incidence of graft-versus-host disease (GVHD). B. More rapid engraftment. C. Lower T-cell content. D. Less stringent human leukocyte antigen (HLA) matching. E. Mobilization with a growth factor, chemotherapy, or adhesion molecule antagonist is not required. 3. Which one of the following is the most common adverse effect of plerixafor + granulocyte colony-stimulating factor (G-CSF) mobilization for peripheral blood HPC collection? B. Blindness. C. Diffuse redness of the skin. D. Dysuria. E. Seizures. 4. Which of one of the following is an immediate adverse event following an autologous, peripheral blood HPC infusion? B. Jaundice. C. Infertility. D. Mucositis. E. Splenic enlargement. 5. Which one of the following sites is the most optimal for harvesting bone marrow? B. Posterior iliac crest. C. Anterior iliac crest. D. Femur. E. Ribs. 6. Which one of the following preharvest peripheral blood cell counts best predicts the yield of a peripheral blood HPC collection by apheresis? B. Total nucleated cells. C. Total mononuclear cells. D. Polymorphonuclear cells. E. CD38+ cells. 7. Which one of the following solutions is used to cryopreserve HPCs? A. Glycerol + DMSO + autologous plasma. B. Glycerol + Adsol. C. Glycerol + autologous plasma. D. DMSO + Adsol. E. DMSO + autologous plasma. 8. Which one of the following is the most common adverse effect of mobilizing HPCs with G-CSF? B. Bronchospasm. C. Jaundice. D. Splenic enlargement. E. Sweet syndrome. 9. Samples for transmissible disease testing of allogeneic HPC donors must be collected within: B. 7 days. C. 14 days. D. 30 days. E. 60 days. 10. Which one of the following doses is generally considered the minimum number of stem cells required for a single transplant? B. 3 × 108 CD38+ cells/kg. C. 2 × 106 CD34+ cells/kg. D. 2 × 106 CD34+ cells/mL. E. 3 × 107 CD38+ cells/mL. 11a. For which one of the following disorders is plerixafor specifically approved for use by the U.S. Food and Drug Administration (FDA)? A. Multiple myeloma and Hodgkin lymphoma. B. Non-Hodgkin lymphoma and Hodgkin lymphoma. C. Acute leukemia and multiple myeloma. D. Multiple myeloma and non-Hodgkin lymphoma. E. Acute leukemia and healthy donors. 11b. Which one of the following is the mechanism of action of plerixafor? A. Stimulates stem cell proliferation. B. Antagonizes C-X-C chemokine receptor type 4 (CXCR4). C. Acts as a ligand for the c-kit (CD117) receptor. D. Induces shedding of membrane bound stem cell factor (SCF). E. Irreversibly inhibits thymidylate synthase. 12. An A-negative patient with a negative antibody screen received a stem cell transplant from a B-positive donor 1 week ago. If the patient requires transfusion, which one of the following types of packed red blood cells should the patient receive? B. A+ C. B– D. B+ E. O– 13. An A-positive patient has a history of anti-K antibodies. The patient receives an O-positive stem cell transplant from a donor who had a negative antibody screen 2 weeks ago. Which one of the following types of red cells should be provided? B. O+ plus K– C. A– D. A+ plus K– E. AB+ plus K– 14. Which one of the following is the best predictor of poor stem cell mobilization? B. Etoposide mobilization. C. Radiotherapy. D. Male gender. E. Fewer than 20 CD34+ cells/μL peripheral blood preharvest count. 15. Which one of the following statements about informed consent for the collection of umbilical cord blood (UCB) hematopoietic stem cells for public cord blood banking is true? A. Informed consent to collect UCB must be obtained from the mother and father of the infant. B. Informed consent to collect UCB is obtained from the mother because of the infant’s inability to give consent and because her blood needs to be tested for transmissible diseases. C. Informed consent should be obtained from the father to perform transmissible disease testing on his blood to improve the safety of the UCB. D. Informed consent for UCB collected is required for UCB collected in utero, but not when collected ex utero. E. Informed consent for UCB is required because the mother and father both require HLA typing. 16. Which one of the following is a requirement for shipping cryopreserved products according to the Foundation for the Accreditation of Cellular Therapy (FACT) standards? B. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –196°C or less for at least 48 hours past the time of delivery to the transplant facility. C. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –150°C or less for at least 24 hours past the time of delivery to the transplant facility. D. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –196°C or less for at least 24 hours past the time of delivery to the transplant facility. E. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –150°C or less until the time of delivery to the transplant facility. 17. Which one of the following HLA loci is most commonly matched for in unrelated allogeneic peripheral blood–derived HPC transplants? B. HLA-DR, -DQ, and -DP. C. HLA-A, -B, -DR, and -DQ D. HLA-A, -C, -DR, -DQ, and -DP. E. HLA-A, -B, -C, -DR, and -DQ. 18. Which one of the following types of red blood cells should be given to a patient who is O+ with a negative antibody screen after receiving an A+ stem cell transplant 2 days ago? B. A+ C. B– D. B+ E. O+ 19. A patient receives an ABO blood group major mismatched HPC transplant. Which one of the following events may be attributed to this type of ABO incompatibility? B. Delayed red blood cell engraftment. C. Delayed hemolysis due to the production of anti-A and/or anti-B antibodies by donor lymphocytes. D. An increased risk for posttransplant Epstein-Barr virus infection. E. Transplant-associated thrombotic microangiopathy. 