Chapter 3 Alexander Kratz; Michael A. Pesce; Jeffrey S. Jhang A. Within the first 4 weeks after hire and annually thereafter. B. Every 6 months during the first 2 years and annually thereafter. C. Every 6 months. D. Before the operator performs patient testing, semiannually during the first year, and annually thereafter. E. Before the operator performs patient testing. 2. Chemiluminescence immunoassays can be used to measure a wide variety of biomarkers. Which one of the following best describes a chemiluminescence assay? A. Measurement of the amount of light absorbed at two different wavelengths. B. Measurement of the amount of emitted light after a molecule is chemically excited from the ground to the excited state. C. Measurement of the amount of emitted light after a molecule is biologically excited from the ground to the excited state. D. Measurement of the amount of emitted light when a molecule in a triplet state returns to the ground state. E. Measurement of the amount of emitted light after a molecule is excited from the ground to the excited state by a short pulse of light. 3. The cloned enzyme donor immunoassay (CEDIA) is a homogeneous assay that is used to quantify small molecules. The reagents consist of two different inactive fragments of the enzyme beta-galactosidase. One fragment is bound to the antigen and is known as the enzyme donor (ED), whereas the other fragment is the enzyme acceptor (EA). The ED fragment must combine with the EA fragment to produce an active enzyme. In the CEDIA assay, which one of the following best explains how the labeled antigen that is bound to the antibody is differentiated from the labeled antigen that is not bound to the antibody? B. The enzyme-labeled antigen that is bound to the antibody reacts at a slower rate with the EA to form the active enzyme than the enzyme-labeled antigen that is not bound to the antibody. C. The enzyme-labeled antigen that is bound to the antibody reacts with the EA to form the active enzyme. D. The enzyme-labeled antigen that is bound to the antibody is physically separated from the enzyme-labeled antigen that is not bound to the antibody and reacts with the EA to form the active enzyme. E. The enzyme-labeled antigen that is bound to the antibody is inhibited from reacting with the EA. The enzyme-labeled antigen that is not bound to the antibody reacts with the EA to form the active enzyme. 4. The majority of laboratory testing errors occur at which one of the following stages? A. During the ordering of tests. B. During the preanalytical phase of testing. C. During the analytical phase of testing. D. During the reporting of the test results. E. During physician interpretation of the test results. 5. Which one of the following is the best definition of the Stokes shift? A. Quenching of fluorescence in the excited state. B. Difference between the wavelengths of the excitation light and emitted light in a fluorescence assay. C. Rate of formation of an antibody-antigen complex. D. Difference between detection time of the internal standard and the compound of interest in a high-performance liquid chromatography (HPLC) assay. E. Distance between the solvent front and the compound of interest in a thin-layer chromatography (TLC) assay. 6. Time-resolved fluorescence immunoassays have been used to measure serum hormone levels. Which one of the following reasons best describes the advantage of time-resolved fluorescence immunoassays over conventional fluorescence immunoassays? B. The fluorescence lifetime of the molecule is increased, resulting in an increase in analytical sensitivity and a decrease in background fluorescence. C. The small Stokes shift that is observed with time-resolved fluorescent immunoassays increases the sensitivity of the assay. D. Fluorescence measurements are rapid, which reduces the background fluorescence. E. Fluorescence is quenched, which reduces the background fluorescence. 7. A 57-year-old man arrived in the emergency department with acute chest pain. He has a history of heavy alcohol use, hypertension, and hyperlipidemia. His electrocardiogram was normal throughout his hospital stay. A chest radiograph showed clear lung fields and no evidence of acute cardiopulmonary pathology. Serum levels of troponin I were measured with a two-site immunometric assay to determine if there was cardiac damage. On admission, his troponin I level was 41 μg/L, and on day 2, his troponin I level was 40 μg/L (reference range: < 0.04 μg/L). Total serum levels of creatine kinase (CK) activity and CK-MB were normal on both days. Which one of the following provides the best explanation of why this patient’s troponin I level was elevated? A. The hook effect caused a false-positive troponin I result. B. Heterophile antibodies interfered with the troponin I assay, causing a false-positive result. C. The patient was correctly diagnosed with an acute myocardial infarction. D. The patient was correctly diagnosed with congestive heart failure. E. There was cross-reactivity of troponin T with the troponin I method. 8. The enzyme-multiplied immunoassay technique (EMIT) is a homogeneous immunoassay that is used to measure low molecular weight molecules. In an EMIT assay, which one of the following best explains how the enzyme-labeled antigen that is bound to the antibody is distinguished from the non–antibody-bound, free enzyme-labeled antigen? A. The enzyme-labeled antigen activity is inhibited by changing the pH of the reaction. B. The antibody-bound enzyme-labeled antigen complex is not as stable as the free, non–antibody-bound enzyme-labeled antigen complex, and the enzyme activity is measured by a kinetic rate reaction. C. Antibody binding to the enzyme-labeled antigen enhances the enzyme activity. D. Antibody binding to the enzyme-labeled antigen sterically inhibits the enzyme activity. E. The free, non–antibody-bound enzyme-labeled antigen is fluorescent. 9. The fluorescent polarization immunoassay (FPIA) is a homogeneous assay for measuring low molecular weight molecules, such as drugs. In the quantification of a drug level by FPIA, which one of the following best explains how the non–antibody-bound fluorescent-labeled drug is differentiated from the antibody-bound fluorescent-labeled drug? A. No fluorescence is observed when antibody binds to the fluorescent-labeled drug. B. Rotation of the non–antibody-bound fluorescent-labeled drug is slower than the antibody-bound fluorescent-labeled drug and depolarizes the emitted light. C. The antibody-bound fluorescent-labeled drug emits less polarized light than the non–antibody-bound fluorescent-labeled drug. D. The antibody-bound fluorescent-labeled drug emits polarized light. The non–antibody-bound fluorescent labeled drug depolarizes the emitted light. E. Rotation of the non–antibody-bound fluorescent-labeled drug is faster than the antibody-bound fluorescent-labeled drug and emits more polarized light. 10. The presence of heterophile antibodies, which usually are human anti-mouse antibodies (HAMA), may interfere with the measurement of an analyte using a competitive binding immunoassay. Which one of the following is the most likely cause of the erroneous analytical result? B. Binding of the HAMA to the analyte that is being measured, thereby causing a falsely lower result. C. Binding of the HAMA to the capture antibody, thereby causing a falsely elevated result. D. Binding of the HAMA to the capture antibody, thereby causing a falsely lower result. E. Binding of HAMA to the labeled analyte, thereby causing a falsely lower result. 