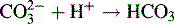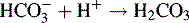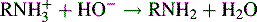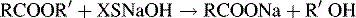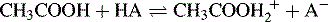3 Titrimetric and chemical analysis methods
Direct acid/base titrations in the aqueous phase
Titrations of the salts of weak bases in mixed aqueous/non-aqueous media
Indirect titrations in the aqueous phase
Karl Fischer titration (coulometric end-point detection)
Automation of wet chemical methods
Applications of FIA in pharmaceutical analysis
Applications
• Provide standard pharmacopoeial methods for the assay of unformulated drugs and excipients and some formulated drugs, e.g. those that lack a strong chromophore.
• Used for standardisations of raw materials and intermediates used in drug synthesis in industry. Suppliers of raw materials may provide these materials at a specified purity which has been assayed titrimetrically to a pharmacopoeial standard.
• Certain specialist titrations, such as the Karl Fischer titration used to estimate water content, are widely used in the pharmaceutical industry.
Advantages
• Capable of a higher degree of precision and accuracy than instrumental methods of analysis, with precisions of ca ± 0.1% being achievable.
• The methods are generally robust.
• Cheap to perform and do not require specialised apparatus.
• They are absolute methods and are not dependent on the calibration of an instrument.
Introduction
Titrimetric methods are still widely used in pharmaceutical analysis because of their robustness, cheapness and capability for high precision. The only requirement of an analytical method that they lack is specificity. This chapter covers the theoretical basis of most of the commonly used methods; the practical aspects of titrations have been covered thoroughly by other textbooks.1,2
Instrumentation and reagents
Primary standards and standard solutions
Primary standards are stable chemical compounds that are available in high purity and which can be used to standardise the standard solutions used in titrations. Titrants such as sodium hydroxide or hydrochloric acid cannot be considered as primary standards since their purity is quite variable. So, for instance, sodium hydroxide standard solution may be standardised against potassium hydrogen phthalate, which is available in high purity. The standardised sodium hydroxide solution (secondary standard) may then be used to standardise a standard solution of hydrochloric acid. Table 3.1 lists some commonly used primary standards and their uses.
Table 3.1 Primary standards and their uses
| Primary standard | Uses |
| Potassium hydrogen phthalate | Standardisation of sodium hydroxide solution |
| Potassium hydrogen phthalate | Standardisation of acetous perchloric acid |
| Potassium iodate | Standardisation of sodium thiosulphate solution through generation of iodine |
| Anhydrous sodium carbonate | Standardisation of hydrochloric acid |
| Zinc metal | Standardisation of EDTA solution |
EDTA, Ethylenediamine tetracetic acid.
Direct acid/base titrations in the aqueous phase
Strong acid/strong base titrations
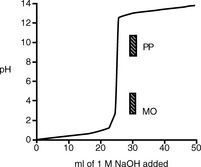
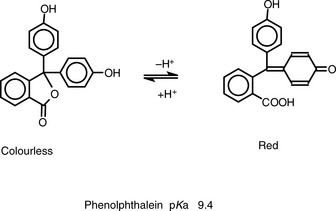
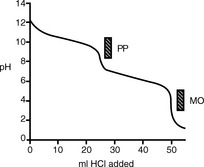
Figure 3.1 shows the titration curve obtained from the titration of a strong acid with a strong base. The pH remains low until just before the equivalence point, when it rises rapidly to a high value. In many titrations a coloured indicator is used, although electrochemical methods of end-point detection are also used. An indicator is a weak acid or base that changes colour between its ionised and un-ionised forms; the useful range for an indicator is 1 pH either side of its pKa value. For example, phenolphthalein (PP) pKa 9.4 (colour changes between pH 8.4 and pH 10.4) undergoes a structural rearrangement as a proton is removed from one of its phenol groups when the pH rises, and this causes the colour change (Fig. 3.2). Methyl orange (MO) pKa 3.7 (colour changes between pH 2.7 and pH 4.7) undergoes a similar pH-dependent structural change. Both these indicators fall within the range of the inflection of the strong acid/strong base titration curve.
There are only a few direct strong acid/strong base titrations carried out in pharmacopoeial assays.
Weak acid/strong base and weak base/strong acid titrations
Figure 3.3 shows a plot of pH when 1 M NaOH is added to 25 ml of a 1 M solution of the weak acid aspirin.
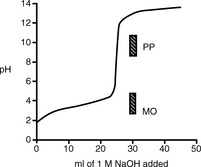
Fig. 3.3 Titration curve for 25 ml of a 1.0 M solution of aspirin (pKa 3.5) titrated with 1.0 M NaOH.
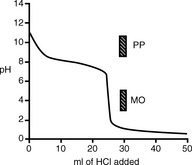
In the example of the titration of quinine with hydrochloric acid (Fig. 3.4), MO is a suitable indicator because it falls within the inflection of the titration curve whereas PP is not suitable.
In a titration of sodium carbonate, the first inflection is indicated by PP and the whole titration by MO (Fig. 3.5).