20. A patient receives an ABO blood group minor mismatched HPC transplant. Which one of the following adverse events may be attributed to this type of ABO incompatibility? B. Delayed red blood cell engraftment. C. Delayed hemolysis due to the production of anti-A and/or anti-B antibodies by donor lymphocytes. D. An increased risk for posttransplant Epstein-Barr virus infection. E. Transplant-associated thrombotic microangiopathy. 21. A 12-year-old boy with sickle cell disease is undergoing an ABO-mismatched bone marrow transplant. The donor is blood group type A and the recipient is type O. Density gradient centrifugation with Ficoll is used to deplete the product of red cells. As shown in Figure 21-1, which one of the following layers contains the granulocytes? B. B C. C D. D Major points of discussion ■ Cell viability is determined by using dye exclusion reagents such as trypan blue dye, fluorescent dyes such as acridine orange and propidium iodide, and 7-AAD. ■ HPC products are tested for bacterial and fungal contamination that may have occurred during collection or processing. HPC products can also be contaminated when patients are bacteremic or septic during the collection. ■ A positive culture result of stem cells collected from bone marrow harvesting does not necessarily lead to immediate discard of the product. Since these cells are “irreplaceable,” they are often transplanted because of emergency need. ■ The CD34 antigen is used to evaluate the “quality” of HPC products. The percentage of CD34+ cells in the product is determined by flow cytometry and is used to calculate the CD34+ cell dose. This is expressed as the number of CD34+ cells per kilogram of recipient body weight. 2. A. Lower incidence of graft-versus-host disease (GVHD). Major points of discussion ■ Bone marrow–derived HPCs can provide an adequate number of CD34+ HPCs for transplantation and this source is associated with less chronic GVHD compared with peripheral blood–derived HPCs; however, they are more difficult to obtain (e.g., requires anesthesia) and are associated with longer neutrophil and platelet engraftment times. ■ Transplantation of peripheral blood–derived HPCs is associated with shorter engraftment times. They are also easier to collect; however, growth factors, chemotherapy, and/or CXCR4 antagonists are necessary to mobilize the HPCs into the circulation. ■ Cord blood–derived HPCs are associated with the lowest incidence of chronic GVHD and have a less-restrictive HLA-matching requirement; however, they contain fewer HPCs and have the longest time to engraftment. ■ The time to engraftment is an important consideration when selecting an HPC source. Shorter engraftment times are associated with fewer infections, fewer transfusions, shorter hospital stays, and lower cost. 3. A. Gastrointestinal symptoms. Major points of discussion ■ Plerixafor is indicated in combination with G-CSF to mobilize HPCs from the marrow to the peripheral blood in patients with multiple myeloma and non-Hodgkin lymphoma undergoing autologous transplantation. ■ Plerixafor is administered subcutaneously after 4 days of G-CSF at a dose of 240 μg/kg approximately 11 hours prior to collection by apheresis. The dose can be repeated for up to 4 additional days with G-CSF. ■ Variability exists in the interval between plerixafor administration and apheresis collection at different institutions. Studies in normal donors suggest there is sustained release of stem cells from 4 to 20 hours after administration of plerixafor with peak release between 10 and 14 hours after administration. ■ The most common (≥ 5%) adverse effects reported in phase III trials of plerixafor + G-CSF are diarrhea, nausea, vomiting, abdominal pain, flatulence, fatigue, injection site erythema, injection site pruritus, bone pain, headache, and paresthesia.3,4 4. A. Bronchospasm. Major points of discussion ■ Treatment of the adverse effects of HPC infusion includes slowing, but not halting, the infusion until the symptoms pass. ■ Premedicating with antihistamines can prevent hypotensive and allergic reactions. Adequate hydration and alkalinization of the urine prior to the infusion may reduce the risk of renal complications from free hemoglobin released by red cell lysis during cryopreservation. ■ The total volume of HPC product infused should not contain more than 1 g/kg DMSO per day. ■ HPC products can be washed prior to infusion to reduce DMSO infusion–related toxicities. 5. A. Sternum. Major points of discussion ■ Bone marrow harvests are performed with the patient under general anesthesia. ■ Red marrow, which contains hematopoietic tissue, is found mainly in flat bones such as the pelvis, sternum, skull, ribs, vertebrae, and scapulae. It can also be found in the epiphyseal ends of long bones such as the femur and humerus. By adolescence, active marrow is usually found only in the cavities of these axial bones. ■ The posterior iliac crest is the most optimal site for bone marrow aspirations in adults, children, and most infants because it is easily accessible, produces a high yield, and causes the least discomfort to the patient compared with other sites. ■ The anterior iliac crest may be used for bone marrow aspirations in adults when access to the posterior iliac crest is limited due to difficulty with positioning, morbid obesity, skin diseases, or previous irradiation. ■ The greater trochanter of the femur, individual vertebral bodies, or ribs may be used for bone marrow aspirations. Obtaining bone marrow from these sites may require surgical consultation and computed tomography guidance. ■ Obtaining bone marrow from a site that has been previously irradiated should be avoided because it is likely to have less overall cellularity. 6. A. CD34+ cells. Major points of discussion ■ CD34 is a cell surface glycoprotein that acts as a surrogate marker of HPCs. ■ The number of CD34+ cells in the peripheral blood is measured prior to the start of apheresis collection. Apheresis collection commences when the number of CD34+ cells is at or above a prespecified threshold (e.g., > 20 CD34+ cells/μL). ■ The dose of stem cells for transplantation is based on the number of CD34+ cells/kg recipient body weight. ■ Pluripotent hematopoietic stem cells are CD34+CD38–. ■ The cellular content of HPCs obtained from peripheral blood differs significantly from those obtained from bone marrow. 7. A. Glycerol + DMSO + autologous plasma. Major points of discussion ■ HPC products can be stored at 4°C to 15°C overnight before processing without compromising graft quality and engraftment. ■ Cryopreservation of HPCs involves suspending the cells in a solution composed of DMSO (10% final concentration) and autologous plasma or another source of protein. ■ Controlled-rate freezing (1° to 2°C/min to –30°C to –50°C, 2° to 10°C/min to –90°C) is used to cryopreserve products in cryopreservation medium. ■ Cryopreserved products may be stored in a mechanical freezer at less than –70°C or in liquid nitrogen freezers (liquid phase at –196°C or vapor phase at < –150°C) for several years without compromising cell viability. 8. A. Bone pain. Major points of discussion ■ G-CSF is the principal hematopoietic growth factor used for HPC mobilization. ■ G-CSF is usually administered once a day by subcutaneous injection in doses ranging from 5 to 20 μg/kg/day. ■ G-CSF is associated with the release of metalloproteases that are hypothesized to cleave at least one receptor–ligand pair that tethers stem cells to the bone marrow stroma. ■ G-CSF causes a predictable increase in circulating CD34+ stem cells and other leukocytes. Peripheral blood stem cell collection can usually begin about 3 to 4 days after the first dose. ■ Symptoms reported with G-CSF administration include bone pain, myalgia, headache, fatigue, insomnia, flu-like symptoms, nausea, sweats, anorexia, fever, chills, emesis, and allergic reactions.6 9. A. 1 day. Major points of discussion ■ Infectious disease testing of HPC donors should be performed within 30 days before collection. ■ Infectious disease testing of HPC products includes HIV 1 and 2, hepatitis C, hepatitis B, HTLV 1 and 2, cytomegalovirus, and Treponema pallidum. Although not required by the FDA, testing for West Nile virus and Chagas disease is commonly included. ■ HPC products can be stored in the vapor phase of liquid nitrogen or placed inside another plastic bag that is sealed before storage to minimize the risk of cross-contamination among HPC products. ■ HPC products from allogeneic donors who are confirmed to be HIV positive are not used. However, other positive disease markers do not necessarily prohibit the use of collections from a particular donor. 10. A. 2 × 1010 CD3+ cells/kg. Major points of discussion ■ CD34 is a cell surface glycoprotein that is used as a surrogate marker for hematopoietic stem cells. ■ Long-term hematopoietic restoration will occur with as few as 1 × 106 CD34+ cells/kg recipient body weight. ■ 2 × 106 CD34+ cells/kg recipient body weight is generally accepted as the minimum dose for a single transplant to ensure reliable engraftment. ■ Larger doses of infused CD34+ cells increase the speed of engraftment. Infusion of 5 × 106 CD34+ cells/kg recipient body weight results in faster engraftment compared with infusion of 2 × 106 CD34+ cells/kg recipient body weight. ■ Other factors aside from infused CD34+ cell dose that affect the speed of engraftment after autologous transplantation include the type and extent of previous myelotoxic therapy and ease of hematopoietic stem cell mobilization. ■ Higher doses of infused CD34+ stem cells in allogeneic transplantation are associated with a higher frequency and severity of chronic, but not usually acute, GVHD in some related donor myeloablative transplants. 11a. A. Multiple myeloma and Hodgkin lymphoma. 11b. A. Stimulates stem cell proliferation. Major points of discussion ■ Plerixafor reversibly inhibits stromal cell–derived factor-1 binding to its receptor CXCR-4. ■ Plerixafor in combination with G-CSF is used to mobilize HPCs to the peripheral blood in patients with multiple myeloma and non-Hodgkin lymphoma undergoing collection for autologous transplantation. ■ Plerixafor is administered subcutaneously after 4 days of G-CSF at a dose of 240 μg/kg approximately 11 hours prior to collection by apheresis. It can be repeated for up to 4 additional days with G-CSF. Studies on intravenous administration of plerixafor have been performed. Variability exists in the interval between plerixafor administration and apheresis collection at different institutions. Studies in normal donors suggest there is sustained release of stem cells from 4 to 20 hours after administering plerixafor with peak release between 10 and 14 hours after administration. ■ Plerixafor is being studied to support mobilization in other conditions such as Hodgkin lymphoma. ■ Plerixafor can mobilize leukemic cells, so it is not FDA approved for use in patients with acute leukemia. However, studies using plerixafor to delocalize leukemic stem cells out of their protective niche and away from their protective signals to make them more vulnerable to chemotherapy agents are under way. ■ Plerixafor is not FDA approved for use in healthy donors for allogeneic transplantation. However, studies have shown that it is safe in family or volunteer HPC donors.1,3-5,7 12. A. A– Major points of discussion ■ Patients undergoing HPC transplantation frequently require transfusion support. ■ ABO incompatibility generally does not disqualify a potential stem cell donor because pluripotent and early committed HPCs lack ABO antigens. ■ A major ABO incompatibility occurs when the recipient has antibodies against blood group antigens present on the surface of the donor’s red blood cells (e.g., type O recipient and type A, B, or AB donor). An acute hemolytic transfusion reaction can result when the product is infused. Red cell reduction of the product prior to the transplant is useful. ■ A minor ABO incompatibility occurs when the donor has antibodies against blood group antigens present on the recipient’s red blood cells (e.g., type AB recipient and type A donor). Plasma reduction of the product is useful. ■ A bidirectional ABO incompatibility occurs when both major and minor incompatibilities are present (e.g., type A recipient and type B donor). 13. A. O– Major points of discussion ■ Patients undergoing HPC transplantation frequently require transfusion support. ■ Red blood cell engraftment may take up to 6 weeks. ■ ABO incompatibility is not a contraindication when selecting potential stem cell donors because pluripotent and early committed HPCs lack ABO antigens. ■ A minor ABO incompatibility occurs when donor antibodies against blood group antigens present on the recipient’s red blood cells are introduced. ■ Removing plasma from the product, which contains the donor isohemagglutinins, may minimize hemolysis in a minor ABO-incompatible stem cell transplant. 14. A. Age less than 5 years. Major points of discussion ■ Predictors of poor mobilization include age greater than 60 years, progressive disease, severe bone marrow involvement, type of disease (e.g., non-Hodgkin lymphoma), number of prior chemotherapy cycles and/or radiotherapy, type of chemotherapy (e.g., methotrexate and lenalidomide), and previously failed mobilization attempts. ■ Options for poor mobilizers include remobilization, addition of plerixafor to a mobilization with G-CSF with or without chemotherapy, and bone marrow harvest. ■ Combined plerixafor and G-CSF mobilization significantly increases the efficacy of remobilization in patients with a previous failed mobilization attempt; it has a success rate of greater than 60%. ■ Remobilization with plerixafor results in timely and stable engraftment with rare and/or manageable side effects in most patients. 15. A. Informed consent to collect UCB must be obtained from the mother and father of the infant. Major points of discussion ■ The first reported use of umbilical cord blood as a source of hematopoietic stem cells was in 1972.8 ■ Informed consent must be obtained from the mother for the collection, processing, testing, storage, and medical use of UCB. ■ To ensure that the UCB does not harbor genetic or transmissible diseases, the mother’s medical history is obtained through an interview or review of her medical record. ■ UCB cannot be obtained from mothers who have first-degree relatives with a history of malignancy or parents who have been treated with chemotherapy. ■ UCB with sickle cell or thalassemia trait may occasionally be stored if they have unique HLA types needed for transplantation. 16. A. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –150°C or less for at least 48 hours past the time of delivery to the transplant facility. Major points of discussion ■ FACT requires that liquid nitrogen dry shipping containers be validated to maintain a temperature of –150°C or less for at least 48 hours past the time of delivery to the transplant facility. ■ AABB (formerly the American Association of Blood Banks) and FACT require continuous monitoring of the temperature in the dry shippers during shipment. ■ The shipping container should include the name, address, and phone number of the shipping and receiving facilities; the phrases “Medical Specimen,” “Do Not X-Ray,” and “Do Not Irradiate” (if applicable); and biohazard labels (as appropriate). ■ Detailed records need to be maintained that include the identity of the shipping facility, date and time the unit was shipped and received, courier, and contents of each shipping container. 17. A. HLA-A, -B, and -C. Major points of discussion ■ Donors and recipients are typed for HLA class I (A, B, and C loci) and class II (DR, DQ, and sometimes DP) alleles. ■ The goal of bone marrow stem cell and peripheral blood stem cell transplantation is to match the HLA-A, -B, -C, and -DRB1 alleles of the prospective donor and recipient. ■ Umbilical cord blood stem cells have less-stringent HLA-matching criteria (HLA-A, -B, and -DRB1 alleles) between donor and recipient. ■ Molecular techniques are used to determine the HLA type of the donor and recipient for optimal assessment of class I and class II allele compatibility. 18. A. A– Major points of discussion ■ Patients undergoing HPC transplantation frequently require transfusion support. ■ ABO incompatibility does not disqualify potential stem cell donors because pluripotent and early committed HPCs lack ABO antigens. ■ A major ABO incompatibility occurs when the recipient has antibodies against blood group antigens present on the surface of the donor’s red blood cells. ■ Major ABO incompatibility poses two challenges. First, there is a potential for acute intravascular hemolysis when ABO-incompatible donor red blood cells are infused with the graft to the recipient. Second, there could be continued production of circulating ABO antibodies by the recipient directed against donor red blood cells and erythroid progenitors produced by the engrafted HPCs, which could lead to pure red cell aplasia in severe cases. ■ Red blood cell depletion removes incompatible red blood cells from the graft to minimize the risk of hemolysis during infusion. 19. A. An acute hemolytic reaction at the time of HPC transplant due to the infusion of incompatible plasma containing anti-A and/or anti-B antibodies. Major points of discussion ■ Delayed red blood cell engraftment is a potential adverse consequence of a major ABO incompatibility. ■ Delayed engraftment is due to production anti-A and/or anti-B antibodies by residual plasma cells in the recipient that survive the preparative conditioning regimen. ■ In the context of a major ABO-mismatched HPC transplant, an acute hemolytic reaction at the time of HPC infusion may also occur due to the presence of ABO-incompatible red blood cells in the donor product and circulating anti-A and/or anti-B antibodies in the recipient. ■ Red blood cells can be depleted from the donor product in the setting of a major ABO mismatched HPC transplant, typically by performing simple red cell depletion, density gradient red cell depletion, or CD34 selection.2 20. A. An acute hemolytic reaction at the time of HPC infusion due to the presence of incompatible red blood cells in the donor product. Major points of discussion ■ The passenger lymphocyte syndrome can also be seen in solid organ transplants (e.g., liver and kidney) when there is a minor ABO blood group mismatch (e.g., transplantation of an organ from a blood group O donor into a blood group A recipient). ■ Hemolysis due to the passenger lymphocyte syndrome can also be seen with antibodies against non-ABO blood group antigens. For example, following transplantation from an Rh(D)– donor with anti-D antibodies into an Rh(D)+ recipient. However, these cases are unusual and the degree of hemolysis is usually mild and well compensated. ■ A hemolytic transfusion reaction at the time of HPC transplantation due to infusion of incompatible plasma containing anti-A and/or anti-B is another complication of ABO minor-mismatched HPC transplantation. Nonetheless, the hemolysis seen in this case is typically much less dramatic than that seen in ABO major-mismatched transplants. ■ Depleting the plasma from HPC product can prevent hemolysis at the time of infusion due to a minor incompatibility.2 21. A. A. Major points of discussion ■ Approximately half of all HPC transplants are ABO mismatched. ■ ABO-mismatched transplants can be major, minor, or bidirectional mismatches. ■ Major mismatches occur when the donor red cells are incompatible with the recipient plasma. This occurs with type A, B, or AB donors and type O recipients. Major mismatches are also seen in A or B recipients receiving a product from an AB or B donor or an AB or A donor, respectively. ■ Major ABO mismatches are at risk for immediate hemolysis at the time of infusion. Red cell depletion of the product is the most common way to prevent this. In addition, delayed engraftment or pure red cell aplasia can be complications of major mismatches. ■ Red cell depletion can be performed by simple centrifugation or density-gradient centrifugation by manual, semi-automated, or automated methods. Manual density gradient centrifugation depletes over 95% of red cells while recovering 50% to 70% of the mononuclear cells. ■ Minor mismatches occur when the donor plasma is incompatible with the recipient red cells, such as with a type O donor and a type A, B, or AB recipient. In addition, minor mismatches can occur with a type A or B donor and AB recipient. ■ Minor mismatches are at risk for immediate hemolysis that can be prevented with plasma depletion. In addition, passenger lymphocyte syndrome and delayed hemolytic transfusion reactions can be complications of minor mismatches. ■ Bidrectional mismatches occur when a major and minor mismatch occur simultaneously, such as in a type A donor and type B recipient or a type B donor and type A recipient. These products are both depleted of red cells and plasma prior to transplantation.2
Transfusion Medicine
Cellular Therapy
Rationale: HPCs collected from peripheral blood have the highest incidence of chronic GVHD among the three sources of HPCs (i.e., peripheral blood, bone marrow, cord blood).
B. More rapid engraftment.
Rationale: HPCs obtained from peripheral blood lead to more rapid neutrophil and platelet engraftment in patients compared with HPCs obtained from bone marrow and cord blood. Rapid engraftment is important because short engraftment times are associated with fewer infections, fewer transfusions, shorter hospital stays, and lower cost. HPCs obtained from cord blood have the longest time to engraftment. HPCs from the bone marrow engraft faster than cord blood–derived HPCs but slower than peripheral blood–derived HPCs.
C. Lower T-cell content.
Rationale: HPCs collected from peripheral blood have a higher T-cell content compared with HPCs from bone marrow and cord blood. Higher T-cell content increases the risk of GVHD.
D. Less stringent human leukocyte antigen (HLA) matching.
Rationale: The HLA type of the donor must be closely matched to the recipient for both peripheral blood– and bone marrow–derived HPCs. The requirements are less restrictive for cord blood–derived HPCs.
E. Mobilization with a growth factor, chemotherapy, or adhesion molecule antagonist is not required.
Rationale: A low level of HPCs is present in the circulation; however, mobilization with a growth factor, chemotherapy, adhesion molecule (e.g., CXCR4) antagonist, or a combination of the three is necessary to obtain sufficient HPCs from the peripheral blood for transplantation.
Rationale: The two most commonly reported adverse effects of plerixafor administration are (1) gastrointestinal symptoms such as diarrhea, nausea, vomiting, abdominal pain, and flatulence and (2) injection site erythema. Other adverse effects reported in 5% or more patients who received plerixafor + G-CSF in phase III trials include bone pain, headache, and paresthesias.
B. Blindness.
Rationale: Blindness was not reported as an adverse effect in phase III trials of plerixafor + G-CSF in patients with multiple myeloma and non-Hodgkin’s lymphoma.
C. Diffuse redness of the skin.
Rationale: Injection site erythema is a commonly reported adverse effect of plerixafor administration. Redness of the skin is a less common side effect.
D. Dysuria.
Rationale: Dysuria is not a common side effect of plerixafor. Gastrointestinal symptoms are more common.
E. Seizures.
Rationale: Seizures are not a common side effect of plerixafor. Gastrointestinal symptoms are more common.
Rationale: Mild allergic to anaphylactic reactions, possibly due to DMSO, can be seen with HPC infusions.
B. Jaundice.
Rationale: Jaundice can be seen in acute GVHD of the liver, which is not seen with autologous HPC transplantation.
C. Infertility.
Rationale: Infertility can result from high-dose chemotherapy, but it is not a side effect of HPC infusion.
D. Mucositis.
Rationale: Mucositis is a common adverse effect of chemotherapy. It is not an immediate adverse event associated with HPC infusion.
E. Splenic enlargement.
Rationale: Splenic enlargement and rupture can occur in allogeneic donors and patients after receiving G-CSF. It is not associated with HPC infusion.
B. Posterior iliac crest.
Rationale: This site provides the most practical and productive site for marrow collection.
C. Anterior iliac crest.
D. Femur.
E. Ribs.
Rationale for A, C, D, and E: These sites may be used because they have red marrow, which consists of hematopoietic tissue, but the posterior iliac crest is the most practical and productive site for marrow collection.
Rationale: CD34 is a surrogate marker for HPCs. Higher levels of circulating peripheral blood CD34+ cells prior to the start of collection are associated with higher product yields.
B. Total nucleated cells.
Rationale: Total nucleated or white blood cell counts are not as predictive as the CD34+ cell count in determining the success of a peripheral blood HPC collection.
C. Total mononuclear cells.
Rationale: Total mononuclear cell counts are not used to predict peripheral blood stem cell harvest yields.
D. Polymorphonuclear cells.
Rationale: Polymorphonuclear cell counts or neutrophil counts are not used to predict peripheral blood stem cell harvest yields.
E. CD38+ cells.
Rationale: CD38 is a marker of cell activation. It is not used to predict peripheral blood stem cell harvest yields.
B. Glycerol + Adsol.
C. Glycerol + autologous plasma.
D. DMSO + Adsol.
E. DMSO + autologous plasma.
Rationale: Glycerol is a cryopreservative solution for red blood cells. Adsol is an additive solution for red blood cells collected in citrate-phosphate-dextrose. DMSO and autologous plasma or another source of protein comprises the cryopreservative solution used for HPCs.
Rationale: The most commonly reported adverse effects of G-CSF mobilization are bone pain, myalgia, headache, and fatigue (> 50%).
B. Bronchospasm.
Rationale: Allergic reactions (e.g., hives, erythema, bronchospasm, anaphylaxis) can be seen with G-CSF mobilization, but they are not as common as bone pain.
C. Jaundice.
Rationale: Liver changes are not a common side effect of G-CSF.
D. Splenic enlargement.
Rationale: Enlargement of the spleen can be seen with G-CSF treatment but is rare. Activities that can lead to traumatic splenic rupture should be avoided.
E. Sweet syndrome.
Rationale: Drug-induced febrile neutrophilic dermatosis is rare.
B. 7 days.
C. 14 days.
D. 30 days.
E. 60 days.
Rationale: Infectious disease testing of HPC donors should be performed within 30 days of collection.
B. 3 × 108 CD38+ cells/kg.
C. 2 × 106 CD34+ cells/kg.
D. 2 × 106 CD34+ cells/mL.
E. 3 × 107 CD38+ cells/mL.
Rationale: 2 × 106 CD34+ cells/kg recipient body weight is generally considered the minimum number of stem cells required for a single transplant. CD3 is a T-cell marker; CD34 is a stem cell marker; and CD38 is a marker founds on B cells and natural killer cells.
B. Non-Hodgkin lymphoma and Hodgkin lymphoma.
C. Acute leukemia and multiple myeloma.
Rationale: Plerixafor is not approved for patients with acute leukemia because it has the ability to mobilize leukemic cells, but it is approved for use in patients with multiple myeloma.
D. Multiple myeloma and non-Hodgkin lymphoma.
Rationale for A, B, and D: Plerixafor is approved for use in patients with multiple myeloma and non-Hodgkin lymphoma based on two pivotal phase III trials. It is not approved for use in patients with Hodgkin lymphoma.
E. Acute leukemia and healthy donors.
Rationale: Plerixafor is not approved for healthy donors or for use in patients with acute leukemia because it has the ability to mobilize leukemic cells.
Rationale: G-CSF and granulocyte-monocyte colony-stimulating factor (GM-CSF) work through this mechanism.
B. Antagonizes C-X-C chemokine receptor type 4 (CXCR-4).
Rationale: Plerixafor is a CXCR-4 antagonist.
C. Acts as a ligand for the c-kit (CD117) receptor.
Rationale: SCF binds c-kit. It is not clinically used as a mobilization regimen.
D. Induces shedding of membrane-bound stem cell factor (SCF).
Rationale: Osteoclast metalloproteinase MMP-9 induces shedding of SCF cytokine into the bone marrow. It is not clinically used as a mobilization regimen.
E. Irreversibly inhibits thymidylate synthase.
Rationale: 5-Fluorouricil is used as a chemotherapeutic agent.
B. A+
Rationale for A and B: The passenger anti-B antibodies in the stem cell graft would hemolyze type A red blood cells.
C. B–
D. B+
Rationale for C and D: The patient’s anti-B antibodies would hemolyze type B red blood cells.
E. O–
Rationale: Type O red blood cells are compatible with both the patient’s and donor’s plasma and would not be hemolyzed.
B. O+ plus K–
C. A–
D. A+ plus K–
E. AB+ plus K–
Rationale: This is a minor ABO-incompatible transplant and the recipient has not engrafted. The red blood cells should be compatible with both the donor and recipient plasma. Because the patient has a history of anti-K antibodies, K– red blood cells should be given.
Rationale: Age greater than 60 years is a predictor of poor stem cell mobilization.
B. Etoposide mobilization.
Rationale: More than three prior chemotherapy cycles was found to predict poor stem cell mobilization.
C. Radiotherapy.
Rationale: Previous radiotherapy is a predictor of poor stem cell mobilization.
D. Male gender.
Rationale: Female gender is a predictor of lower yields.
E. Fewer than 20 CD34+ cells/μL peripheral blood preharvest count.
Rationale: More than 20 CD34+ cells/μL would predict a good yield.
B. Informed consent to collect UCB is obtained from the mother because of the infant’s inability to give consent and because her blood needs to be tested for transmissible diseases.
Rationale: See Major Points of Discussion.
C. Informed consent should be obtained from the father to perform transmissible disease testing on his blood to improve the safety of the UCB.
Rationale for A and C: Informed consent from the father is not necessary and will not add to the safety of the UCB. Only the mother’s consent is required.
D. Informed consent for UCB collected is required for UCB collected in utero, but not when collected ex utero.
Rationale: UCB that is collected in utero is less costly than UCB that is collected ex utero because dedicated UCB bank collection personnel are not needed on site. Informed consent is required for both types of collections.
E. Informed consent for UCB is required because the mother and father both require HLA typing.
Rationale: UCB for allogeneic transplantation must be typed for HLA class I (A and B loci) and class II (DRB1 loci). HLA-C and DQB loci typing are recommended as well. The mother and father are not routinely typed.
B. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –196°C or less for at least 48 hours past the time of delivery to the transplant facility.
C. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –150°C or less for at least 24 hours past the time of delivery to the transplant facility.
D. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –196°C or less for at least 24 hours past the time of delivery to the transplant facility.
E. Liquid nitrogen dry shippers have to be validated to maintain a temperature of –150°C or less until the time of delivery to the transplant facility.
Rationale: Liquid nitrogen dry shippers should be validated to maintain a temperature of –150°C or less for at least 48 hours past the time of delivery to the transplant facility.
B. HLA-DR, -DQ, and -DP.
C. HLA-A, -B, -DR, and -DQ.
D. HLA-A, -C, -DR, -DQ, and -DP.
E. HLA-A, -B, -C, -DR, and -DQ.
Rationale: Stem cell transplant donors and recipients are typed for their HLA-A, -B, -C, -DR, and -DQ alleles. In some cases, HLA-DP may be typed as well.
B. A+
Rationale: The patient’s anti-A antibodies would hemolyze type A red blood cells.
C. B–
D. B+
Rationale: Type B red blood cells would be hemolyzed by the patient’s and donor’s passenger anti-B antibodies.
E. O+
Rationale: Type O red blood cells are compatible with both the patient’s and donor’s isoagglutinins and would not be hemolyzed.
Rationale: This is a complication of an ABO minor-mismatched HPC transplant.
B. Delayed red blood cell engraftment.
Rationale: This is a potential adverse consequence of a major ABO incompatibility due to anti-A and/or anti-B antibody production by residual recipient plasma cells that survived the preparative conditioning regimen.
C. Delayed hemolysis due to the production of anti-A and/or anti-B antibodies by donor lymphocytes.
Rationale: This phenomenon, due to passenger lymphocyte syndrome, is a complication of an ABO minor-mismatched HPC transplant.
D. An increased risk for posttransplant Epstein-Barr virus infection.
E. Transplant-associated thrombotic microangiopathy.
Rationale: These complications are not related to whether the HPC transplantation is mismatched for the ABO blood group.
Rationale: This is a complication of an ABO major-mismatched HPC transplant.
B. Delayed red blood cell engraftment.
Rationale: This is a potential adverse consequence of a major ABO incompatibility due to anti-A and/or anti-B antibody production by residual recipient plasma cells that survived the preparative conditioning regimen.
C. Delayed hemolysis due to the production of anti-A and/or anti-B antibodies by donor lymphocytes.
Rationale: This phenomenon can occur due to the passenger lymphocyte syndrome.
D. An increased risk for posttransplant Epstein-Barr virus infection.
E. Transplant-associated thrombotic microangiopathy.
Rationale: These complications are not related to whether the HPC transplantation is mismatched for the ABO blood group.
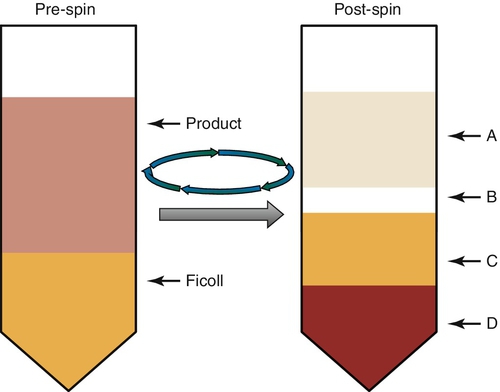
Rationale: The uppermost layer contains plasma and platelets.
B. B.
Rationale: This layer contains the peripheral blood mononuclear cells, which is the layer containing the stem cells. After washing this layer, these cells are used for the transplant.
C. C.
Rationale: The Ficoll separates the erythrocytes from the peripheral blood mononuclear cells.
D. D.
Rationale: This layer contains both the red cells and the granulocytes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