11a. An 18-year-old girl presents to the emergency department with a 2-week history of nausea, vomiting, and vaginal spotting. Serum human chorionic gonadotropin (hCG) was measured with a one-step, two-site immunometric assay and was 20 IU/L (reference range: < 25 IU/L). In this case, the physician called the laboratory director and suggested that the hCG result was incorrect because the patient was diagnosed as having a hydatidiform mole and should have an extremely high circulating hCG level. Which one of the following best explains this false-negative serum hCG result? A. Interference by heterophile antibodies (i.e., HAMAs). B. The hook effect. C. A matrix effect. D. Inhibition of hCG by maternal antibodies. E. Binding of hCG to human immunoglobulin (Ig) G. 11b. In this case, which one of the following is the best approach the laboratory can take to ensure that the correct hCG result is reported (which turned out to be an hCG level of 3,500,000 IU/L)? A. Repeat the hCG assay using another serum sample. B. Measure the hCG level using a different method. C. Precipitate the interfering heterophile antibodies in the sample using polyethylene glycol and remeasure the hCG level. D. Perform a serial dilution of the sample and remeasure the hCG levels using the diluted samples. E. Add mouse immunoglobulins to the sample to remove the interference from heterophile antibodies and remeasure the hCG level. 12. Which one of the following best describes reflectance photometry? A. Reflectance photometry is used to detect serum electrolyte concentrations. B. Reflectance photometry is used in DNA testing. C. Reflectance photometry measures the light intensity emitted from the excited state of the molecule. D. In reflectance photometry, there is a linear relationship between the intensity of light reflected and concentration of the analyte. E. In reflectance photometry, the sample concentration is calculated by comparing the reflectance of a white standard to the light reflected by the sample. 13. A falsely increased result for a given analyte in samples containing a heterophile antibody, usually a HAMA, can be obtained with some two-site immunometric assays. This can best be explained by which one of the following mechanisms? A. Binding of HAMA to the capture antibody. B. Binding of HAMA to the analyte being measured. C. Binding of HAMA to both the capture antibody and the labeled antibody. D. Binding of the HAMA to the labeled antibody. E. Binding of the HAMA to both the capture antibody and the analyte that is being measured. 14. Which one of the following statements is true about the activated clotting time (ACT)? B. The test is sensitive to deficiencies in coagulation factors in the intrinsic and final common pathways. C. The test is performed on plasma. D. Clotting times are usually between 50 and 100 seconds. E. The test is often used for managing outpatients treated with Coumadin. 15. Heparin therapy during cardiac surgery can be monitored using either the ACT or heparin assays. Which one of the following statements best describes the principle(s) on which these two assays are based? B. Both assays are based on adding large amounts of thromboplastin to the sample. C. The ACT assay is based on adding a strong contact activator of the clotting cascade to whole blood. The heparin assay is based on titrating the amount of protamine needed to neutralize the heparin present in the sample. D. The ACT assay is based on adding kaolin or Celite to the sample. The heparin assay is based on adding glass beads to the sample. E. The ACT assay is based on adding a strong contact activator of the clotting cascade to whole blood. The heparin assay use antibodies specific for heparin to determine heparin levels. 16. Which one of the following statements is true about the microscopic detection of amniotic fluid crystallization? A. It is classified as a waived test. B. It is positive in the presence of amniotic fluid at any gestational age. C. It is more sensitive for the presence of amniotic fluid than the nitrazine paper test. D. It is more specific for the presence of amniotic fluid than the nitrazine paper test. E. It is rendered unreliable by the presence of even small amounts of blood. 17. Which one of the following is the best example of an advantage of point-of-care testing (POCT), as opposed to testing in a centralized laboratory? A. Improved turnaround time for the test. B. Enhanced competency of the operator performing the assay. C. Decreased test cost. D. Improved test accuracy. E. Improved test precision. 18. Many modern POCT devices have built-in barcode readers. Which one of the following best describes the purpose of these barcode readers? A. Patient identification only. B. Operator identification only. C. Reagent and/or cartridge identification only. D. Quality control material identification only. E. Identification of patients, operators, reagents, cartridges, and quality control material. 19. The appropriate definition, reporting, and documentation of critical values are important issues in the management of clinical laboratories. Which one of the following statements best describes critical values in the context of POCT? B. Critical values from POCTs performed in hospitals are the responsibility of the performing clinical care provider, not of the clinical laboratory. C. Critical values in POCT, and the intervention taken in response to them, must be documented. D. Critical values in POCT are never repeated. E. The documentation of critical values from POCT instruments always requires a separate entry in the patient’s medical record. 20. POCT programs in hospitals are routinely inspected by which one of the following? A. The Department of Health of the state in which the hospital is located. B. A “deemed” organization, such as The Joint Commission or the College of American Pathologists. C. The Centers for Medicare and Medicaid Services. D. A professional organization, such as the American Association for Clinical Chemistry. E. The Food and Drug Administration. 21. Which one of the following statements best describes the use of POC glucose meters? A. They should not be used to manage diabetes because they are not sufficiently accurate. B. They should not be used to screen for diabetes. C. They should not be used to diagnose diabetes. D. Their results are not affected by lipemia. E. They all use the same chemical reaction for measuring glucose. 22. According to the Clinical Laboratory Improvement Amendments of 1988 (CLIA’88), POCT programs must keep records of all quality control activities for which one of the following time intervals? B. At least 1 year. C. As long as the program is active. D. At least 5 years. E. For the amount of time required by state law. 23. Many modern POCT instruments can be connected to a hospital’s data network. The connection can be either through docking stations or through a wireless connection. Which one of the following currently represents the best advantage of a wireless connection versus a cable-based docking connection? A. Setting up wireless connections is always cheaper. B. A wireless network is safer. C. Wireless connections provide faster availability of results in the electronic medical record. D. The wireless connection can be bidirectional and allow both uploading of patient data and physician orders to the POCT device. E. A wireless connection can be turned off more easily if the security of the network has been compromised. 24. Provider-performed microscopy procedures are tests performed by a health care provider using a microscope. Which one of the following statements best describes how these procedures are usually performed? A. After the patient leaves the provider’s office. B. By physicians and nurses. C. For the determination of white blood cell differentials in peripheral blood smears. D. On specimens that require only limited sample handling or processing. E. With bright-field, fluorescence, or phase-contrast microscopy. 25. The nitrazine paper test is used to detect the presence of amniotic fluid and to confirm the premature rupture of membranes. Which one of the following is a true statement about this test? A. The nitrazine paper test has a wide pH range. B. The nitrazine paper test has a sensitivity and specificity of greater than 95%. C. The nitrazine paper test has a sensitivity of 40% and a specificity of greater than 75%. D. The nitrazine paper test has a sensitivity of 95% and a specificity of greater than 40%. E. The reasons for false-positive nitrazine tests are unknown. 26. Many POCT analyzers use conductivity-based methods for determining hemoglobin and hematocrit. Which one of the following best describes the major limitation of this method compared with the optical method? A. Conductivity-based methods take longer to perform than optical methods. B. Conductivity-based methods are more difficult to calibrate than optical methods. C. Conductivity-based methods can be significantly influenced by plasma composition, including electrolyte and protein concentrations. D. Conductivity-based methods are less accurate at higher hematocrit levels. E. Conductivity-based methods are less accurate at lower hematocrit levels. 27. Which one of the following statements best describes the relationship between hematocrit and POCT blood glucose measurements? B. A high hematocrit leads to overestimation of blood glucose levels; a low hematocrit leads to underestimation of blood glucose levels. C. High and low hematocrit levels both lead to overestimation of blood glucose levels. D. High and low hematocrit levels both lead to underestimation of blood glucose levels. E. There is no effect of hematocrit on blood glucose determination using POCT devices. 28. Which one of the following statements best describes the limitation of both thromboelastography and thromboelastometry? A. They do not measure platelet function and platelet count. B. They do not measure fibrinogen levels. C. They do not measure the final common pathway of the coagulation cascade. D. They cannot be performed on citrated blood samples. E. Results obtained with these methods often do not correlate well with results obtained by standard coagulation tests, such as the prothrombin time and the partial thromboplastin time. 29. Hospital-wide electronic order entry systems with onsite label printing and specimen collection scanning facilitate electronic capture of various time element(s) of the preanalytical process. Which one of the following best describes what is possible using this approach? B. Capturing order and collection times. C. Capturing critical value result times. D. Identifying result corrections and capturing result update times. E. Capturing accession, order, and collection times. 30. The clinical staff members at your hospital complain that it takes too long to receive laboratory test results from the primary reference laboratory you use. You investigate and determine that the reference laboratory faxes results to your laboratory in a timely fashion; however, due to your own staffing issues, there are major delays in the transcription of these results into your laboratory information system (LIS). You determine that direct electronic interfacing of your LIS with the reference laboratory would eliminate these delays. However, hospital administration informs you that the money needed for this interface is not available in the current budget. Which one of the following contains the best arguments you should make to hospital administration? B. Electronic interfacing of the LIS with the reference laboratory will eliminate the need for laboratory employees to manually transcribe results; this will reduce overtime and/or staffing costs and, thereby, save the hospital money. In addition, by decreasing the turnaround times for these results, patients may be discharged faster and the average length of stay may decrease, thereby allowing the hospital to improve revenue. C. Electronic interfacing of the LIS with the reference laboratory will decrease courier costs. D. Electronic interfacing of the LIS with the reference laboratory will decrease the time required to transport samples to the reference laboratory. E. Electronic interfacing of the LIS with the reference laboratory will increase transcription errors in preanalytical functions in your laboratory. 31. A byte is a unit of measurement for information storage on computers. How many unique combinations of letters, numbers, or special characters can 1 byte produce? B. 8. C. 64. D. 128. E. 256. 32. Which one of the following is an advantage of adding computerized physician order entry (CPOE) to an existing electronic health record (EHR) system versus implementing a new EHR with CPOE? B. Greater cost of supporting an older system. C. Greater disruption of operations. D. Greater variety of options. E. Increased number of user devices required. 33. Which one of the following would be the final step in purchasing a new LIS? A. Determine functional requirements. B. Form a working group. C. Negotiate the contract. D. Send out a request for information (RFI). E. Send out a request for proposals (RFP). 34. If all power sources to a computer are turned off, which one of the following storage devices is most likely to lose its data? B. Hard disk drive. C. Solid-state drive. D. Random-access memory (RAM). E. Read-only memory (ROM). 35. Which one of the following is an example of an output device? B. Keyboard. C. Monitor. D. Mouse. E. Scanner. 36. Which one of the following is the best example of an input device? B. Label printer. C. LCD projector. D. Monitor. E. Scanner. 37. In your laboratory, the Cockcroft-Gault equation for creatinine clearance is calculated in the laboratory information system and reported along with the serum creatinine. Which one of the following best describes how accuracy checks for calculations should be performed? A. Accuracy of the calculations and reports should be reviewed on a periodic basis. B. Accuracy of the calculations and reports need to be checked only when a system change is made. C. Calculations shared by multiple hospitals using the same laboratory information should be checked by every site. D. Checks of calculation accuracy do not have to be performed if calculations are made by middleware. E. Checks of calculation accuracy do not have to be performed if calculations are made by the analyzer. 38. Which one of the following best describes the purpose of Logical Observation Identifiers Names and Codes (LOINC)? A. Links human-readable terms to concepts. B. Standard used for the transfer of information associated with blood transfusion. C. Communications protocol used for sending and receiving data across the Internet. D. Seventh level of the seven-layer communications model for open systems interconnection. E. Universal identifiers for laboratory and other clinical observations. 39. Which one of the following best describes the main function of the Massachusetts General Hospital Utility Multi-Programming System (MUMPS)? A. It is a utility software to optimize a computer system. B. It allocates computer resources to more than one application (i.e., multitasking). C. It is an Internet protocol. D. It is a laboratory information system. E. It is a programming language. 40. Which one of the following applications is best solved by a neural network? B. Delta check. C. Calculations. D. Image-based white blood cell differential. E. Reflex test ordering. 41. Which one of the following is the preferred method for inputting data into an electronic health record (EHR)? A. Automated interface to other systems. B. Free text manual data entry. C. Scanning documents with subsequent optical character recognition translation. D. Structured form manual data entry. E. Transcription of physician dictation. 42. Which one of the following represents the best advantage of an EHR over a paper-based system? A. Smaller financial investment. B. Fewer organizational challenges. C. Catastrophic loss of all patient data. D. Smaller time commitment for physician entry. E. Increased availability of records. 43. Which one of the following best describes the Health Information Technology for Economic and Clinical Health (HITECH) Act? B. Promotes the adoption and meaningful use of electronic health records. C. Protects identifiable patient information from being used to identify patient safety events. D. Requires all federal employees to take an information security awareness program. E. Requires federal agencies to develop, document, and implement security of information systems. 44. Which one of the following best describes the seventh level of the seven-layer communications model for Open Systems Interconnection (OSI)? B. Data layer. C. Network layer. D. Physical layer. E. Session layer. 45. Packet-filtering routers and an application gateway are two types of firewalls. Which one of the following represents the best advantage of packet-filtering over application gateway firewalls? A. Easier auditing of incoming traffic. B. Less processing overhead. C. Easier logging of incoming traffic. D. Better security. E. Ability to relay packets without examining them. 46. Which one of the following best describes the purpose of the Federal Information Security Management Act of 2002 (FISMA)? B. Establishes national standards for the security and maintenance of confidentiality of protected health information. C. Requires all EHRs to maintain a log of users who access a health record and what the user accessed. D. Requires federal agencies to develop and implement plans to ensure information and information system security. E. Requires all hospitals to be certified by a federal agency prior to implementing an EHR. 47. Middleware versions must have a short cycle time to adjust to rapid changes in which one of the following? B. LIS upgrades. C. Instrument interface engine upgrades. D. User-defined expert rules. E. Server hardware upgrades. 48. Which one of the following issues must be resolved before implementation of total laboratory automation? A. Multiple different types of specimen containers are used throughout the hospital. B. Many samples arrive hand-labeled accompanied by a paper requisition. C. The available capital budget does not contain enough funds to purchase the automation line. D. The Laboratory Information System (LIS) assigns the same specimen number to all specimens collected from a single patient phlebotomy. E. The laboratory desires to continue to use instrumentation from different vendors in different sections of the laboratory. 49. Which one of the following represents the best reason to implement total laboratory automation in a clinical laboratory? A. It does not require a major financial investment. B. Little planning is required for implementation. C. It can increase production capacity severalfold without adding space or personnel. D. Usually, no architectural work is required. E. Organizational changes are usually not required. Major points of discussion ■ According to the CAP checklist, competency can be assessed by the following: 2. Monitoring the recording and reporting of test results, including, as applicable, reporting critical results. 3. Review of intermediate test results or worksheets, quality control records, proficiency testing results, and preventive maintenance records. 4. Direct observation of performance of instrument maintenance and function checks. 5. Assessment of test performance through testing previously analyzed specimens, internal blind testing samples, or external proficiency testing samples. 6. Evaluation of problem-solving skills. ■ Other elements of competency may be assessed, as applicable. For nonwaived tests, all six elements described above must be assessed at each assessment event. For waived tests, it is not necessary to assess all elements at each assessment event; the laboratory may select which elements to assess. ■ Ongoing supervisory review is an acceptable method of assessing competency for certain elements (as examples, direct observation of test performance, instrument maintenance, and problem-solving skills). Competency assessment may be documented in various ways, including a checklist completed by a supervisor.21 2. A. Measurement of the amount of light absorbed at two different wavelengths. Major points of discussion ■ The chemical reactions must be exothermic to generate the energy required for this transition. The reactions usually involve an oxidation step using hydrogen peroxide or other strong oxidants to provide the high energy needed to excite the electrons. ■ When the electrons return to the ground state, the amount of light generated is measured. In chemiluminescence assays, electrons are in an excited state without the absorption of radiation. ■ A heterogeneous chemiluminescence immunoassay assay involves the addition of the sample containing the antigen, the capture antibody, and the antigen labeled with an organic molecule. After incubation and separation of the bound from the free labeled antigen, hydrogen peroxide is added, which reacts with the organic molecule and raises the electrons to the excited state. The amount of light emitted is related to the concentration of the analyte in the sample. ■ One major advantage of a chemiluminescence assay is increased analytical sensitivity. Because there is no need for radiation of the sample, the problems associated with light scattering are eliminated, thereby resulting in a low background. Another advantage is that chemiluminescence assays usually have a wide linear range.5,25 3. A. The enzyme-labeled antigen that is bound to the antibody is inhibited by changing the pH of the solution. Major points of discussion ■ By using genetic engineering, two inactive fragments of the enzyme beta-galactosidase are produced by Escherichia coli. These fragments include an EA that lacks the complementary enzyme donor (ED) fragment required for enzyme activity. Combination of the EA and ED fragments is needed for enzyme activity. ■ In the CEDIA assay, the antigen is bound to the ED fragment and competes with free antigen in the sample for binding sites on the antigen-specific antibody. ■ When the antigen that is bound to the ED binds to the antibody, the combination of the ED with the EA fragment to produce the active enzyme is inhibited. However, if the antigen bound to the ED does not bind to the antibody, then the ED will combine with the EA to produce the active enzyme, which then reacts with the enzyme substrate to produce a chromagen. ■ With the CEDIA assay, there is a direct relationship between the concentration of the free antigen (i.e., the low molecular weight analyte to be quantified) and the amount of chromagen produced. Higher levels of the free antigen in the sample lead to higher levels of non–antibody-bound ED fragment, which combine with the EA to produce higher levels of active enzyme.3,15 4. A. During the ordering of tests. Major points of discussion ■ Most laboratory errors occur during phlebotomy and front-end processing of samples. The manual phlebotomy process—which involves collecting specimen labels, identifying the patient by examining the wristband, matching the labels with the patient, collecting blood using the correct order of draw, and correctly labeling the tubes—is highly susceptible to error. Standardizing the phlebotomy process can greatly reduce these errors. ■ The most common sample processing errors are mislabeling the sample, incorrectly preparing aliquot samples (e.g., pouring from the wrong sample), mislabeling aliquot tubes, and misplacing samples in the laboratory. Automation of sample processing eliminates most of these errors. ■ Errors in physician orders commonly happen because of the similarity of test names, lack of ordering physician knowledge about tests, duplicate orders, and transcription errors. The key to improving test ordering accuracy is to implement computerized order entry by the physician. ■ Delays in transporting the specimen to the laboratory can compromise test results. To minimize postcollection variation, specimens should be delivered, processed, and stored promptly after collection. For example, glucose levels decrease at a rate of 5% to 7% per hour in whole blood maintained at room temperature. Transportation of specimens to the laboratory often significantly delays processing. Mechanical transport systems, typically with pneumatic tubes, are used by some laboratories to expedite specimen delivery. ■ The concept of “critical values” refers to a pathophysiological state that can be life threatening for a patient unless appropriate therapy is initiated rapidly. Failure to report critical values expeditiously can compromise patient care and result in legal actions against the physician, the hospital, and the laboratory. ■ Misinterpretation of test results means that the health care provider received the correct result but did not take the correct action based on the result. Interpretive reports can prevent this type of error and should include a description of the abnormal result, possible reasons for an abnormal result, suggestions for further testing, and a statement of need for treatment.4,7 5. A. Quenching of fluorescence in the excited state. Major points of discussion ■ When the electrons return to the ground state, the energy of the photon emitted is lower than the one that was initially absorbed and emits light at a longer wavelength. ■ The Stokes shift is the difference between the excitation and emission wavelengths. ■ If the Stokes shift is small, the excitation and emission spectra can overlap, causing an increase in background fluorescence due to the scattering effects of the excitation light. ■ A large Stokes shift will reduce the amount of incident light arriving at the detector and will improve the sensitivity of the assay. The difference between the excitation and emission wavelengths should be at least 50 nm but ideally should be more than 200 nm.14 6. A. The fluorescence lifetime of the molecule is decreased, resulting in an increase in analytical sensitivity and a decrease in background fluorescence. Major points of discussion ■ When the Eu3 + organic complex is excited by light irradiation, the organic ligand absorbs energy and electrons are raised from the ground to a singlet excited state. While in the excited state, these electrons can pass to a triplet state. ■ If the ligand is chelated to Eu3 +, there can be an energy transfer from the triplet excited state of the ligand to the Eu3 + ion, which then can move to an excited singlet state and emit fluorescence that is characteristic of Eu3 +. ■ The major advantage of time-resolved immunoassay is its long fluorescence lifetime. In conventional fluorescence assays, the fluorescence lasts about 1 μsec, whereas in time-resolved assays, the fluorescence can be measured at intervals of 400 to 800 μsec. This prolonged fluorescence eliminates the noise created by background fluorescence in biological samples, cuvettes, and solvents. As a result, the sensitivity of the assay is increased. ■ Another advantage of time-resolved fluorescent assays is the long Stokes shift. The Stokes shift in some time-resolved assays can be greater than 200 nm. As a result, there is no overlap between the excitation and emission wavelengths. For example, the excitation and emission wavelengths for a Eu3 + organic complex are 295 and 612 nm, respectively.28,29 7. A. The hook effect caused a false-positive troponin I result. Major points of discussion ■ Heterophile antibodies can arise from • Administering mouse monoclonal antibodies for diagnostic imaging or therapeutics • Exposure to animals • Vaccination • Blood transfusions • Maternal transfer across the placenta to an unborn child ■ The prevalence of heterophile antibodies in healthy blood donors can range from 0.7% to 3.1% and is approximately 3% to 4% in hospitalized populations. ■ HAMAs interfere with some assays for analytes, such as cancer antigen 125 (CA-125), troponin I, troponin T, CK-MB, thyroid-stimulating hormone (TSH), T4, T3, luteinizing hormone (LH), follicle-stimulating hormone (FSH), human chorionic gonadotropin (hCG), carcinoembryonic antigen (CEA), prostate-specific antigen (PSA), prolactin, hepatitis B surface antigen, C-reactive protein (CRP), progesterone, and alpha-fetoprotein (AFP). ■ The clinical laboratory can detect the presence of a heterophile antibody by measuring the analyte using another immunochemical method that does not use the same antibodies that were used in the original method. If a heterophile antibody is present, a much lower analytical result will typically be obtained with the second method. ■ Heterophile antibodies can also be identified by serially diluting the sample. Heterophile antibody–positive samples frequently yield aberrant dilution results. ■ The heterophile antibody can also be removed by using protein G or protein A immobilized on agarose (Sepharose) beads or by precipitation with polyethylene glycol.6,9,19 8. A. The enzyme-labeled antigen activity is inhibited by changing the pH of the reaction. Major points of discussion ■ An enzyme (usually glucose-6-phosphate dehydrogenase) is labeled with a drug (i.e., enzyme-bound antigen) and competes with the free drug in the sample (i.e., the free antigen) for binding sites on a drug-specific antibody. After the reaction is complete, enzyme substrate is added and the amount of enzyme product produced is measured spectrophotometrically. ■ The enzyme-labeled drug that is bound to the antibody is sterically inhibited from reacting with the enzyme substrate. Only the free, non–antibody-bound enzyme-labeled drug reacts with the enzyme substrate. ■ The concentration of the drug in the patient sample is directly related to the amount of enzyme product produced. ■ The EMIT assay can be used with any spectrophotometer and is the most common procedure used for quantifying serum drug levels.22 9. A. No fluorescence is observed when antibody binds to the fluorescent-labeled drug. Major points of discussion ■ If a fluorescent molecule absorbs polarized light to produce the excited state, the emitted light will also be polarized if the fluorophore does not rotate during its time in the excited state. If the fluorophore rotates in the excited state before fluorescence emission takes place, the emitted light will be depolarized. ■ The principle of FPIA involves competition between a fluorescent-labeled antigen (i.e., the drug; fluorescein is the most common fluorophore) and the antigen (i.e., free drug) in the sample for binding sites on an antigen-specific (i.e., drug-specific) antibody. ■ The antibody-bound fluorescent-labeled antigen is a large molecule and rotates slowly in the excited state. Therefore, the polarization of the emitted light is not significantly changed from the polarized light that was used to excite the molecule. ■ The non–antibody-bound fluorescent-labeled antigen is a small molecule and rotates rapidly in the excited state before fluorescence light is emitted. In this case, the emitted light is depolarized. ■ In an FPIA assay, the amount of polarized light emitted is inversely related to the sample concentration of the analyte to be measured. At low analyte concentrations, more of the fluorescent-labeled antigen will bind to the antibody, resulting in a large amount of emitted polarized light.27 10. A. Binding of the HAMA to the analyte that is being measured, thereby causing a falsely elevated result. Major points of discussion ■ There is an inverse relationship between the amount of labeled analyte that binds to the capture antibody and the concentration of analyte in the sample. At low analyte concentrations, more of the labeled analyte binds to the capture antibody and a high signal is observed. ■ If a HAMA is present and binds to the capture antibody, it prevents formation of the labeled analyte–capture antibody complex, thereby resulting in a lower than expected signal and a higher than expected analytical result. ■ The interference of HAMA with competitive binding assays is not as frequent as with two-site immunometric assays because of the greater affinity of the analyte for the antibody binding sites. ■ Falsely elevated results have been observed for competitive binding assays for free T4, T4, and inhibin A. Addition of blocking agents (e.g., mouse or animal immunoglobulins) is used to prevent binding of HAMA to the capture antibody. 11a. A. Interference by heterophile antibodies (i.e., HAMAs). 11b. A. Repeat the hCG assay using another serum sample. Major points of discussion ■ The high-dose hook effect occurs in one-step immunometric assays when the sample and signal antibody are simultaneously added to the capture antibody and incubated. The presence of large amounts of the antigen in the sample limits the number of antigen-antibody complexes that can be formed because excess antigen saturates the capture and signal antibodies and blocks the formation of the antigen-antibody complex, which then results in a lower signal than expected. If the concentration level obtained falls in the linear range of the assay, the sample is not diluted and a falsely low concentration will be reported. ■ In this case, the sample was serially diluted with the zero concentration hCG calibrator. The hCG level obtained after dilution was 3,500,000 IU/L. Because of this very high hCG level, very little of the antibody-antigen complex was formed, resulting in a normal hCG level. ■ The use of a two-step immunometric assay could eliminate the high-dose hook effect. It involves adding the sample to the capture antibody, an incubation step, and a subsequent washing step that removes the excess antigen. Adding the signal antibody in the second step of the assay would result in a signal that is above the linear range of the assay. ■ In this case, the sample would be diluted and the correct result reported. However, manufacturers are reluctant to use this technique because they would have to change the reagent formulation, which would increase the reporting time for the analyte and would involve approval by the FDA. ■ If the hook effect is undetected, a significantly lower analyte value will be reported, which can result in patient mismanagement. The laboratory should have a dilution protocol to test for the hook effect when using these types of assays.1,2 12. A. Reflectance photometry is used to detect serum electrolyte concentrations. Major points of discussion ■ Light from a lamp passes through a filter wheel that selects the wavelength of interest. The light is directed to the test surface of the reagent strip or dry film slide. Some light is absorbed by the chromophore on the test surface and some light is reflected. The amount of light reflected is focused onto a photomultiplier. ■ In reflectance photometry, light can be directed onto the reagent strip at a 45-degree angle and the reflected light detected at a 90-degree angle. The amount of the reflected light depends on the amount of chromophore formed and is related to the concentration of the analyte in the sample. ■ The relationship between the amount of reflected light and the concentration of the analyte is not linear. To calculate the sample concentration, the signal from the reflected light of the sample is compared with the signal from a reference white standard. The signal from the white standard is set to 100% because all light is reflected. The reflection density, which is the ratio of the reflected light from the white standard and the light reflected by the sample, is obtained and an algorithm is used to linearize the relationship of this reflectance signal/concentration ratio. ■ Reflectance photometry is used in POC testing, in urinalysis testing using reagent strips, in automated analyzers (e.g., the Vitros system from Ortho Diagnostics, which uses dry-film slide technology for measuring routine chemistry analytes), and in therapeutic drug monitoring.8 13. A. Binding of HAMA to the capture antibody. Major points of discussion ■ The normal reaction of an analyte with both the capture and labeled antibodies in a two-site immunometric assay is shown in Figure 3-1, A, in reaction scheme a. Sample and labeled antibody are added to a capture antibody, thereby resulting in an antibody-antigen complex. The concentration of the analyte in the sample is directly related to the amount of antibody-antigen complex that is formed. The positive interference from heterophile antibodies (e.g., HAMA) is shown in Figure 3-1, A, in reaction scheme b. The heterophile antibody will bind to both the capture and labeled antibodies at either the Fc or Fab positions on the reagent IgG molecules and cause a falsely elevated result.
General Laboratory
Instrumentation, Analytic Techniques, Automation, Point of Care Testing, and Informatics
Rationale: This is the definition of a bichromatic measurement of an analyte.
B. Measurement of the amount of emitted light after a molecule is chemically excited from the ground to the excited state.
Rationale: This is the definition of chemiluminescence
C. Measurement of the amount of emitted light after a molecule is biologically excited from the ground to the excited state.
Rationale: This is the definition of bioluminescence
D. Measurement of the amount of emitted light when a molecule in a triplet state returns to the ground state.
Rationale: This is the definition of phosphorescence.
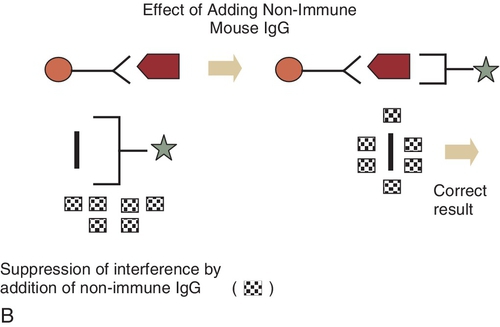
E. Measurement of the amount of emitted light after a molecule is excited from the ground to the excited state by a short pulse of light.
Rationale: This is the definition of fluorescence.
Rationale: This homogeneous reaction occurs at a single pH.
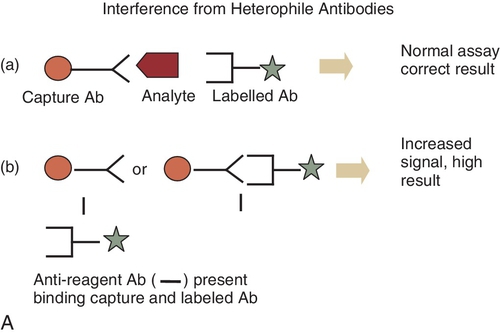
B. The enzyme-labeled antigen that is bound to the antibody reacts at a slower rate with the EA to form the active enzyme than the enzyme-labeled antigen that is not bound to the antibody.
Rationale: The enzyme-labeled antigen that is bound to the antibody does not react with the EA at all.
C. The enzyme-labeled antigen that is bound to the antibody reacts with the EA to form the active enzyme.
Rationale: The enzyme-labeled antigen that is bound to the antibody is inhibited from binding to the EA.
D. The enzyme-labeled antigen that is bound to the antibody is physically separated from the enzyme-labeled antigen that is not bound to the antibody and reacts with the EA to form the active enzyme.
Rationale: There is no physical step separating the bound antigen from the free antigen because the CEDIA assay is a homogeneous immunoassay.
E. The enzyme-labeled antigen that is bound to the antibody is inhibited from reacting with the EA. The enzyme-labeled antigen that is not bound to the antibody reacts with the EA to form the active enzyme.
Rationale: See Major Points of Discussion.
Rationale: Approximately 1% to 2% of errors occur when laboratory tests are ordered.
B. During the preanalytical phase of testing.
Rationale: Most errors (68% to 75%) occur in the preanalytical phase of testing.
C. During the analytical phase of testing.
Rationale: Approximately 10% to 32% of errors occur during the analytical testing phase.
D. During the reporting of the test results.
Rationale: Approximately 2% to 5% of errors occur when test results are reported.
E. During physician interpretation of the test results.
Rationale: Approximately 3% to 5% of errors occur because of physician misinterpretation of test results.
Rationale: Quenching is the loss of fluorescence due to interactions between the fluorophore and its molecular environment.
B. Difference between the wavelengths of the excitation light and emitted light in a fluorescence assay.
Rationale: The Stokes shift should be large for the design of a useful clinical assay.
C. Rate of formation of an antibody-antigen complex.
Rationale: This kinetic rate is not the Stokes shift.
D. Difference between detection time of the internal standard and the compound of interest in a high-performance liquid chromatography (HPLC) assay.
Rationale: In HPLC, the internal standard should elute close to the compound of interest.
E. Distance between the solvent front and the compound of interest in a thin-layer chromatography (TLC) assay.
Rationale: In TLC, the retention factor value is the distance traveled by the compound, divided by the distance traveled by the solvent front. This is a useful characteristic of every compound that can be separated by TLC.
Rationale: The fluorescence lifetime is significantly increased in time-resolved fluorescence assays.
B. The fluorescence lifetime of the molecule is increased, resulting in an increase in analytical sensitivity and a decrease in background fluorescence.
Rationale: This is the main advantage of time-resolved fluorescence assays.
C. The small Stokes shift that is observed with time-resolved fluorescent immunoassays increases the sensitivity of the assay.
Rationale: There is a large Stokes shift with time-resolved fluorescence assays.
D. Fluorescence measurements are rapid, which reduces the background fluorescence.
Rationale: Fluorescence measurements are prolonged in time-resolved fluorescent immunoassays.
E. Fluorescence is quenched, which reduces the background fluorescence.
Rationale: Quenching is not a significant issue in time-resolved fluorescence immunoassays.
Rationale: The hook effect would produce a lower-than-expected result
B. Heterophile antibodies interfered with the troponin I assay, causing a false-positive result.
Rationale: The heterophile antibodies are human anti-animal antibodies that bind to the reagent antibodies used in the assay, thereby interfering with their function.
C. The patient was correctly diagnosed with an acute myocardial infarction.
Rationale: The patient did not have an acute myocardial infarction because the troponin I value did not change with time and the CK, CK-MB, and electrocardiogram were all normal.
D. The patient was correctly diagnosed with congestive heart failure.
Rationale: Measurement of brain natriuretic peptide (BNP) or N-terminal prohormone-BNP (NT-proBNP) are used to assess patients for congestive heart failure.
E. There was cross-reactivity of troponin T with the troponin I method.
Rationale: Troponin I methods are very specific and measure only troponin I with little or no cross-reactivity for troponin T.
Rationale: This homogeneous assay is performed at a single pH.
B. The antibody-bound enzyme-labeled antigen complex is not as stable as the free, non–antibody-bound enzyme-labeled antigen complex, and the enzyme activity is measured by a kinetic rate reaction.
Rationale: The enzyme-labeled antibody complex is stable and the enzyme activity is not measured by a kinetic assay.
C. Antibody binding to the enzyme-labeled antigen enhances the enzyme activity.
Rationale: Actually, antibody binding sterically inhibits the enzymatic activity of the antigen-labeled enzyme.
D. Antibody binding to the enzyme-labeled antigen sterically inhibits the enzyme activity.
Rationale: See Major Points of Discussion.
E. The free, non–antibody-bound enzyme-labeled antigen is fluorescent.
Rationale: No fluorescence measurements are involved in EMIT assays.
Rationale: Fluorescence is observed when the fluorescent-labeled drug is bound to the antibody.
B. Rotation of the non–antibody-bound fluorescent-labeled drug is slower than the antibody-bound fluorescent-labeled drug and depolarizes the emitted light.
Rationale: Rotation of the free, non–antibody-bound fluorescent-labeled drug depolarizes the emitted light because it rotates faster than the antibody-bound fluorescent-labeled drug.
C. The antibody-bound fluorescent-labeled drug emits less polarized light than the non–antibody-bound fluorescent-labeled drug.
Rationale: The antibody-bound fluorescent-labeled drug rotates slowly and emits polarized light. Rotation of the non–antibody-bound fluorescent-labeled drug depolarizes the emitted light.
D. The antibody-bound fluorescent-labeled drug emits polarized light. The non–antibody-bound fluorescent labeled drug depolarizes the emitted light.
Rationale: See Major Points of Discussion.
E. Rotation of the non–antibody-bound fluorescent-labeled drug is faster than the antibody-bound fluorescent-labeled drug and emits more polarized light.
Rationale: Rotation of the non–antibody-bound fluorescent-labeled drug depolarizes the emitted light.
Rationale: Binding of HAMA to an analyte is not a common occurrence.
B. Binding of the HAMA to the analyte that is being measured, thereby causing a falsely lower result.
Rationale: HAMA usually will not bind to an analyte.
C. Binding of the HAMA to the capture antibody,6,19 thereby causing a falsely elevated result.
Rationale: See Major Points of Discussion.
D. Binding of the HAMA to the capture antibody, thereby causing a falsely lower result.
Rationale: Binding of HAMA to the capture antibody will cause an elevated result.
E. Binding of HAMA to the labeled analyte, thereby causing a falsely lower result.
Rationale: HAMA usually will not bind to a nonimmunoglobulin analyte.
Rationale: If heterophile antibodies are present, a higher-than-expected result is usually obtained.
B. The hook effect.
Rationale: The hook effect describes the situation when a sample with an extremely high analyte concentration produces a result below that of the highest calibrator.
C. A matrix effect.
Rationale: This will usually not cause the very low levels of hCG observed with this sample.
D. Inhibition of hCG by maternal antibodies.
Rationale: hCG is usually not inhibited by maternal antibodies.
E. Binding of hCG to human immunoglobulin (Ig) G.
Rationale: hCG is usually not bound to IgG.
Rationale: A similar hCG result would be obtained by simply repeating the process.
B. Measure the hCG level using a different method.
Rationale: Because the correct hCG result is very high, measuring hCG with another method would most likely also yield a lower-than-expected hCG result.
C. Precipitate the interfering heterophile antibodies in the sample using polyethylene glycol and remeasure the hCG level.
Rationale: Because heterophile antibodies are not present, this would not affect the hCG result.
D. Perform a serial dilution of the sample and remeasure the hCG levels using the diluted samples.
Rationale: When hydatiform moles are present, serum hCG levels are very high and a hook effect can be observed. Serial dilutions of the sample are used to determine the correct hCG level.
E. Add mouse immunoglobulins to the sample to remove the interference from heterophile antibodies and remeasure the hCG level.
Rationale: The same hCG result would be obtained because heterophile antibodies are not the cause of these low hCG values.
Rationale: Serum electrolytes are measured using ion-specific electrodes.
B. Reflectance photometry is used in DNA testing.
Rationale: DNA testing typically uses other types of methods, such as those involving capillary electrophoresis, polymerase chain reaction, etc.
C. Reflectance photometry measures the light intensity emitted from the excited state of the molecule.
Rationale: This defines fluorescence.
D. In reflectance photometry, there is a linear relationship between the intensity of light reflected and concentration of the analyte.
Rationale: There is a nonlinear relationship between the intensity of the reflected light and sample concentration.
E. In reflectance photometry, the sample concentration is calculated by comparing the reflectance of a white standard to the light reflected by the sample.
Rationale: See Major Points of Discussion.
Rationale: HAMA must bind to both the capture and labeled antibody to produce a signal.
B. Binding of HAMA to the analyte being measured.
Rationale: HAMA does not typically bind to nonimmunoglobulin analytes.
C. Binding of HAMA to both the capture antibody and the labeled antibody.
Rationale: In this case, a signal will be generated and a falsely increased result will be reported.
D. Binding of the HAMA to the labeled antibody.
E. Binding of the HAMA to both the capture antibody and the analyte that is being measured.
Rationale D and E: To produce a signal, the HAMA needs to bind to both the capture and labeled antibodies.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


General Laboratory: Instrumentation, Analytic Techniques, Automation, Point of Care Testing, and Informatics
Figure 3-1 A and B, Schematic diagram of mechanisms of interference of reagent antibodies by heterophile antibodies. Ab, antibodies; IgG, immunoglobulin G.