Titrations of the salts of weak bases in mixed aqueous/non-aqueous media
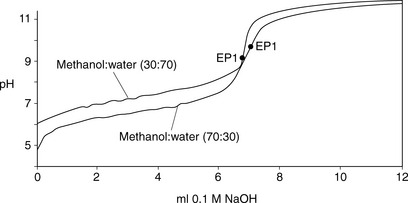
The advantage of adding a water-miscible solvent such as methanol to the titration is twofold. Firstly, the addition of the organic solvent effectively lowers the pKa value of the base, since the ionised form of the base is less stable in a mixed solvent system where the dielectric constant is lower, and, secondly, the organic solvent keeps the base in solution as it is converted to its free base form during the titration. An example of this can be seen for the titration of lidocaine (lignocaine) hydrochloride in methanol/water mixtures (Fig. 3.6), where the size of the inflection in the titration curve increases when moving from 30% methanol to 70% methanol. This is a very convenient procedure for many organic bases.
Indirect titrations in the aqueous phase
Estimation of esters by back titration
Excess of sodium hydroxide is added to the ester. The following reaction occurs:
The XSNaOH is back titrated with HCl using PP as an indicator.
Saponification value
The following data were obtained for a sample of cod liver oil:
Weight of oil taken for analysis = 2.398 g
Ethanolic KOH (molecular weight 56.1) used in determination = 0.986 M
Amount of ethanolic KOH used for hydrolysis and in blank titration = 25 ml
Amount of 0.470 M HCl required to neutralise excess KOH = 35.2 ml
Amount of 0.470 M HCl required in the titration of blank = 52.3 ml
Calculation
Amount of KOH used initially = 52.3 × 0.47 = 24.6 mmole
Amount of HCl required to neutralise excess KOH = 35.20 × 0.470 = 16.5 mmole
Amount of KOH used in hydrolysis = 24.6 – 16.5 = 8.1 mmole × molecular weight = mg
Amount of KOH used in the hydrolysis = 8.1 × 56.1 = 454.0 mg
Amount of KOH/g of fixed oil used in the hydrolysis = 454/2.398 = 189.3 mg
Calculate the saponification value of a sample of castor oil from the following data:
• Weight of oil taken for analysis = 2.535 g
• Ethanolic KOH used in the hydrolysis = 1.03 M
• Amount of KOH used in hydrolysis = 25 ml
• Amount of 0.514 M HCl required to neutralise excess KOH = 34.2 ml
• Amount of 0.514 M HCl required in the titration of blank = 50.2 ml.
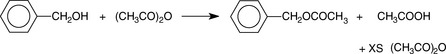
Estimation of alcohols and hydroxyl values by reaction with acetic anhydride (AA)
Alcohols can be determined by reaction with excess acetic anhydride (AA) (Fig. 3.7). This is a useful titrimetric method because the alcohol group is difficult to estimate by any other means.
The excess AA and acetic acid may be back titrated with NaOH using PP as an indicator.
The number of mg of KOH required to neutralise a blank titration of the reagents – the number of mg KOH required to neutralise excess AA + acetic acid after reaction with 1 g of the test substance.
The following data were obtained for a sample of castor oil:
Weight of castor oil taken for analysis = 1.648 g
Volume of acetic anhydride used for the reaction = 5 ml
Molarity of ethanolic KOH used to neutralise the excess AA + acetic acid = 0.505 M
Volume of ethanolic KOH required to titrate 5 ml of reagent = 53.5 ml
Volume of ethanolic KOH required to neutralise excess AA + acetic acid after reaction with the castor oil = 44.6 ml.
Number of mmoles of KOH used in the blank titration = 53.5 × 0.505 = 27.0
Number of mg of KOH used in the titration of the blank = 27.0 × 56.1 = 1515
Number of mmoles of KOH used in titration of AA + acetic acid = 44.6 × 0.505 = 22.5
Number of mg KOH used in titration of excess AA + acetic acid = 22.5 × 56.1 = 1262
Non-aqueous titrations
Theory
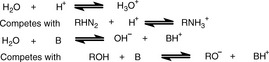
The theory is, very briefly, as follows: water behaves as both a weak acid and a weak base; thus, in an aqueous environment, it can compete effectively with very weak acids and bases with regard to proton donation and acceptance, as shown in Figure 3.8.
Non-aqueous titration of weak bases
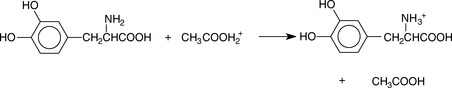
Perchloric acid is the strongest of the common acids in acetic acid solution, and the titration medium usually used for non-aqueous titration of bases is perchloric acid in acetic acid. Addition of acetic anhydride, which hydrolyses to acetic acid, is used to remove water from aqueous perchloric acid. Weak bases compete very effectively with acetic acid for protons. Oracet blue, quinalidine red and crystal violet (very weak bases) are used as indicators in this type of titration. A typical analysis is shown in Figure 3.9 for L-3,4-dihydroxyphenylalanine (LDOPA).
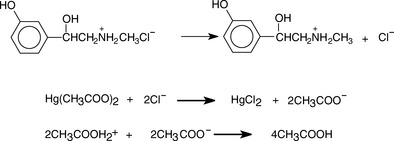
When the base is in the form of a salt of a weak acid, removal of an anionic counter ion prior to titration is not necessary, e.g. for salts of bases with weak acids such as tartrate, acetate or succinate. However, when a base is in the form of a chloride or bromide salt, the counter ion has to be removed prior to titration. This is achieved by the addition of mercuric acetate; the liberated acetate is then titrated with acetous perchloric acid. This is illustrated in Figure 3.10 for the example of phenylephrine HCl.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree